Abstract
Emergent records propose that Aspergillus niger endophytic fungus is a vital source for various bioactive molecules possessing many biological properties. The current study was designed to inspect the antibacterial and anti-Toxoplasma potentials of Ficus retusa-derived endophytic fungi. After isolation and identification (using 18S rRNA gene sequencing) of A. niger endophytic fungus, LC/MS was utilized for identification and authentication of the chemical profile of the A. niger endophyte extract. Then, the fungal extract was assessed for its antibacterial and antibiofilm activities against Klebsiella pneumoniae clinical isolates. Additionally, its efficacy against Toxoplasma gondii was elucidated in vivo. The fungal extract displayed antibacterial activity against K. pneumoniae isolates with minimum inhibitory concentration values of 64–512 µg/mL. It also possessed a membrane potential dissipating effect using flow cytometry. Moreover, it formed distorted cells with rough surfaces and deformed shapes using a scanning electron microscope (SEM). Regarding its antibiofilm activity, it resulted in a dysregulation of the genes encoding biofilm formation (fimH, mrkA and mrkD) using qRT-PCR in nine K. pneumoniae isolates. The in vivo anti-Toxoplasma potential was demonstrated by decreasing the mortality rate of mice and reducing the tachyzoites’ count in the peritoneal fluids and liver impression smears of mice. In addition, the deformities of the parasite decreased, as revealed by SEM and the inflammation in tissues diminished. Thus, A. niger endophytic fungi could be a valuable source of antibacterial and anti-Toxoplasma compounds.
Introduction
With the global antibiotic resistance crisis, Klebsiella pneumoniae has emerged as one of the most important antibiotic-resistant pathogens associated with severe infections that harshly creep up human health [Citation1]. K. pneumoniae can cause various infections, such as pneumonia, urinary tract infections, surgical wound infections, cystitis and severe community-acquired infections, such as pyogenic liver abscesses, necrotizing pneumonia and endogenous endophthalmitis. Furthermore, it is an important cause of life-threatening severe infections with high morbidity and mortality, including endocarditis and septicaemia [Citation2]. This is due to the limited treatment options, which pose a significant threat to the future of infectious diseases [Citation3].
The scientific community is challenged to find novel alternatives to combat these drug-resistant microbes. Researchers have explored natural products as prospective sources of antimicrobial agents [Citation4]. In this regard, there is an increasing interest in investigating endophytic fungi due to their diverse nature and potentials as a significant source of secondary bioactive metabolites that would be helpful in our battle against harmful antibiotic-resistant microbes [Citation5,Citation6]. Endophytic fungi primarily exist in all medicinal plants. They live in the inner tissues and inside their hosts’ plant cells without triggering any adverse effects or damage [Citation7]. Endophytic fungi synthesize bioactive components such as phenols, alkaloids, terpenoids, lignans, lactones, isocoumarins, steroids, phenylpropanoids and quinones [Citation8]. These secondary metabolites could have substantial bactericidal, fungicidal, cytotoxic and antioxidant activities. Therefore, fungal endophytes could be explored as an alternative strategy to combat multidrug-resistant (MDR) pathogens [Citation5].
Toxoplasmosis is a worldwide disease exacerbated by the obligate intracellular protozoan Toxoplasma gondii. It is among the most prevalent infections in people [Citation9]. Consuming cyst-infected, raw or undercooked can cause infection in humans. It is an opportunistic infection, and although it is asymptomatic in most individuals, it could be a life-threatening disease in immunocompromised patients [Citation10].
Despite the fact that the admixture of pyrimethamine and sulfadiazine is considered the first-choice treatment for toxoplasmosis, it is intolerant by many patients due to its severe side effects. It can cause severe allergy, haematological toxicity and bone marrow suppression in addition to hepatic and renal toxicity [Citation11]. Therefore, it is urgently necessary to look for novel and safe bioactive components from natural sources such as endophytic fungi to develop new therapeutic drugs for human diseases such as toxoplasmosis [Citation12,Citation13].
Herein, we aimed to isolate, identify and phytochemically analyse the endophytic fungi isolated from F. retusa plant. Then, its in vitro antimicrobial activity against K. pneumonia clinical isolates and its in vivo anti-Toxoplasma activity will be investigated.
Materials and methods
Chemicals and culture media
All media were obtained from Oxoid (Basingstoke, UK). Spiramycin was obtained from Pharaonia Pharmaceuticals (Cairo, Egypt). The other chemicals were purchased from Merck (Kenilworth, NJ).
Collection of plant samples
The endophytic fungi were isolated from the healthy leaves of Ficus retusa L. collected in September 2021 from the botanical garden of the Faculty of Pharmacy. The plant identification was graciously verified by Dr. Esraa Ammar, Plant Ecology Department, Faculty of Science, Tanta University. A voucher specimen (PG-A-END-D-02) was kept at the Pharmacognosy Department in Tanta University (see section ‘Isolation and identification of the endophytic fungi’ in supplementary file).
Preparation of the fungal extract
The fresh mycelia in each 10 PDA petri dish were inoculated into a pre-autoclaved Erlenmeyer flask containing 100 g Asian rice in 110 mL sterile water. The cotton-plugged fermentation flasks were incubated for three weeks under static conditions at room temperature, away from light. The fungus was extracted with ethyl acetate (3 × 1 L) by sonication at 50 °C for 30 min. The combined ethyl acetate extracts were filtered and concentrated to dryness using a rotary vacuum evaporator at 45 °C and prepared for further biological assay [Citation14] (see section ‘LC–ESI-MS/MS and metabolomics analysis’ in supplementary file).
Antibacterial activity of the endophytic fungal extract
Bacteria
Thirty-two K. pneumoniae isolates obtained from laboratories of Tanta University Hospitals were utilized for screening the antibacterial activities of the isolated endophytic fungus, A. niger. The clinical isolates were identified by microscopical examination and standard biochemical tests [Citation15]. Klebsiella pneumoniae (ATCC 13883) was used as a reference strain (see section ‘Agar well diffusion method: determination of the minimum inhibitory concentration (MIC) of the endophytic fungal extract’ [Citation16] in supplementary file).
Membrane depolarization
Membrane depolarization of the tested bacterial isolates was determined using (bis‐(1,3‐dibutylbarbituric acid)trimethine oxonol) or DiBAC4(3), a fluorescent stain. The staining of the bacterial cells was analysed using FACSVerse flow cytometer (BD Biosciences, Franklin Lakes, NJ) before and after treatment with the fungal extract (at 0.5 MIC values).
Scanning electron microscope (SEM)
The impact of the endophytic fungal extract (at 0.5 MIC values) on bacterial morphology and ultrastructure was studied using SEM. Both treated and untreated K. pneumoniae isolates were examined, and SEM investigations were performed as previously described [Citation17] (see section ‘Crystal violet assay’ in supplementary file).
Antibiofilm activity of the endophytic fungal extract
Real-time PCR
The effect of the endophytic fungal extract on the biofilm-related genes (fimH, mrkA and mrkD) was detected using qRT-PCR, and the housekeeping gene was rpoA [Citation18]. The used primers’ sequences are accessible in Table S1. Briefly, total RNA was extracted using the GeneJET RNA purification kit (Thermo Scientific, Waltham, MA) as described by the manufacturer, cDNA was made using the power cDNA synthesis kit (iNtRON Biotechnology, Seongnam, Republic of Korea). Then, the formed cDNA was amplified using the Power SYBR® Green master mix (Thermo Scientific, Waltham, MA) in a Rotor-Gene Q 5plex machine (Qiagen, Hilden, Germany). For the calculation of the relative gene expression, we used the 2−ΔΔCt method. The gene expression in the tested isolates before treatment was one.
In vivo anti-Toxoplasma activity of the fungal extract
Ethical statement
The study protocol was approved and carried out according to the Laboratory Animal Centre for Research Ethics Committee guidelines at Faculty of Medicine, Alexandria University (code number: 0305750).
Parasites
The RH HXGPRT (–) virulent strain of T. gondii strain was maintained by serial passage in Swiss Albino mice in the Medical Parasitology Department, Faculty of Medicine, Alexandria University.
Experimental design
This experiment used 18 laboratory-bred Swiss Albino mice weighing 20–25 g and aged from four to six weeks. Mice were randomly grouped into the following groups:
Group I (positive control infected non-treated): six mice infected by intraperitoneal injection (IP) of 2500 tachyzoites.
Group II: six mice IP infected with 2500 tachyzoites were then spiramycin-treated in an oral dose of 200 mg/kg/day for five days [Citation19].
Group III: six mice IP infected with 2500 tachyzoites were then treated with endophytic fungal extract in an IP dose of 12.5 mg/kg/day for five days [Citation20].
All mice were euthanized by cervical dislocation on the sixth day post-infection.
Parasitological investigations
The following parameters were conducted in the three groups of mice:
Estimation of the mortality rate (MR) in the experimental groups. The MR was estimated at the sacrificed time allocated for each group of mice.
Counting the mean number of T. gondii tachyzoites in 1 mL of the peritoneal fluid using haemocytometer (HBG®, Gießen, Germany) [Citation21].
Counting the mean number of T. gondii tachyzoites using a light microscope in liver impression smears. The number of tachyzoites of 10 different fields from each mouse was calculated, and then the mean number of each subgroup was determined [Citation21,Citation22].
SEM analysis
Tachyzoites were obtained from the peritoneal exudates of mice. Tachyzoites were isolated from the peritoneal fluid by centrifugation at 1000 × g for 10 min. Then, the supernatant containing tachyzoites was collected and centrifuged at 2000 × g for another 10 min. The pellet of tachyzoites was fixed in buffered glutaraldehyde phosphate (2.5%) and stored at 4 °C. The fixed specimens were washed three times with buffered saline, then dehydrated in ascending concentrations of ethanol and left to dry. Then, samples were dried, mounted on aluminium stubs, coated with 20-nanometer gold particles in an ion sputter evaporator (JFC-1100E, JEOL, Tokyo, Japan) and examined using SEM (JSM-200 IT, JEOL, Tokyo, Japan) [Citation21]. The ultrastructure of the parasites was examined in the treated groups (groups II and III) compared to the corresponding control (group I).
Histopathological study
Formalin-fixed paraffin-embedded liver tissues were processed for histopathological examination. The paraffin-embedded blocks were sectioned at 5 µm for routine haematoxylin and eosin (H&E) staining. The inflammatory score was determined by the number of layers of inflammatory cells as follows: mild <2, moderate 2–4 and severe >4 [Citation23] (see section ‘Statistical data analysis’ in supplementary file).
Results
A. niger endophytic fungi
According to the macroscopic and microscopic characters of the isolated endophytic fungus and the 18S rRNA gene sequencing results, this fungus was identified as A. niger, as presented in . The accession number of the DNA sequence was ON100824, as shown in .
Figure 1. Phylogenetic tree of the isolated endophytic fungus according to its 18S rRNA gene sequence. The yellow highlight indicates the location of the isolated strain named A. niger.
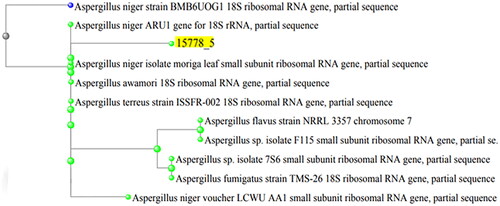
Table 1. Identification data of A. niger based on the 18S rRNA gene sequencing.
LC–ESI-MS/MS analysis
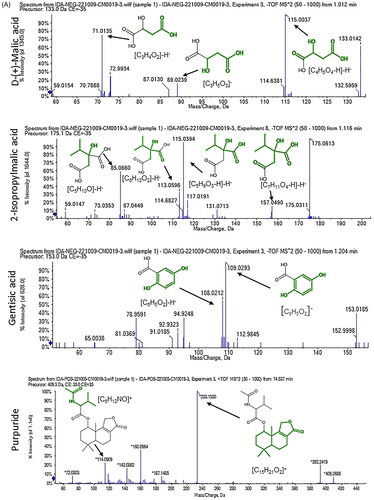
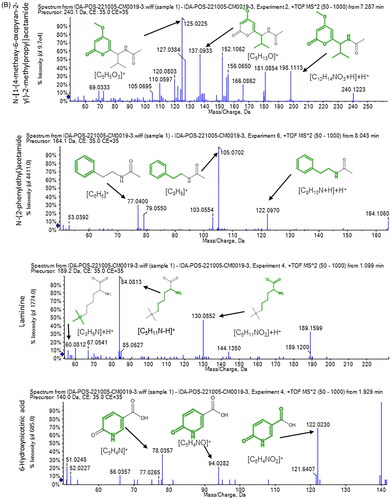
A total of 33 compounds were tentatively identified in A. niger extract using LC–ESI-MS/MS in positive and negative modes. The main substances are amino acids, carboxylic acids, coumarins and flavonols. The metabolite profile is presented in and Figures S1 and S2. While shows mass/mass spectra that display the pattern of some selected metabolites’ fragmentation.
Figure 2. Mass/mass spectra showed a fragmentation pattern of some selected compounds.
Table 2. List of tentatively identified metabolites in A. niger extract analysed by LC–ESI-MS/MS.
In vitro antibacterial activity
Susceptibility of K. pneumoniae isolates to the fungal extract
The fungal extract revealed an inhibition zone around the tested isolates in the agar well diffusion assay, which means it possesses an antibacterial potential against K. pneumoniae isolates. Thus, the MIC values of the fungal extract were elucidated using the broth microdilution technique. As presented in Table S2, the fungal extract’s MIC values ranged from 64 to 512 μg/mL.
Impact of the fungal extract on the membrane depolarization
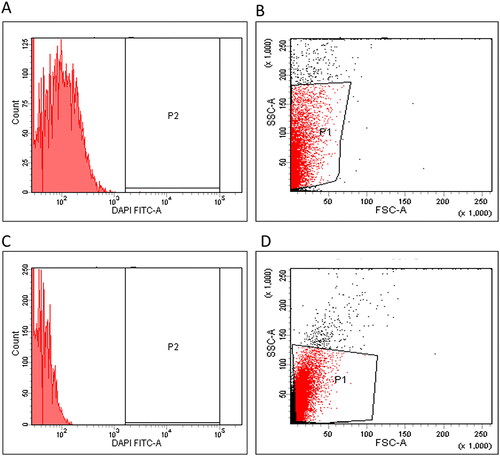
The membrane depolarization was investigated in K. pneumoniae isolates using DiBAC4(3) stain. It is a membrane potential-sensitive stain capable of entering the depolarized cells where it binds to the existing proteins and causes an increased absorbed fluorescence. Here, treatment with the fungal extract was observed to produce a considerable decline in the membrane potential in 31.25% of K. pneumoniae isolates, as revealed in the representative example in .
Impact on the bacterial morphology
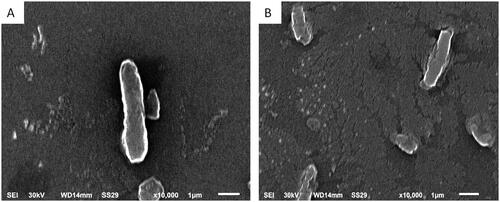
To elucidate the changes induced by the fungal extract on the ultrastructure and the morphology of the tested K. pneumoniae isolates, we examined them using SEM. It revealed some structural alteration in treated cells compared to untreated cells. These alterations included a distortion of some bacterial cells, shape deformation and roughness of cell surfaces, as demonstrated in .
Antibiofilm activity of the fungal extract
The impact of the fungal extract on the phenotypic biofilm formation was assessed using crystal violet method. The fungal extract decreased the number of K. pneumoniae isolates with a strong and moderate biofilm-forming capacity from 53.13% to 18.75%, as presented in .
Table 3. Impact of the fungal extract on the biofilm-forming capacity of K. pneumoniae isolates.
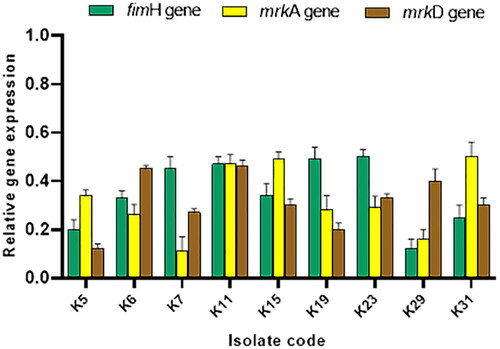
The influence of the fungal extract on the expression of the genes encoding biofilm formation (fimH, mrkA and mrkD) in K. pneumoniae isolates was evaluated using qRT-PCR. As revealed in , the fungal extract resulted in a downregulation of the studied genes in 28.13% of K. pneumoniae isolates.
In vivo anti-Toxoplasma activity
Parasitological study
Mortality rate estimation
There was a marked reduction in the MR of mice treated with polyphenols (16.7%) compared to the control group (41.7%) ().
Table 4. The mortality rate in the investigated groups.
Parasitic load in the peritoneal fluids
The mean count of T. gondii tachyzoites of (134.17 ± 99.17) in the peritoneal fluids of mice treated with the fungal extract decreased significantly (p < .05) compared to the control (1013.33 ± 485.78) as shown in .
Table 5. The mean parasitic count in the peritoneal fluid.
Parasitic load in liver
A significant (p < .05) reduction was observed in the mean count of T. gondii tachyzoites in the liver impression smears of mice treated with the fungal extract (9.17 ± 4.88) compared to the control (17.33 ± 3.56) ( and ).
Figure 6. T. gondii tachyzoites in the liver impression smears (blue arrows) of (A) group I, (B) group II, and (C) group III.
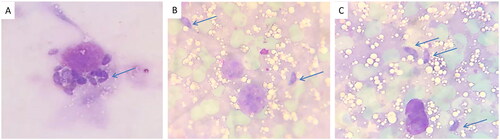
Table 6. The mean parasitic count in liver smears.
Morphological studies
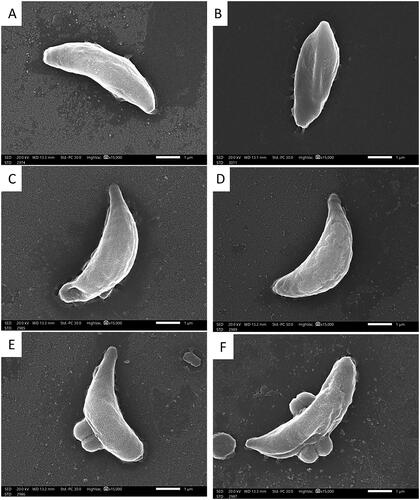
Using SEM, the tachyzoites of T. gondii in the infected control group (group I) appeared elongated, crescent-shaped with a rounded pole at one end and a pointed pole at the other with a smooth regular surface (). While in group II (treated with spiramycin), the tachyzoites were deformed with irregular surfaces. In addition, multiple ridges and papules were noticed on their surfaces (). In group III (treated with the fungal extract), large papules and dimples were noticed on the tachyzoites’ surface (). Also, multiple grooves and ridges were noticed on their surfaces ().
Figure 7. SEM micrographs of T. gondii tachyzoites of (A, B) group I appeared crescent-shaped with a rounded pole at one end and a pointed pole at the other with smooth regular surface (×15,000). (C, D) Group II appeared with multiple ridges and papules on their surfaces (×15,000). (E) Group III showing many papules and dimples (×15,000). (F) Group III shows multiple grooves and ridges on their surfaces (×15,000).
Histopathological results
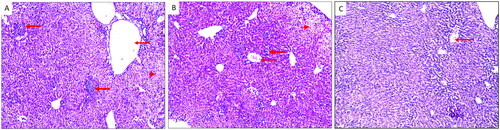
In the infected control group (group I), there was severe inflammatory cellular infiltrates with dilated central veins and areas of hydropic degeneration of the hepatocytes. While in the groups treated with spiramycin (group II) and the fungal extract (group III), there were mild inflammatory infiltrations with normal central veins ().
Figure 8. H&E-stained liver sections of (A) group I showing severe inflammatory cellular infiltrates (thick arrow) together with dilated central vein (thin arrow) and areas of hydropic degeneration of the hepatocytes (arrowhead) (×100). (B) Group II showing moderate inflammatory cellular infiltrates (thick arrow) together with moderately dilated central veins (thin arrow) and areas of hydropic degeneration of the hepatocytes (arrowhead) (×100). (C) Group III showing mild inflammation with normal central veins (thin arrow) (×100).
Discussion
It is well known that many endophytic microorganisms, particularly fungi, can produce multiple bioactive compounds that protect the plant against different pathogens. Many research articles have reported that the natural agents synthesized by the endophytic fungi possess an inhibitory potential against a wide range of plant and animal pathogens [Citation24]. Thus, isolation, identification and phytochemical analysis of the endophytic mycobiota are needed to investigate its therapeutic potential.
Both positive and negative ionization modes of LC–ESI-MS/MS analysis of A. niger endophytic extract resulted in the detection of 33 bioactive metabolite compounds, 20 in the positive ionization mode and 13 in the negative mode. The identified metabolites belong to several phytochemical classes, such as amino acids, carboxylic acids, coumarins and flavonols, in agreement with the previous literature [Citation4,Citation25]. The major identified compounds in positive mode are N-[1-(4-methoxy-6-oxopyran-2-yl)-2-methylbutyl] acetamide, N-[1-(4-methoxy-6-oxopyran-2-yl)-2-methylpropyl] acetamide, purpuride, glycine-betaine, 3,4-dihydroxy-l-phenylalanine and choline. On the other hand, compounds such as spiculisporic acid, mannitol, gluconate and citric acid were identified as majors in the negative mode. Our results are in line with previous research concerning antibacterial effects of different compounds as purpuride [Citation26] and spiculisporic acid [Citation27,Citation28] found in natural extracts.
In recent times, the dissemination of antimicrobial resistance among pathogenic bacteria has pressed an increased need for the elucidation of novel compounds with a broad spectrum of bioactivity to serve as an effective therapeutic option for MDR bacteria [Citation29]. Screening the crude fungal extracts’ antimicrobial potential allows us to isolate and identify the compounds responsible for such bioactivity. Nevertheless, several studies have demonstrated that some separate compounds are significantly less active than the combined extract that contains these compounds [Citation30–32]. This could be explained by the synergism between these compounds when present in the crude extract [Citation30].
Herein, we investigated the in vitro antibacterial activity of A. niger endophytic extract against K. pneumoniae clinical isolates. Remarkably, the fungal extract revealed a range of MICs of 64–512 μg/mL. As K. pneumoniae is regarded as remarked human pathogenic bacteria that can cause a variety of diseases and infections [Citation33], it is essential to explore the probable mechanism of action of A. niger endophytic fungal extract against K. pneumoniae. So, we elucidated its effect on the membrane depolarization and the morphology of the tested isolates.
Owing to the cellular role of the cell membrane and its relatively easy accessibility, it is an substantial target for many antimicrobials [Citation34,Citation35]. Thus, we elucidated the fungal extract effect on the membrane depolarization using DiBAC4(3) stain. The fungal extract resulted in a considerable decline in the membrane potential in 31.25% of K. pneumoniae isolates. Disturbing the function of the bacterial membrane, such as dissipating its potential, can inhibit many critical cellular processes and produce a severe assault on the bacterial cell [Citation36]. Also, the fungal extract affected the cellular morphology of the tested K. pneumoniae isolates, resulting in the treated cells’ deformation. The SEM is usually used to investigate bacterial cell morphological changes due to its high resolution [Citation37,Citation38].
A biofilm is a group of bacteria living together in extracellular polymeric matrix and adhering to a biotic or abiotic source. It is a major virulence factor that helps bacterial cells colonize the human body and produce infection [Citation39]. Thus, antibiofilm agents are highly needed to combat bacterial infections. Here, the fungal extract exhibited antibiofilm potential against the biofilm-forming isolates. To investigate the antibiofilm mechanism of action at a molecular level, we performed qRT-PCR. This technique was conducted to elucidate the fungal extract effect on the biofilm gene expression. Interestingly, the fungal extract downregulated the studied genes (fimH, mrkA and mrkD) in 28.13% of K. pneumoniae isolates.
We also assessed the use of the crude extract of A. niger endophytic fungus isolated from Ficus retusa in treating mice infected with toxoplasmosis. The efficacy of treatment was gauged by monitoring the MR, the parasite load in peritoneal fluids, and liver impression smears of mice. In addition, the morphological changes in T. gondii tachyzoites were investigated by SEM. The histopathological changes in the liver of mice were also investigated.
The fungal extract improved the MR of the treated mice relative to the control group. This decrease can be explained by the inhibitory impact of the bioactive constituents of the fungal extract on the tachyzoites replication.
With respect to the parasite load, it is related to the density of the infection in tissues. There was a substantial reduction (p < .05) in the mean count of T. gondii tachyzoites in the peritoneal fluids and liver impression smears of mice relative to the infected control group. This indicates the high efficacy of the fungal extract in eradicating T. gondii tachyzoites.
Severe deformities were seen in the shape of tachyzoites group III, when the morphological changes in the T. gondii tachyzoites were examined by SEM in the peritoneal exudates of the investigated groups. As stated by Hammouda et al., these malformations may be referred to as interference with DNA synthesis or the parasite’s folic acid cycle. Similar morphological changes were stated by Mady et al. [Citation40] and Gaafar et al. [Citation41], who reported similar results using different treatments of acutely infected mice with toxoplasmosis.
Regarding the histopathological changes in the livers of mice, there was a marked reduction of inflammation in mice treating with fungal extract relative to the infected control group. Also, there was mild inflammatory infiltration and minimal congestion of the central veins of group III. This result indicates the efficacy of the fungal extract in decreasing T. gondii tachyzoites and subsequent decrease in inflammation in liver tissue. Comparable results were published by Anand et al. [Citation42] and Mady et al. [Citation40] treating acute toxoplasmosis in mice.
Conclusions
The rising worldwide dissemination of infectious diseases caused by bacteria and parasites has become a significant challenge for the humankind’s future. A. niger endophytic fungus derived from Ficus retusa plant was elucidated in the current study for its potential activity against K. pneumoniae and T. gondii. This was performed after its chemical profile investigation using LC–MS/MS. The fungal crude extract exhibited inhibitory potential towards K. pneumoniae as revealed using flow cytometry (affected the membrane depolarization), SEM (affected the cell morphology) and qRT-PCR (affected the gene expression of biofilm). In addition, it revealed an inhibitory potential towards T. gondii using in vivo studies. This was proofed by decreasing the MR of mice and reducing the tachyzoites count in the peritoneal fluids and liver impression smears of mice, in addition to the deformities of the parasite by SEM and decreased inflammation in tissues. Hence, further work shall be conducted in the future to elucidate the prospective usage of the isolated endophytic fungi in the clinical setting. Moreover, additional research is required for these isolated endophytes to aid in producing specific biologically active compounds on a large scale.
Author contributions
Ehssan Moglad: conception, design and interpretation of the data. Engy Elekhnawy, Walaa A. Negm, Duaa Eliwa, Salwa Sami Younis, Basma Mohamed Elmansory and Omnia Momtaz Al-Fakhrany: conception, design, methodology, interpretation of the data, drafting of the paper, and revising it critically for intellectual content. Sebaey Mahgoub and Eman A. Ahmed: conception, design and methodology. All authors reviewed the final version of the manuscript.
Supplemental Material
Download MS Word (177.8 KB)Acknowledgements
The authors thank Dr. Nehal Abd El-Ghaffar Heabah.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Navon-Venezia S, Kondratyeva K, Carattoli A. Klebsiella pneumoniae: a major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol Rev. 2017;41(3):252–275.
- Tutelyan A, Shlykova D, Voskanyan SL, et al. Molecular epidemiology of hypervirulent K. pneumoniae and problems of health-care associated infections. Bull Exp Biol Med. 2022;172(5):507–522.
- Attallah NG, El-Kadem AH, Negm WA, et al. Promising antiviral activity of Agrimonia pilosa phytochemicals against severe acute respiratory syndrome coronavirus 2 supported with in vivo mice study. Pharmaceuticals. 2021;14(12):1313.
- Abdelkader DH, Negm WA, Elekhnawy E, et al. Zinc oxide nanoparticles as potential delivery carrier: green synthesis by Aspergillus niger endophytic fungus, characterization, and in vitro/in vivo antibacterial activity. Pharmaceuticals. 2022;15(9):1057.
- Ababutain IM, Aldosary SK, Aljuraifani AA, et al. Identification and antibacterial characterization of endophytic fungi from Artemisia sieberi. Int J Microbiol. 2021;2021:1–11.
- Bilal L, Asaf S, Hamayun M, et al. Plant growth promoting endophytic fungi Aspergillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis. 2018;76(2):117–127.
- Jia M, Chen L, Xin H-L, et al. A friendly relationship between endophytic fungi and medicinal plants: a systematic review. Front Microbiol. 2016;7:906.
- Hamzah TNT, Lee SY, Hidayat A, et al. Diversity and characterization of endophytic fungi isolated from the tropical mangrove species, Rhizophora mucronata, and identification of potential antagonists against the soil-borne fungus, Fusarium solani. Front Microbiol. 2018;9:1707.
- Flegr J, Prandota J, Sovičková M, et al. Toxoplasmosis – a global threat. Correlation of latent toxoplasmosis with specific disease burden in a set of 88 countries. PLOS One. 2014;9(3):e90203.
- Robert-Gangneux F, Dardé M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–296.
- Dhapte V, Kadam V, Pokharkar V. Pyrimethamine nanosuspension with improved bioavailability: in vivo pharmacokinetic studies. Drug Deliv Transl Res. 2013;3(5):416–420.
- Palanichamy P, Krishnamoorthy G, Kannan S, et al. Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egypt J Basic Appl Sci. 2018;5(4):303–312.
- Manganyi MC, Tchatchouang C-DK, Regnier T, et al. Bioactive compound produced by endophytic fungi isolated from Pelargonium sidoides against selected bacteria of clinical importance. Mycobiology. 2019;47(3):335–339.
- Talukdar R, Padhi S, Rai AK, et al. Isolation and characterization of an endophytic fungus Colletotrichum coccodes producing tyrosol from Houttuynia cordata Thunb. using ITS2 RNA secondary structure and molecular docking study. Front Bioeng Biotechnol. 2021;9:650247.
- Odds F. Biochemical tests for identification of medical bacteria. J Clin Pathol. 1981;34(5):572–572.
- Negm WA, El-Kadem AH, Elekhnawy E, et al. Wound-healing potential of rhoifolin-rich fraction isolated from Sanguisorba officinalis roots supported by enhancing re-epithelization, angiogenesis, anti-inflammatory, and antimicrobial effects. Pharmaceuticals. 2022;15(2):178.
- Chatterjee S, Ghosh R, Mandal NC. Production of bioactive compounds with bactericidal and antioxidant potential by endophytic fungus Alternaria alternata AE1 isolated from Azadirachta indica A. Juss. PLOS One. 2019;14(4):e0214744.
- Ashwath P, Deekshit VK, Rohit A, et al. Biofilm formation and associated gene expression in multidrug-resistant Klebsiella pneumoniae isolated from clinical specimens. Curr Microbiol. 2022;79(3):1–10.
- Grujić J, Djurković-Djaković O, Nikolić A, et al. Effectiveness of spiramycin in murine models of acute and chronic toxoplasmosis. Int J Antimicrob Agents. 2005;25(3):226–230.
- Bottari NB, Baldissera MD, Tonin AA, et al. Sulfamethoxazole–trimethoprim associated with resveratrol for the treatment of toxoplasmosis in mice: influence on the activity of enzymes involved in brain neurotransmission. Microb Pathog. 2015;79:17–23.
- El-Zawawy LA, El-Said D, Mossallam SF, et al. Triclosan and triclosan-loaded liposomal nanoparticles in the treatment of acute experimental toxoplasmosis. Exp Parasitol. 2015;149:54–64.
- Thiptara A, Kongkaew W, Bilmad U, et al. Toxoplasmosis in piglets. Ann N Y Acad Sci. 2006;1081(1):336–338.
- El-Kowrany SI, Abd El Ghaffar AE-S, Shoheib ZS, et al. Evaluation of nitazoxanide as a novel drug for the treatment of acute and chronic toxoplasmosis. Acta Trop. 2019;195:145–154.
- Golias HC, Polonio JC, dos Santos Ribeiro MA, et al. Tibouchina granulosa (Vell.) Cogn (Melastomataceae) as source of endophytic fungi: isolation, identification, and antiprotozoal activity of metabolites from Phyllosticta capitalensis. Braz J Microbiol. 2020;51(2):557–569.
- Shaker KH, Zohair MM, Hassan AZ, et al. LC–MS/MS and GC–MS based phytochemical perspectives and antimicrobial effects of endophytic fungus Chaetomium ovatoascomatis isolated from Euphorbia milii. Arch Microbiol. 2022;204(11):1–13.
- Ge H, Shi M, Ma M, et al. Evaluation of the antiproliferative activity of 106 marine microbial metabolites against human lung cancer cells and potential antiproliferative mechanism of purpuride G. Bioorg Med Chem Lett. 2021;39:127915.
- Wang R, Guo ZK, Li XM, et al. Spiculisporic acid analogues of the marine-derived fungus, Aspergillus candidus strain HDf2, and their antibacterial activity. Antonie Van Leeuwenhoek. 2015;108(1):215–219.
- Kumla D, Dethoup T, Buttachon S, et al. Spiculisporic acid E, a new spiculisporic acid derivative and ergosterol derivatives from the marine-sponge associated fungus Talaromyces trachyspermus (KUFA 0021). Nat Prod Commun. 2014;9(8):1934578X1400900.
- Brown ED, Wright GD. Antibacterial drug discovery in the resistance era. Nature. 2016;529(7586):336–343.
- Becker K, Stadler M. Recent progress in biodiversity research on the Xylariales and their secondary metabolism. J Antibiot. 2021;74(1):1–23.
- Caicedo NH, Kumirska J, Neumann J, et al. Detection of bioactive exometabolites produced by the filamentous marine cyanobacterium Geitlerinema sp. Mar Biotechnol. 2012;14(4):436–445.
- Charria-Girón E, Espinosa MC, Zapata-Montoya A, et al. Evaluation of the antibacterial activity of crude extracts obtained from cultivation of native endophytic fungi belonging to a tropical montane rainforest in Colombia. Front Microbiol. 2021;12:2515.
- Bengoechea JA, Sa Pessoa J. Klebsiella pneumoniae infection biology: living to counteract host defences. FEMS Microbiol Rev. 2019;43(2):123–144.
- Te Winkel JD, Gray DA, Seistrup KH, et al. Analysis of antimicrobial-triggered membrane depolarization using voltage sensitive dyes. Front Cell Dev Biol. 2016;4:29.
- Elekhnawy E, Sonbol F, Abdelaziz A, et al. An investigation of the impact of triclosan adaptation on Proteus mirabilis clinical isolates from an Egyptian University Hospital. Braz J Microbiol. 2021;52(2):927–937.
- Gray DA, Wenzel M. Multitarget approaches against multiresistant superbugs. ACS Infect Dis. 2020;6(6):1346–1365.
- Alotaibi B, Negm WA, Elekhnawy E, et al. Antibacterial, immunomodulatory, and lung protective effects of Boswellia dalzielii oleoresin ethanol extract in pulmonary diseases: in vitro and in vivo studies. Antibiotics. 2021;10(12):1444.
- El-Banna T, Abd El-Aziz A, Sonbol F, et al. Adaptation of Pseudomonas aeruginosa clinical isolates to benzalkonium chloride retards its growth and enhances biofilm production. Mol Biol Rep. 2019;46(3):3437–3443.
- Abdelgawad MA, Hamed AA, Nayl AA, et al. The chemical profiling, docking study, and antimicrobial and antibiofilm activities of the endophytic fungi Aspergillus sp. AP5. Molecules. 2022;27(5):1704.
- Mady RF, El-Hadidy W, Elachy S. Effect of Nigella sativa oil on experimental toxoplasmosis. Parasitol Res. 2016;115(1):379–390.
- Gaafar M, Mady R, Diab R, et al. Chitosan and silver nanoparticles: promising anti-Toxoplasma agents. Exp Parasitol. 2014;143:30–38.
- Anand N, Sehgal R, Kanwar RK, et al. Oral administration of encapsulated bovine lactoferrin protein nanocapsules against intracellular parasite Toxoplasma gondii. Int J Nanomedicine. 2015;10:6355.