?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
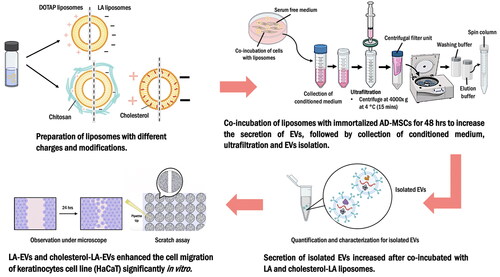
Extracellular vesicles (EVs) are small vesicles that are naturally released by cells and play a crucial role in cell-to-cell communication, tissue repair and regeneration. As naturally secreted EVs are limited, liposomes with different physicochemical properties, such as 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) and linoleic acid (LA) with modifications have been formulated to improve EVs secretion for in vitro wound healing. Various analyses, including dynamic light scattering (DLS) and transmission electron microscopy (TEM) were performed to monitor the successful preparation of different types of liposomes. The results showed that cholesterol–LA liposomes significantly improved the secretion of EVs from immortalized adipose-derived mesenchymal stem cells (AD-MSCs) by 1.5-fold. Based on the cell migration effects obtained from scratch assay, both LA liposomal-induced EVs and cholesterol–LA liposomal-induced EVs significantly enhanced the migration of human keratinocytes (HaCaT) cell line. These findings suggested that LA and cholesterol–LA liposomes that enhance EVs secretion are potentially useful and can be extended for various tissue regeneration applications.
Graphical Abstract
Introduction
Extracellular vesicles (EVs) are nanosized membrane vesicles that are actively released by cells. It consists of three major subtypes, including exosomes, microvesicles (MVs) and apoptotic body [Citation1–4], which can be differentiated by their size, release pathways, biogenesis and cargo [Citation5]. The vesicles produced by cell are generally referred as EVs and are classified according to their sizes, as proposed by the International Society of Extracellular Vesicles (ISEV) [Citation6]. Studies have showed that the contents or cargo of EVs, including lipids, nuclei acids and proteins, play a crucial role in stem cell biology and tumour progression via signalling pathways, which promote organogenesis and tumorigenesis [Citation7,Citation8]. Additionally, EVs complex cargo such as mRNA, miRNAs, transcription factors and oncogenes have been found to induce migration and proliferation of fibroblasts and play a significant role in tissue repair and regeneration [Citation8–13]. However, the commercialization and application of naturally secreted EVs in regenerative medicine are being constrained by their low yield [Citation14].
The most common methods used in improving EV secretion include three-dimensional (3D) culture, physiological alteration, physical stimulation and cell membrane rupture [Citation15,Citation16]. However, most methods for stimulating EVs have their drawbacks [Citation17]. The extensive variety of components contained in the matrices makes the building of 3D scaffolds potentially challenging and laborious. Furthermore, the repeatability of 3D biomimetic scaffolds is weak as they differ from batch to batch. Assay optimization is also required since not all tests can be performed in such culturing conditions [Citation18,Citation19]. Hence, modification of chemical stimulant, such as liposomes, could be an optimal alternative method to improve the secretion of EV.
Liposomes are the most popular form of spherical synthetic vesicles that can be established from fatty acids and natural non-toxic phospholipids [Citation20,Citation21]. The surface of liposomes can be modified by the choice of bilayer lipids as well as electrophoretic properties to enhance their stability. Liposomes has been shown to interact with cell surfaces in a physiochemically dependent manner and resulting in cell stimulation [Citation22]. In addition, the secretion of EVs from cancer cells may be improved by modifying the physicochemical properties of liposomes, as demonstrated in previous studies [Citation12,Citation20,Citation22,Citation23].
1,2-Dioleoyl-3-trimethylammonium propane (DOTAP) liposomes, which carry a positive charge, are known to be taken up more efficiently by cancer cells compared to neutral liposomes. Interestingly, linoleic acid (LA) liposomes with negatively charged have also been observed to be taken up by cells, suggesting a promising approach for stimulating EVs [Citation24,Citation25]. However, it has yet to be determined whether DOTAP and LA liposomes, which have different physicochemical properties, can induce the release of EVs from immortalized adipose-derived mesenchymal stem cells (AD-MSCs). Therefore, the aim of this study was to investigate the potential of DOTAP and LA liposomes with different physicochemical properties and modifications in stimulating the secretion of EVs from immortalized AD-MSCs for in vitro wound healing effects.
Materials and methods
Materials
Acetic acid, chloroform and hydrochloric acid were obtained from R&M Chemicals, Kuala Lumpur, Malaysia. Low-molecular-weight chitosan and cholesterol were obtained from Sigma-Aldrich (St. Louis, MO). Linoleic acid and DOTAP were obtained from Fluka (Buchs, Switzerland) and Avanti Polar Lipids (Alabaster, AL), respectively. Analytical grade sodium nitrate was obtained from HmbG Chemicals (Kuala Lumpur, Malaysia). Sodium hydroxide (reagent grade, ≥98%, pellets (anhydrous)) was obtained from Merck (Solna, Sweden). Low glucose Dulbecco’s modified Eagle medium (DMEM), foetal bovine serum (FBS), penicillin–streptomycin (Pen–strep) (10,000 U/ml) and GlutaMax were obtained from Gibco (Carlsbad, CA). Immortalized AD-MSCs, ASC52telo were obtained from America Type Culture Collection (ATCC) (Manassas, VA). Human keratinocytes (HaCaT) cell line and optimized DMEM were obtained from AddexBio (San Diego, CA). Pierce™ bovine serum albumin (BSA) standard (2 mg/ml) was obtained from Thermo Fisher Scientific (Waltham, MA). Cell culture falcon tubes, dishes and plates were obtained from NEST Biotechnology Co. (Wuxi, China). Dulbecco’s phosphate-buffered saline (DPBS) was obtained from Sangon Biotechnology (Shanghai, China). Minisart® syringe filter, surfactant-free cellulose acetate (SFCA) and pore size of 0.8 µm were obtained from Sartorius (Goettingen, Germany). Amicon® Ultra-15 centrifugal filter devices-10 kDa and exoEasy Maxi Kit were obtained from Merck (Darmstadt, Germany) and Qiagen (Hilden, Germany). Coomassie Brilliant Blue (CBB) dye was obtained from Nacalai Tesque Inc. (Kyoto, Japan).
Sample preparation
Preparation of low molecular weight soluble chitosan
A 0.10% (weight/volume) (w/v) chitosan solution was prepared by dissolving 0.025 g of chitosan powder in 0.25 ml of 1% (v/v) acetic acid. Next, 0.10 M of sodium nitrite solution was added dropwise into the chitosan solution and stirred with a magnetic stirrer for 1 h at room temperature (25 °C). The solution was then adjusted to pH 8–9 with 1 M of sodium hydroxide solution. The reaction mixture containing chitosan was then adjusted to pH 7 with 1 M of hydrochloric acid. Then, chitosan was precipitated by using acetone and centrifuged at 5000 rpm for 2 min. The thin film of soluble chitosan was formed after being dried in an oven at 45 °C for 24 h [Citation26].
Preparation of 1,2-dioleoyl-3-trimethylammonium propane liposomes
0.157 g of DOTAP was dissolved in 5 ml of chloroform in a round-bottom flask. The chloroform was evaporated using the rotary evaporator at 50 °C for 1 h and a thin film was formed in the round-bottom flask on the rotary evaporator. Subsequently, the obtained thin film was vacuum dried in the dark overnight at room temperature and rehydrated with 1.5 ml of 500 mM phosphate-buffered saline (PBS) 1×, pH 7.5 and 13.5 ml of deionized water the next day. The solution was then shaken and sonicated for 15 min at 25 °C [Citation26].
Preparation of chitosan-coated DOTAP liposomes
0.157 g of DOTAP was dissolved in 5 ml of chloroform in a round-bottom flask. The chloroform was evaporated by using the rotary evaporator at 50 °C for 1 h and a thin film formed in the round-bottom flask on the rotary evaporator. Subsequently, the obtained thin film was vacuum dried in in the dark overnight at room temperature and rehydrated with 1.5 ml of 500 mM PBS 1×, pH 7.5 and 13.5 ml of deionized water the next day. The solution was then added with 0.10% (w/v) of chitosan solution and sonicated at 25 °C for 15 min [Citation26].
Preparation of cholesterol–DOTAP liposomes
Both DOTAP and cholesterol (5:4) were dissolved together in 5 ml of chloroform to form thin film [Citation27]. The chloroform was evaporated using the rotary evaporator at 50 °C for 1 h. The thin film was formed in the round-bottom flask on the rotary evaporator. Subsequently, the obtained thin film was dried in vacuum in the dark overnight at room temperature and rehydrated with 1.5 ml of 500 mM PBS 1×, pH 7.5 and 13.5 ml of deionized water the next day. The solution was then shaken and sonicated for 15 min at 25 °C [Citation28,Citation29].
Preparation of linoleic acid liposomes
In a round-bottom flask, 2.5 ml of 500 mM PBS 1×, pH 7.5, was added. Subsequently, 194 µl of LA (Fluka, Buchs, Switzerland) was added and adjusted to pH 9. The liposome solution in the round-bottom flask was then stirred for 4 h and sonicated at 25 °C for 5 min. The pH of solution was adjusted to pH 7.5 and the solution was transferred to a volumetric flask. Then, the volumetric flask was added with deionized water until it reached the meniscus line of the volumetric flask. The LA liposomes were shaken and kept at room temperature in the dark for 24 h [Citation26].
Preparation of chitosan-coated LA liposomes
In a round-bottom flask, 2.5 ml of 500 mM PBS 1×, pH 7.5, was added. Subsequently, 194 µl of LA (Fluka, Buchs, Switzerland) was added and adjusted to pH 9. The liposome solution in the round-bottom flask was then stirred for 4 h and sonicated at 25 °C for 5 min. The pH of solution was adjusted to pH 7.5 and 0.10% (w/v) of chitosan solution was added dropwise into the flask. Subsequently, the solution was adjusted to pH 7 and transferred to a volumetric flask. Then, the volumetric flask was added with deionized water until the meniscus line of the volumetric flask was reached. The chitosan–coated linoleic acid (chitosan–coated LA) liposomes were shaken and kept at room temperature in the dark for 24 h [Citation26].
Preparation of cholesterol–LA liposomes
Both LA and cholesterol (2:1) were dissolved together in 5 ml of chloroform to form thin film [Citation30]. The chloroform was evaporated using the rotary evaporator at 50 °C for 1 h. The thin film was formed in the round-bottom flask on the rotary evaporator. Subsequently, the obtained thin film was dried in vacuum in the dark overnight at room temperature and rehydrated with 2.5 ml of 500 mM PBS 1×, pH 7.5 and 22.5 ml of deionized water the next day. The solution was then shaken and sonicated for 15 min at 25 °C [Citation26,Citation28,Citation29].
Characterization of liposomes
Size and zeta potential
Liposome samples were placed into disposable cuvette respectively for analysis. The dynamic light scattering (DLS) measurements were performed with a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Water viscosity at 25 °C (0.8872 cP) and the refractive index of the solution of 1.33 were used to interpret the measurements. Samples were analysed in three repeats, each consisting of 12 scattering measurements [Citation31].
Transmission electron microscopy (TEM)
Liposomes suspensions were processed by using copper grids to adsorb liposome particles respectively from the suspension. The samples were stained in 2.5% uranyl acetate for 30 s and dried for 3–5 min. The specimens were then observed under a JEM-2100F High Resolution Transmission Electron Microscope (JEOL, Tokyo, Japan), operated at 80 kV [Citation26].
Co-incubation of different types of liposomes in cell culture for maximum extracellular vesicle secretion
Immortalized AD-MSCs, ASC52telo from America Type Culture Collection (ATCC, Manassas, VA) were cultured in a 10 cm2 petri dish supplemented with low glucose DMEM (Gibco, Carlsbad, CA), 10% of FBS (Gibco, Carlsbad, CA), 1% of GlutaMax (Gibco, Carlsbad, CA) and 1% Pen–strep (Gibco, Carlsbad, CA). The cells were then incubated in a 5% carbon dioxide (CO2), 37 °C incubator. Subsequently, the culture medium was changed to serum-free medium (SFM) and co-incubated with five different concentrations (0 mM, 0.05 mM, 0.10 mM, 0.20 mM and 1 mM) of different types of liposomes respectively for 48 h once the immortalized AD-MSCs have reached a confluence of 80%. The negative control was treated similarly by co-incubating with SFM. The conditioned medium in 10 cm2 petri dish was collected and transferred to sterile falcon tube after 48 h incubation for further ultrafiltration and EVs isolation processes [Citation22].
Ultrafiltration
A minimum of 40 ml of culture medium for each condition was collected after 48 h and filtered through Minisart® syringe filter 0.80 µm to remove the contaminating liposomes, apoptotic bodies and cell debris [Citation32]. Subsequently, 10 ml of filtered conditioned medium were transferred into the Amicon® Ultra-15 centrifugal filter devices-10 kDa (Merck, Darmstadt, Germany), and concentrated further to 200 µl using hybrid refrigerated centrifuge at 4000 × g at 4 °C for 15 min. The concentrated EVs were then collected and further purified through exoEasy Maxi Kit (Qiagen, Hilden, Germany) [Citation32,Citation33].
exoEasy Maxi Kit
The kit provided membrane affinity spin columns and specialized buffers to efficiently isolate and purify EVs from the conditioned medium [Citation33]. Briefly, XBP buffer was added to bind the concentrated EVs (1:1) to an affinity spin column. The mixture was then centrifuged for 1 min at 500 × g. Subsequently, XWP buffer was added to remove the residual from the column by centrifuged at 5000 × g for 5 min and the flow-through was discarded. The EVs were eluted with XE buffer.
Bradford protein assay
The secretion of EVs from each condition was quantified using the Bradford protein assay. Bovine serum albumin at different concentrations (0, 50, 100, 200, 400, 800, 1000 and 2000 µg/ml) was used as a standard to produce calibration curve. For sample measurement, each well of 96-wells plate was added with 90 µl of CBB dye (Nacalai Tesque Inc., Kyoto, Japan) and 10 µl of samples (isolated EVs). Subsequently, the 96-wells plate was covered with aluminium foil and incubated in dark for 15 min. The absorbance reading was then determined using a microplate reader at 595 nm after 15 min. The assay was performed in triplicates [Citation22].
Fluorochrome staining
A control study has been performed to confirm that the collected EVs were free of liposomes. The optimum dosage, 0.20 mM liposomes was added to SFM under similar experimental conditions and incubated for 48 h. The collected medium filtered was through a Minisart® syringe filter of 0.80 µm. The filtered conditioned medium was transferred into the Amicon® Ultra-15 centrifugal filter devices-10 kDa (Merck, Darmstadt, Germany). Subsequently, 1% acridine orange dye was added to the samples and followed by purification step by using exoEasy Maxi Kit. The recovered eluate was examined under a fluorescence microscope at an emission wavelength of 480 nm to confirm the absence of liposomes right after purification [Citation34].
Characterization of EVs
Size and zeta potential
EV samples were placed into disposable cuvette respectively for analysis. The DLS measurements were performed with a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). Water viscosity at 25 °C (0.8872 cP) and the refractive index of the solution of 1.33 were used to analyse the measurements. Samples were analysed in three repeats, each consisting of 12 scattering measurements [Citation31].
Transmission electron microscopy
EV suspensions were processed by using copper grids to adsorb liposome particles respectively from the suspension. The samples were stained in 2.5% uranyl acetate for 30 s and dried for 3–5 min. The specimens were then observed under a JEM-2100F High Resolution Transmission Electron Microscope (JEOL, Tokyo, Japan) operated at 80 kV [Citation26].
Scratch assay
Human keratinocytes were seeded in a 24-well plate with optimized DMEM (AddexBio, San Diego, CA), 10% FBS (Gibco, Carlsbad, CA), 1% Pen–strep (Sigma, St. Louis, MO). Briefly, a scratch was made by scraping the bottom of each well in straight lines using a sterile 10 µl pipette tip and washed with DPBS to remove cell debris. Subsequently, the medium was then changed to SFM conditions and cells were treated with different dosage and types of liposomal-induced EVs, as well as EVs without liposomal stimulation. Additionally, higher dose of liposomes alone was also treated with cells as control. Then, cell images were captured every 2 h using an Olympus microscope (Olympus America Inc., Waltham, MA) by live imaging for 24 h [Citation35]. Cells were maintained in a plate incubator at 37 °C with 5% CO2. The percentage of wound closure at each time point was calculated using the following formula:
Statistical analysis
Statistical analysis of the experimental data was conducted using GraphPad Prism 9 (La Jolla, CA). Each experiment was performed at least three times. The statistical analysis was analysed by using the Kruskal–Wallis test, followed by post hoc test for multiple comparison test. The statistical analysis was considered statistically significant if p < .05.
Results
Stability of different types of liposomes
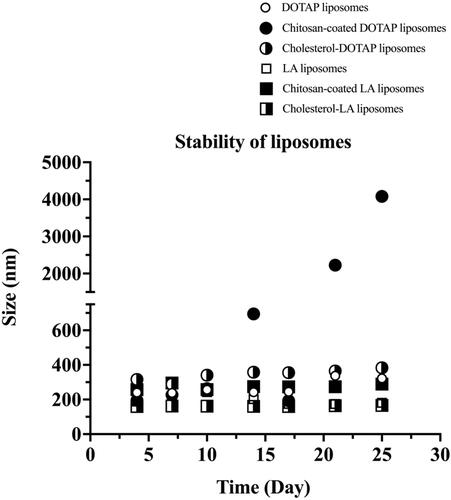
In this study, the stability of different types of liposomes was evaluated by monitoring their z-average size for a month of storage at 25 °C. The z-average size for all different types of prepared liposomes throughout the 30 days of storage time is shown in . The size of all liposomes was found to increase slowly during the first 10 days of storage time. Meanwhile, the size of all liposomes remained unchanged on the day 14–17 of storage time, except for chitosan–coated DOTAP liposomes, which increased rapidly after day 14.
Characterization of liposomes
Size and zeta potential
In general, the z-average size of liposomes on day 14 increased in the following order: cholesterol–linoleic acid (cholesterol–LA) liposomes (nm) < LA liposomes (nm) < DOTAP liposomes (nm) < chitosan–coated LA liposomes (nm) < cholesterol–DOTAP liposomes (nm) < chitosan–coated DOTAP liposomes (nm) as shown in . The z-average size for DOTAP, chitosan–coated DOTAP and cholesterol–DOTAP liposomes on day 14 was 242.3 ± 0.8 nm, 694 ± 20 nm and 358 ± 1 nm, respectively, whereas the z-average size for LA, chitosan–coated LA and cholesterol–LA liposomes on day 14 was 202.2 ± 0.1 nm, 275 ± 5 nm and 161.8 ± 0.6 nm, respectively. The size of both chitosan-coated DOTAP and LA liposomes were slightly bigger than the non-coated liposomes as the liposomes were coated with a layer of chitosan.
Table 1. Characterization of the size and zeta potential for different types of liposomes.
The zeta potentials for DOTAP, chitosan–coated DOTAP, cholesterol–DOTAP, LA, chitosan–coated LA and cholesterol–LA liposomes were 22.3 ± 0.8 mV, 24.9 ± 0.7 mV, 26 ± 1 mV, −31 ± 2 mV, −40.0 ± 0.8 mV and −57.8 ± 0.7 mV, respectively, as shown in .
Transmission electron microscopy
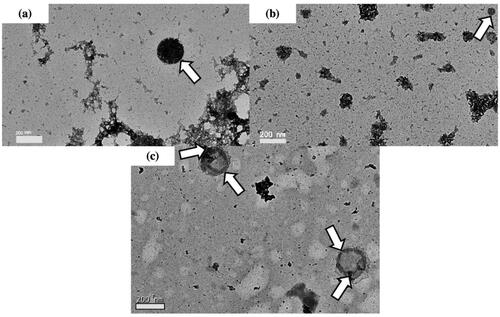
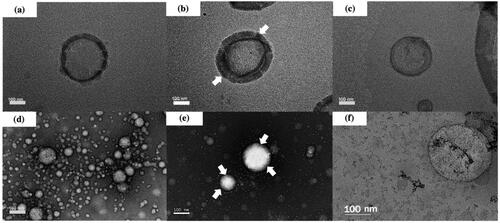
TEM study was performed to illustrate the morphological structure of DOTAP, chitosan–coated DOTAP, cholesterol–DOTAP, LA, chitosan–coated LA and cholesterol–LA liposomes. The TEM images clearly showed that all liposomes were spherical in shape as shown in , respectively. The presence of chitosan thickened the lipid layer of DOTAP and LA liposomes, resulting an increased in size as shown in , respectively.
Figure 2. Transmission electron microscopy of (a) DOTAP liposomes, (b) chitosan–coated DOTAP liposomes, (c) cholesterol–DOTAP liposomes, (d) LA liposomes, (e) chitosan–coated LA liposomes and (f) cholesterol–LA liposomes with a magnification of ×25,000. The chitosan coating layer is indicated by arrows.
Effect on the immortalized AD-MSCs extracellular vesicles secretion and cell viability
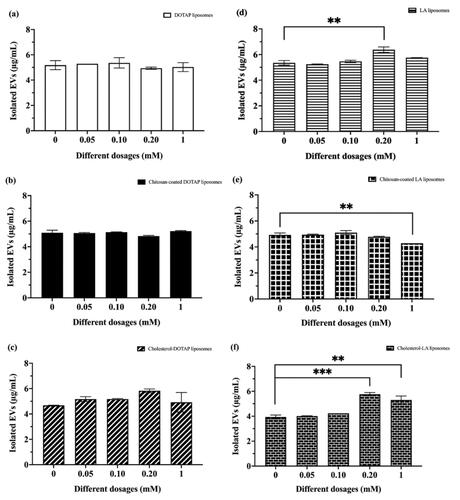
The secretion of isolated EVs from immortalized AD-MSCs was examined by incubating with five different dosages and different types of liposomes for 48 h. The secretion of isolated EVs was found to increase 1.19-fold and 1.5-fold after incubating with 0.20 mM LA and cholesterol–LA liposomes respectively for 48 h as shown in . Likewise, the secretion of isolated EVs did not increase when both DOTAP and LA liposomes were modified with chitosan as shown in , respectively.
Figure 3. The secretion of isolated EVs from immortalized adipose-derived mesenchymal stem cells after being treated with different dosages and types of liposomes: (a) DOTAP liposomes, (b) chitosan–coated DOTAP liposomes, (c) cholesterol–DOTAP liposomes, (d) LA liposomes, (e) chitosan–coated LA liposomes and (f) cholesterol–LA liposomes. Data are represented as mean ± SD (n = 3), **p < .01, ***p < .001.
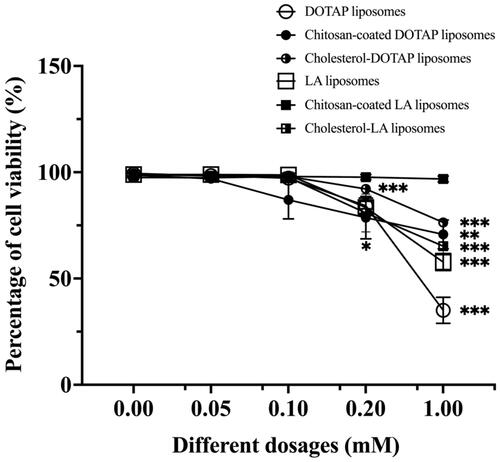
The cell viability of immortalized AD-MSCs after being treated with different dosages and types of liposomes was examined as presented in . The cell viability was 98.76% and 97.55% after co-incubation with 0.10 mM LA and cholesterol–LA liposomes for 48 h. The results demonstrated that the maximal incubation dose for both LA and cholesterol–LA liposomes was 0.20 mM as the cell viability declined to 83.38% and 80.84% after 48 h. The results showed that all percentage of cell viability remained above 50% after being treated with different dosages and types of liposomes, except for the percentage of cell viability after treated with 1 mM of DOTAP liposomes for 48 h had decreased gradually.
Effect of treated liposomes were separated from the conditioned medium during purification
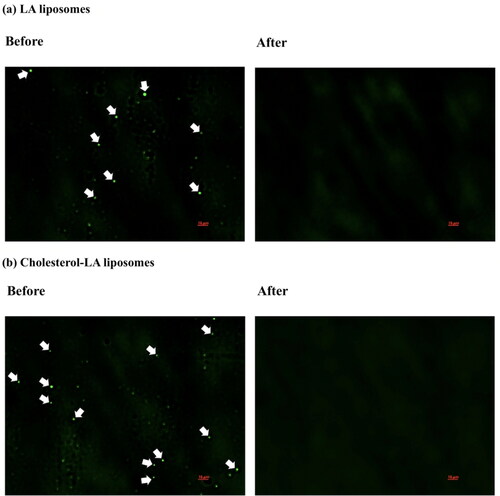
In this investigation, the presence of both LA and cholesterol–LA liposomes was identified by 1% acridine orange staining as a positive control. For the negative control sample, 0.20 mM LA and cholesterol–LA liposomes were present in SFM, respectively. After 48 h of incubation, the samples were filtered, ultrafiltered and purified using the exoEasy Maxi kit. illustrates the absence of the acridine orange-stained LA and cholesterol–LA liposomes under fluorescence microscope observation. These results showed that the series of filtration, ultrafiltration and exoEasy Maxi kit were successful in removing liposomes from samples.
Figure 5. Fluorescence microscopy of (a) LA liposomes before and after being separated from the conditioned medium and (b) cholesterol–LA liposomes before and after being separated from the conditioned medium. The green spherical shape represents the presence of liposomes in conditioned medium before separation.
Characterization of EVs
Size and zeta potential
The z-average size for EVs without liposomal stimulation (non-induced EVs), LA-EVs and cholesterol–LA-EVs was 280 ± 10 nm, 557 ± 20 nm and 320 ± 9 nm respectively as shown in . The size for all EVs was within the range of 0.1–1.0 µm. Meanwhile, the zeta potential of all EVs was negatively charged as shown in . There was no significant difference found between the zeta potential of non-induced EVs and liposomal-induced EVs.
Table 2. Characterization of the size and zeta potential for different types of liposomal-EVs with wound healing properties.
Transmission electron microscopy
The morphology of all isolated EVs was evaluated using TEM. The TEM images clearly showed that all EVs were spherical in shape and identical to the non-induced EVs as shown in .
Effect of liposomal-induced EVs on wound closure by using human keratinocytes
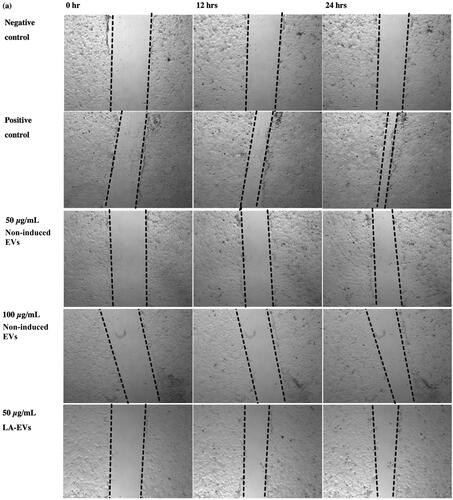
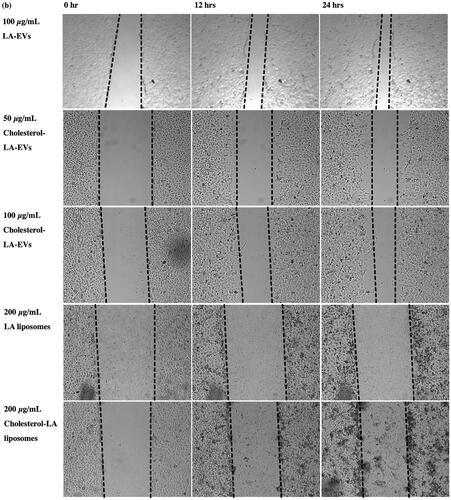
The scratch assay was performed to evaluate the functional properties of liposomal-induced EVs using HaCaT. The selected dosages of 50 µg/ml and 100 µg/ml of EVs were applied for an in vitro scratch assay [Citation36]. All liposomal-induced EVs showed different percentages of wound closure increment in HaCaT over time as compared to the negative control and non-induced EVs, as presented in . The percentage of wound closure for negative control (SFM) in HaCaT after 12 h and 24 h was 13.33% and 18.57%, respectively. Meanwhile, positive control with 10% FBS resulted in wound closure percentages of 27.51% and 45.05% after 12 h and 24 h, respectively. The efficiency of wound closure was doubly compared to SFM conditions. The wounded area in HaCaT treated with 50 µg/ml and 100 µg/ml non-induced EVs exhibited almost similar percentages of wound closure after 24 h, which were 22.82% and 23.19%, respectively. In addition, 50 µg/ml of cholesterol–LA-EVs exhibited 33.53% of wound closure significantly after 24 h. The efficiency of wound closure was around 74% compared to positive control (10% FBS). The use of higher dose LA and cholesterol–LA liposomes (control) only in HaCaT did not show any migratory effects after the treatment. The images of wound closure in HaCaT were taken at 0, 12 and 24 h, as shown in .
Figure 7. The percentage of wound closure in HaCaT after treatment with different dosages and types of liposomal-induced EVs. Data are represented as mean ± SEM (n = 3), *p < .05, **p < .01 .
Figure 8. Images of wound closure in HaCaT after treatment with different dosages and types of liposomal-induced EVs. All cells were treated under serum free conditions, except for the positive control.
Discussion
Liposomes with different physicochemical properties were prepared and their respective efficiency in inducing EVs secretion was studied. All the liposomes achieved stability between days 14 and 17 with no significant changes in their sizes, except for chitosan–coated DOTAP liposomes, where the size increased from day 14 onwards. The result was in accordance with several studies, which observed changes in size probably due to the occurrence of flocculation [Citation26,Citation37–39] as chitosan–coated DOTAP liposomes underwent aggregation, transforming their size from day 14 onwards [Citation21,Citation38,Citation40–42].
The properties of liposomes with and without 0.10% chitosan and cholesterol were further investigated where the morphology of all liposomes was examined using TEM. Similar to the previous study [Citation43], the obtained results showed that all liposomes were spherical in shape with a coating layer found on the surface of the LA and DOTAP liposomes. There was an increase in zeta potential of chitosan–coated DOTAP and chitosan–coated LA liposomes when compared to the non-coated ones. The random arrangement of β-(1-4)-linked d-glucosamine and N-acetyl-d-glucosamine increased their positively charged linear polysaccharides (PS) resulting in an increase in zeta potential [Citation44]. Similar zeta potential increments were also observed for both cholesterol–DOTAP and cholesterol–LA liposomes when compared to the non-modified liposomes, demonstrating an upsurge in stability for both liposomes and successful incorporation of cholesterol into the membrane bilayer.
The secretion of EVs from immortalized AD-MSCs was obtained after 48 h of incubation with and without liposomal stimulation. These results showed that only LA and cholesterol–LA liposomes significantly induced EVs secretion whereas, neither chitosan–coated LA nor chitosan–coated DOTAP liposomes induced EVs secretion. The presence of 0.20 mM of LA and cholesterol–LA liposomes promoted EVs secretion at a ratio of 1.19-fold and 1.5-fold, respectively. The secretion of EVs was reported to increase by 1.3-fold under hypoxic conditions [Citation45]. Furthermore, the application of cholesterol–LA liposomes was observed to induce a more pronounced release of EVs. This could be due to the binding ability of liposomes bind to the cell surface and subsequently undergoing endocytosis, which influences the behaviour of individual cellular collectives [Citation46–50].
In addition, different electrostatic charges are induced across the cell membrane due to the motion of resting membrane potential, which is a consequence of the flow of a few distinct ion species across the plasma membrane via various ion channels and transporters [Citation51]. The findings showed that both LA and cholesterol–LA liposomes (anionic liposomes) uptake may be mediated by a combination of electrostatic charge interactions and protein transporters [Citation52].
Negatively charged cholesterol–LA liposomes can promote favourable and strong electrostatic interactions with the cell membrane that attributes with a positive charged surface [Citation53–55], and are more likely to bind to the cell membrane [Citation1,Citation40,Citation56–64], resulting in more EVs secretion. Similar results were reported in previous studies where negatively charged liposomes could effectively bind to the cell membrane to deliver more clodronate and gallium in macrophages and other cell lines [Citation56,Citation65–68].
Likewise, DOTAP liposomes exhibited similar pattern with chitosan–coated LA and chitosan–coated DOTAP liposomes, as they were also positively charged and repelled from the positively charged membrane of immortalized AD-MSCs cells [Citation69–72], in which explained lesser EVs secretion [Citation73,Citation74]. The suppressive effect of modified chitosan could be recognized as an approach for treating malignancies, as EVs play a disputed role in tumour progression [Citation75].
In cell viability study, 1 mM of DOTAP liposomes was found to induce cytotoxicity in a dose-dependent manner; therefore, stimulatory effect on EVs secretion was not observed. Cationic liposomes possessed toxicity which explained the above results since factors such as surface and interface effects, quantum size impact and agglomeration ability played a crucial role in affecting cell toxicity [Citation22,Citation76,Citation77]. High levels of negatively charged plasma proteins such as apolipoproteins, glycoproteins and glycosaminoglycans (GAGs) may lead to micelle formation and cytotoxicity in cationic liposomes [Citation76]. These discrepancies in toxicity patterns are most likely aggravated by variances in membrane composition, differences in phagocytic activity and liposomal processing, which then interfered with the membrane function and integrity of the cell or subcellular compartments [Citation78].
Fluorochrome staining was further carried out to further confirm the separation of liposomes and the conditioned medium during EVs isolation. These staining indicated that both LA and cholesterol–LA liposomes were successfully separated from the conditioned medium as there was no green spherical shape was observed under the fluorescence microscope.
The sizes for all isolated EVs were found to be within the range of 0.1–1.0 µm, indicating that the successfully isolated EVs may be exploited for cell–cell communication [Citation79].
The zeta potential of EVs is a measure of charge stability that can be determined from the electrophoretic mobility of a suspension and affects particle–particle interactions [Citation80–85]. In this study, the zeta potential of all isolated EVs was negatively charged due to the presence of negatively charged phospholipids and proteins on the EV membrane [Citation86–88]. The range of zeta potential for EVs isolated from various cancer and normal cells was −8 mV to 54 mV, as shown in previous studies [Citation89]. The zeta potential of cancer cell EVs was found to be higher than the normal cell EVs. This could be due to the high level of sialic acid residues found in the terminal positions of mucins, glycolipids and other glycoproteins that have ionized carboxylates at physiological pH, which can contribute significantly to the net negative charge in biological cells [Citation89–91]. Furthermore, EVs with low zeta potential are highly susceptible to aggregation, while EVs with higher charges are less likely to aggregate and are potentially more stable [Citation92]. There was no significant difference in zeta potential non-induced EVs and all liposomal-induced EVs. Thus, this suggests that all the isolated EVs have the same stability. The morphology of all isolated EVs was confirmed by the TEM. All the isolated EVs were spherical in shape, which is identical to previous studies [Citation93,Citation94].
To date, there were no study on enhancing in vitro wound healing by using liposomal-induced EVs. The low secretion of EVs from AD-MSCs cells have shown to limit their clinical applications [Citation14]. Studies have revealed that EVs are recognized as a type of cell communication, which is essential for wound healing as communication events are intensified at the edge of wound [Citation95,Citation96]. The paracrine actions of MSCs were responsible for most of the regeneration as these cells may induce.
Besides generating a variety of growth factors, lowering oxidative stress and apoptosis, boosting angiogenesis, and having antimicrobial capabilities may also hasten wound healing [Citation97–99]. It has been shown that the effectiveness of topical application of EVs produced from MSCs in promoting skin healing were more than MSCs alone [Citation100]. The healing and regeneration abilities of MSCs were hypothesized to be mediated by EVs [Citation101,Citation102] and the significant amounts of ceramide, sphingomyelin and cholesterol which made up the lipid raft were found on the surface of EVs. Lipid elements including prostaglandins and phosphatidylserine may also be important for determining how the EVs operated. The mRNAs and miRNAs that were presented in the EVs have the potential to alter the activities of those cells as well as the expression of proteins after being taken up by the recipient cells [Citation103,Citation104]. Additionally, EVs derived from AD-MSCs could reduce high-glucose-induced oxidative stress, increase angiogenesis, lessen mitochondrial functional impairment and inflammatory response, as well as promote diabetic wound healing [Citation105].
Consequently, a scratch assay was performed using HaCaT cell line to validate the functional properties of EVs without liposomal stimulation and liposomal-induced EVs. Both LA-EVs and cholesterol–LA-EVs showed similar migratory efficiencies in HaCaT compared to the positive control. All liposomal-induced EVs were found to promote the migration of HaCaT cell line indicating that the non-induced EVs and all liposomal-induced EVs with negatively charge had retained healing properties and accelerated cell migration in vitro. The mechanism of liposomal-induced EVs for enhancing wound closure in HaCaT could be facilitated by electrostatic interactions and mediated by transporter or receptor [Citation103]. Phosphatidylserine such as lysophosphatidylcholine positively regulates the curvature of the outer membrane, whereas cardiolipin can be found in EVs negatively regulates the curvature of the inner membrane [Citation106]. EVs that comprised phosphatidylserine might act as a pro-coagulant during the platelet formation process as it can aid lesions [Citation107]. Those diverse lipidomic compositions may be detected in various subtypes of EVs. For instance, phosphatidylserine have shown to translocate to the outer portion of lipid bilayer [Citation108,Citation109].
Overall, the results in this study showed that the low secretion of EVs derived from immortalized AD-MSCs after stimulation with LA and cholesterol–LA liposomes has been improved and was a promising treatment approach for wound healing in vitro. This implies that LA-EVs and cholesterol–LA-EVs were positively correlated with the keratinocytes cell line, resulting in enhancing the cell migration in vitro. Low secretion of EVs derived from immortalized AD-MSCs was improved after stimulation with both LA and cholesterol–LA liposomes, with enhanced cell migration in HaCaT cell line.
Conclusions
The findings of this study showed that co-incubating with LA and cholesterol–LA liposomes (anionic liposomes), as compared to other liposomes (cationic), significantly improved the low secretion of EVs derived from immortalized AD-MSCs. Such findings suggest that the surface charge of liposomes is a crucial factor in enhancing the interaction between liposomes and immortalized AD-MSCs, promoting their stimulatory effect on EVs secretion. Steric barriers, on the other hand, hinder liposomes from interacting with cells, preventing the stimulatory effect on EV secretion. Moreover, both LA-EVs and cholesterol–LA-EVs were able to accelerate wound closure in vitro in HaCaT cell line.
Further research in regenerative medicine is essential to develop a more comprehensive understanding of the molecular mechanisms underlying healing processes. Specifically, it is crucial to investigate how EVs derived from immortalized AD-MSCs influence each stage of healing and its associated cells. It is required to examine the composition of liposomal-induced EVs and carry out clinical studies to determine the overall efficacy of this approach in treating chronic wounds and their anti-inflammatory effects.
Author contributions
Conceptualization and methodology, F.F.L., H.W.T., I.C. and M.M.; carrying out experiments, J.W.C. and F.F.L.; data analysis and interpretation, J.W.C., I.C., H.W.T., F.F.L. and M.M.; statistical analysis and figures preparation, J.W.C., F.F.L. and H.W.T.; manuscript preparation, J.W.C., F.F.L., H.W.T., I.C. and M.M; supervision, I.C., H.W.T. and F.F.L.; cell lines, chemical and reagent purchasing, F.F.L. All authors contributed, critically reviewed the manuscript and approved the final version. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors are grateful for all administrative and purchasing support provided by MAHSA University Research Management Centre (RMC). Laboratory work was performed at the Translational Core Laboratory (TCL), Faculty of Medicine, and Department of Chemistry, Faculty of Science, University of Malaya.
Disclosure statement
The authors declare no conflict of interest.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Desideri E, Ciccarone F, Ciriolo MR, et al. Extracellular vesicles in endothelial cells: from mediators of cell-to-cell communication to cargo delivery tools. Free Radic Biol Med. 2021;172:508–520. doi: 10.1016/j.freeradbiomed.2021.06.030.
- Gurunathan S, Kang M-H, Qasim M, et al. Biogenesis, membrane trafficking, functions, and next generation nanotherapeutics medicine of extracellular vesicles. Int J Nanomedicine. 2021;16:3357–3383. doi: 10.2147/IJN.S310357.
- Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9(1):63. doi: 10.1186/s13287-018-0791-7.
- Kletukhina S, Neustroeva O, James V, et al. Role of mesenchymal stem cell-derived extracellular vesicles in epithelial–mesenchymal transition. Int J Mol Sci. 2019;20:4813. doi: 10.3390/ijms20194813.
- Ozaki Tan SJ, Floriano JF, Nicastro L, et al. Novel applications of mesenchymal stem cell-derived exosomes for myocardial infarction therapeutics. Biomolecules. 2020;10:707. doi: 10.3390/biom10050707.
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750.
- Veerman RE, Akpinar GG, Eldh M, et al. Immune cell-derived extracellular vesicles – functions and therapeutic applications. Trends Mol Med. 2019;25(5):382–394. doi: 10.1016/j.molmed.2019.02.003.
- Yang Q, Diamond MP, Al-Hendy A. The emerging role of extracellular vesicle-derived miRNAs: implication in cancer progression and stem cell related diseases. J Clin Epigenet. 2016;2:13.
- Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. doi: 10.1038/s41392-020-00261-0.
- Kholia S, Ranghino A, Garnieri P, et al. Extracellular vesicles as new players in angiogenesis. Vascul Pharmacol. 2016;86:64–70. doi: 10.1016/j.vph.2016.03.005.
- Muralikumar M, Jain SM, Ganesan H, et al. Current understanding of the mesenchymal stem cell-derived exosomes in cancer and aging. Biotechnol Rep. 2021;31:e00658. doi: 10.1016/j.btre.2021.e00658.
- Sakai-Kato K, Yoshida K, Takechi-Haraya Y, et al. Physicochemical characterization of liposomes that mimic the lipid composition of exosomes for effective intracellular trafficking. Langmuir. 2020;36(42):12735–12744. doi: 10.1021/acs.langmuir.0c02491.
- Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016;49(1):3–13. doi: 10.1111/cpr.12233.
- Varderidou-Minasian S, Lorenowicz MJ. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: challenges and opportunities. Theranostics. 2020;10(13):5979–5997. doi: 10.7150/thno.40122.
- Ng CY, Kee LT, Al-Masawa ME, et al. Scalable production of extracellular vesicles and its therapeutic values: a review. Int J Mol Sci. 2022;23:7986. doi: 10.3390/ijms23147986.
- Syromiatnikova V, Prokopeva A, Gomzikova M. Methods of the large-scale production of extracellular vesicles. Int J Mol Sci. 2022;23:10522. doi: 10.3390/ijms231810522.
- Li X, Corbett AL, Taatizadeh E, et al. Challenges and opportunities in exosome research—perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3(1):011503. doi: 10.1063/1.5087122.
- Antoni D, Burckel H, Josset E, et al. Three-dimensional cell culture: a breakthrough in vivo. Int J Mol Sci. 2015;16(3):5517–5527. doi: 10.3390/ijms16035517.
- Duval K, Grover H, Han L-H, et al. Modeling physiological events in 2D vs. 3D cell culture. Physiology. 2017;32(4):266–277. doi: 10.1152/physiol.00036.2016.
- Dwivedi C, Sahu R, Tiwari SP, et al. Role of liposome in novel drug delivery system. J Drug Deliv Ther. 2014;4(2):116–129. doi: 10.22270/jddt.v4i2.768.
- Van Der Koog L, Gandek TB, Nagelkerke A. Liposomes and extracellular vesicles as drug delivery systems: a comparison of composition, pharmacokinetics, and functionalization. Adv Healthc Mater. 2022;11(5):e2100639. doi: 10.1002/adhm.202100639.
- Emam SE, Ando H, Lila ASA, et al. A novel strategy to increase the yield of exosomes (extracellular vesicles) for an expansion of basic research. Biol Pharm Bull. 2018;41(5):733–742. doi: 10.1248/bpb.b17-00919.
- Emam SE, Ando H, Lila ASA, et al. Liposome co-incubation with cancer cells secreted exosomes (extracellular vesicles) with different proteins expressions and different uptake pathways. Sci Rep. 2018;8(1):14493. doi: 10.1038/s41598-018-32861-w.
- Garcia-Hernandez A, Leal-Orta E, Ramirez-Ricardo J, et al. Linoleic acid induces secretion of extracellular vesicles from MDA-MB-231 breast cancer cells that mediate cellular processes involved with angiogenesis in HUVECs. Prostaglandins Other Lipid Mediat. 2021;153:106519. doi: 10.1016/j.prostaglandins.2020.106519.
- Miatmoko A, Asmoro FH, Azhari AA, et al. The effect of 1, 2-dioleoyl-3-trimethylammonium propane (DOTAP) addition on the physical characteristics of β-ionone liposomes. Sci Rep. 2023;13(1):4324. doi: 10.1038/s41598-023-31560-5.
- Tan HW, Misran M. Characterization of fatty acid liposome coated with low-molecular-weight chitosan. J Liposome Res. 2012;22(4):329–335. doi: 10.3109/08982104.2012.700459.
- Grace VMB, Wilson DD, Guruvayoorappan C, et al. Liposome nano-formulation with cationic polar lipid DOTAP and cholesterol as a suitable pH-responsive carrier for molecular therapeutic drug (all-trans retinoic acid) delivery to lung cancer cells. IET Nanobiotechnol. 2021;15(4):380–390. doi: 10.1049/nbt2.12028.
- Seo JW, Mahakian LM, Tam S, et al. The pharmacokinetics of Zr-89 labeled liposomes over extended periods in a murine tumor model. Nucl Med Biol. 2015;42(2):155–163. doi: 10.1016/j.nucmedbio.2014.09.001.
- Shashidhar G, Manohar B. Nanocharacterization of liposomes for the encapsulation of water soluble compounds from cordyceps sinensis CS1197 by a supercritical gas anti-solvent technique. RSC Adv. 2018;8(60):34634–34649. doi: 10.1039/c8ra07601d.
- Hallan SS, Marchetti P, Bortolotti D, et al. Design of nanosystems for the delivery of quorum sensing inhibitors: a preliminary study. Molecules. 2020;25:5655. doi: 10.3390/molecules25235655.
- Jalilian M, Derakhshandeh K, Kurd M, et al. Targeting solid lipid nanoparticles with anisamide for docetaxel delivery to prostate cancer: preparation, optimization, and in-vitro evaluation. Iran J Pharm Res. 2021;20:327.
- Meng Z, Liao Y, Peng Z, et al. Bone marrow mesenchymal stem-cell-derived exosomes ameliorate deoxynivalenol-induced mice liver damage. Antioxidants. 2023;12(3):588. doi: 10.3390/antiox12030588.
- Safadi DE, Lebeau G, Lagrave A, et al. Extracellular vesicles are conveyors of the NS1 toxin during dengue virus and zika virus infection. Viruses. 2023;15:364. doi: 10.3390/v15020364.
- Yefimova S, Kurilchenko IY, Tkacheva T, et al. Comparative study of dye-loaded liposome accumulation in sensitive and resistant human breast cancer cells. Exp Oncol. 2012;34(2):101–106.
- Wm Nor WFSB, Chung I, Said NABM. MicroRNA-548m suppresses cell migration and invasion by targeting aryl hydrocarbon receptor in breast cancer cells. Oncol Res. 2021;28(6):615–629. doi: 10.3727/096504020X16037933185170.
- Zhang Z, Mi T, Jin L, et al. Comprehensive proteomic analysis of exosome mimetic vesicles and exosomes derived from human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2022;13(1):312. doi: 10.1186/s13287-022-03008-6.
- Channarong S, Chaicumpa W, Sinchaipanid N, et al. Development and evaluation of chitosan-coated liposomes for oral DNA vaccine: the improvement of Peyer’s patch targeting using a polyplex-loaded liposomes. Am Assoc Pharm Sci. 2011;12(1):192–200. doi: 10.1208/s12249-010-9559-9.
- Guo J-X, Ping Q-N, Jiang G, et al. Chitosan-coated liposomes: characterization and interaction with leuprolide. Int J Pharm. 2003;260(2):167–173. doi: 10.1016/s0378-5173(03)00254-0.
- Mady MM, Darwish MM. Effect of chitosan coating on the characteristics of DPPC liposomes. J Adv Res. 2010;1(3):187–191. doi: 10.1016/j.jare.2010.05.008.
- Olusanya TO, Haj Ahmad RR, Ibegbu DM, et al. Liposomal drug delivery systems and anticancer drugs. Molecules. 2018;23:907. doi: 10.3390/molecules23040907.
- Yanar F, Mosayyebi A, Nastruzzi C, et al. Continuous-flow production of liposomes with a millireactor under varying fluidic conditions. Pharmaceutics. 2020;12:1001. doi: 10.3390/pharmaceutics12111001.
- Yu JY, Chuesiang P, Shin GH, et al. Post-processing techniques for the improvement of liposome stability. Pharmaceutics. 2021;13:1023. doi: 10.3390/pharmaceutics13071023.
- Fan Y, Marioli M, Zhang K. Analytical characterization of liposomes and other lipid nanoparticles for drug delivery. J Pharm Biomed Anal. 2021;192:113642. doi: 10.1016/j.jpba.2020.113642.
- Nurunnabi M, Revuri V, Huh KM, et al. Polysaccharide based nano/microformulation: an effective and versatile oral drug delivery system. In: Andronescu E, Grumezescu AM, editors. Nanostructures for oral medicine. Netherlands, UK, USA: Elsevier; 2017. p. 409–433.
- Wang J, Bonacquisti EE, Brown AD, et al. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells. 2020;9:660. doi: 10.3390/cells9030660.
- Corbo C, Molinaro R, Taraballi F, et al. Effects of the protein corona on liposome–liposome and liposome–cell interactions. Int J Nanomedicine. 2016;11:3049–3063. doi: 10.2147/IJN.S109059.
- Kang JH, Jang WY, Ko YT. The effect of surface charges on the cellular uptake of liposomes investigated by live cell imaging. Pharm Res. 2017;34(4):704–717. doi: 10.1007/s11095-017-2097-3.
- Petrini M, Lokerse WJ, Mach A, et al. Effects of surface charge, PEGylation and functionalization with dipalmitoylphosphatidyldiglycerol on liposome–cell interactions and local drug delivery to solid tumors via thermosensitive liposomes. Int J Nanomedicine. 2021;16:4045–4061. doi: 10.2147/IJN.S305106.
- Sigismund S, Lanzetti L, Scita G, et al. Endocytosis in the context-dependent regulation of individual and collective cell properties. Nat Rev Mol Cell Biol. 2021;22(9):625–643. doi: 10.1038/s41580-021-00375-5.
- Xia Y, Tian J, Chen X. Effect of surface properties on liposomal siRNA delivery. Biomaterials. 2016;79:56–68. doi: 10.1016/j.biomaterials.2015.11.056.
- Wright SH. Generation of resting membrane potential. Adv Physiol Educ. 2004;28(1–4):139–142. doi: 10.1152/advan.00029.2004.
- George S, Hamblin MR, Kishen A. Uptake pathways of anionic and cationic photosensitizers into bacteria. Photochem Photobiol Sci. 2009;8(6):788–795. doi: 10.1039/b809624d.
- Lombardo D, Calandra P, Bellocco E, et al. Effect of anionic and cationic polyamidoamine (PAMAM) dendrimers on a model lipid membrane. Biochim Biophys Acta. 2016;1858(11):2769–2777. doi: 10.1016/j.bbamem.2016.08.001.
- Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J Control Release. 2019;303:130–150. doi: 10.1016/j.jconrel.2019.04.025.
- Zhao J, Qin L, Song R, et al. Elucidating inhaled liposome surface charge on its interaction with biological barriers in the lung. Eur J Pharm Biopharm. 2022;172:101–111. doi: 10.1016/j.ejpb.2022.01.009.
- Aizik G, Grad E, Golomb G. Monocyte-mediated drug delivery systems for the treatment of cardiovascular diseases. Drug Deliv Transl Res. 2018;8(4):868–882. doi: 10.1007/s13346-017-0431-2.
- Attia N, Mashal M. Mesenchymal stem cells: the past present and future. In: Turksen K, editor. Cell biology and translational medicine, vol 11, Advances in experimental medicine and biology, vol 1312. Cham, Switzerland: Springer. 2020. p. 107–129. doi:10.1007/5584_2020_595.
- Diomede F, Marconi GD, Fonticoli L, et al. Functional relationship between osteogenesis and angiogenesis in tissue regeneration. Int J Mol Sci. 2020;21:3242. doi: 10.3390/ijms21093242.
- Lombardo D, Calandra P, Barreca D, et al. Soft interaction in liposome nanocarriers for therapeutic drug delivery. Nanomaterials. 2016;6:125. doi: 10.3390/nano6070125.
- Lürick A, Gao J, Kuhlee A, et al. Multivalent Rab interactions determine tether-mediated membrane fusion. Mol Biol Cell. 2017;28(2):322–332. doi: 10.1091/mbc.E16-11-0764.
- Manzanares D, Ceña V. Endocytosis: the nanoparticle and submicron nanocompounds gateway into the cell. Pharmaceutics. 2020;12:371. doi: 10.3390/pharmaceutics12040371.
- Monteiro N, Martins A, Reis RL, et al. Liposomes in tissue engineering and regenerative medicine. J R Soc Interface. 2014;11(101):20140459. doi: 10.1098/rsif.2014.0459.
- Ramasubramanian L, Kumar P, Wang A. Engineering extracellular vesicles as nanotherapeutics for regenerative medicine. Biomolecules. 2019;10:48. doi: 10.3390/biom10010048.
- Wonder E, Simón-Gracia L, Scodeller P, et al. Competition of charge-mediated and specific binding by peptide-tagged cationic liposome–DNA nanoparticles in vitro and in vivo. Biomaterials. 2018;166:52–63. doi: 10.1016/j.biomaterials.2018.02.052.
- Daraee H, Etemadi A, Kouhi M, et al. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):381–391. doi: 10.3109/21691401.2014.953633.
- Mukhtar M, Ali H, Ahmed N, et al. Drug delivery to macrophages: a review of nano-therapeutics targeted approach for inflammatory disorders and cancer. Expert Opin Drug Deliv. 2020;17(9):1239–1257. doi: 10.1080/17425247.2020.1783237.
- Nikolova MP, Kumar EM, Chavali MS. Updates on responsive drug delivery based on liposome vehicles for cancer treatment. Pharmaceutics. 2022;14:2195. doi: 10.3390/pharmaceutics14102195.
- Savini F, Bobone S, Roversi D, et al. From liposomes to cells: filling the gap between physicochemical and microbiological studies of the activity and selectivity of host-defense peptides. Pept Sci. 2018;110(5):e24041. doi: 10.1002/pep2.24041.
- Chattopadhyay S, Chen J-Y, Chen H-W, et al. Nanoparticle vaccines adopting virus-like features for enhanced immune potentiation. Nanotheranostics. 2017;1(3):244–260. doi: 10.7150/ntno.19796.
- Jeyagaran A, Lu C-E, Zbinden A, et al. Type 1 diabetes and engineering enhanced islet transplantation. Adv Drug Deliv Rev. 2022;189:114481. doi: 10.1016/j.addr.2022.114481.
- Narauskaitė D, Vydmantaitė G, Rusteikaitė J, et al. Extracellular vesicles in skin wound healing. Pharmaceuticals. 2021;14:811. doi: 10.3390/ph14080811.
- Umar AK, Wathoni N, Zothantluanga JH, et al. Liposome-polymer complex for drug delivery system and vaccine stabilization. Heliyon. 2022;8(2):e08934. doi: 10.1016/j.heliyon.2022.e08934.
- Haeri A, Sadeghian S, Rabbani S, et al. Effective attenuation of vascular restenosis following local delivery of chitosan decorated sirolimus liposomes. Carbohydr Polym. 2017;157:1461–1469. doi: 10.1016/j.carbpol.2016.11.021.
- Taetz S, Nafee N, Beisner J, et al. The influence of chitosan content in cationic chitosan/PLGA nanoparticles on the delivery efficiency of antisense 2′-O-methyl-RNA directed against telomerase in lung cancer cells. Eur J Pharm Biopharm. 2009;72(2):358–369. doi: 10.1016/j.ejpb.2008.07.011.
- Maia J, Caja S, Strano Moraes MC, et al. Exosome-based cell–cell communication in the tumor microenvironment. Front Cell Dev Biol. 2018;6:18. doi: 10.3389/fcell.2018.00018.
- Li Y, Cui X-L, Chen Q-S, et al. Cationic liposomes induce cytotoxicity in HepG2 via regulation of lipid metabolism based on whole-transcriptome sequencing analysis. BMC Pharmacol Toxicol. 2018;19(1):43. doi: 10.1186/s40360-018-0230-5.
- Stefanutti E, Papacci F, Sennato S, et al. Cationic liposomes formulated with DMPC and a gemini surfactant traverse the cell membrane without causing a significant bio-damage. Biochim Biophys Acta. 2014;1838(10):2646–2655. doi: 10.1016/j.bbamem.2014.05.026.
- Romøren K, Thu BJ, Bols NC, et al. Transfection efficiency and cytotoxicity of cationic liposomes in salmonid cell lines of hepatocyte and macrophage origin. Biochim Biophys Acta. 2004;1663(1–2):127–134. doi: 10.1016/j.bbamem.2004.02.007.
- Sharma P, Schiapparelli L, Cline HT. Exosomes function in cell–cell communication during brain circuit development. Curr Opin Neurobiol. 2013;23(6):997–1004. doi: 10.1016/j.conb.2013.08.005.
- Beit-Yannai E, Tabak S, Stamer WD. Physical exosome: exosome interactions. J Cell Mol Med. 2018;22(3):2001–2006. doi: 10.1111/jcmm.13479.
- Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal Biochem. 2014;448:41–49. doi: 10.1016/j.ab.2013.12.001.
- Liu X, Zhang Q, Knoll W, et al. Rational design of functional peptide–gold hybrid nanomaterials for molecular interactions. Adv Mater. 2020;32(37):2000866. doi: 10.1002/adma.202000866.
- Midekessa G, Godakumara K, Ord J, et al. Zeta potential of extracellular vesicles: toward understanding the attributes that determine colloidal stability. ACS Omega. 2020;5(27):16701–16710. doi: 10.1021/acsomega.0c01582.
- Vogel R, Pal AK, Jambhrunkar S, et al. High-resolution single particle zeta potential characterisation of biological nanoparticles using tunable resistive pulse sensing. Sci Rep. 2017;7(1):17479. doi: 10.1038/s41598-017-14981-x.
- Xu W, He W, Du Z, et al. Functional nucleic acid nanomaterials: development, properties, and applications. Angew Chem Int Ed Engl. 2021;60(13):6890–6918. doi: 10.1002/anie.201909927.
- Deregibus MC, Figliolini F, D’antico S, et al. Charge-based precipitation of extracellular vesicles. Int J Mol Med. 2016;38(5):1359–1366. doi: 10.3892/ijmm.2016.2759.
- Kaddour H, Panzner TD, Welch JL, et al. Electrostatic surface properties of blood and semen extracellular vesicles: implications of sialylation and HIV-induced changes on EV internalization. Viruses. 2020;12:1117. doi: 10.3390/v12101117.
- Woo H-K, Cho YK, Lee CY, et al. Characterization and modulation of surface charges to enhance extracellular vesicle isolation in plasma. Theranostics. 2022;12(5):1988–1998. doi: 10.7150/thno.69094.
- Kesimer M, Gupta R. Physical characterization and profiling of airway epithelial derived exosomes using light scattering. Methods. 2015;87:59–63. doi: 10.1016/j.ymeth.2015.03.013.
- Koide R, Hirane N, Kambe D, et al. Antiadhesive nanosome elicits role of glycocalyx of tumor cell-derived exosomes in the organotropic cancer metastasis. Biomaterials. 2022;280:121314. doi: 10.1016/j.biomaterials.2021.121314.
- Pietrobono S, Stecca B. Aberrant sialylation in cancer: biomarker and potential target for therapeutic intervention? Cancers. 2021;13:2014. doi: 10.3390/cancers13092014.
- Soares Martins T, Catita J, Martins Rosa I, et al. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLOS One. 2018;13(6):e0198820. doi: 10.1371/journal.pone.0198820.
- Lyu TS, Ahn Y, Im Y-J, et al. The characterization of exosomes from fibrosarcoma cell and the useful usage of dynamic light scattering (DLS) for their evaluation. PLOS One. 2021;16(1):e0231994. doi: 10.1371/journal.pone.0231994.
- Willis GR, Kourembanas S, Mitsialis SA. Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front Cardiovasc Med. 2017;4:63. doi: 10.3389/fcvm.2017.00063.
- Ehrlich HP. A snapshot of direct cell–cell communications in wound healing and scarring. Adv Wound Care. 2013;2(4):113–121. doi: 10.1089/wound.2012.0414.
- Vu R, Jin S, Sun P, et al. Wound healing in aged skin exhibits systems-level alterations in cellular composition and cell–cell communication. Cell Rep. 2022;40(5):111155. doi: 10.1016/j.celrep.2022.111155.
- Gentile P, Garcovich S. Concise review: adipose-derived stem cells (ASCs) and adipocyte-secreted exosomal microRNA (A-SE-miR) modulate cancer growth and promote wound repair. J Clin Med. 2019;8:855. doi: 10.3390/jcm8060855.
- Hassanshahi A, Hassanshahi M, Khabbazi S, et al. Adipose-derived stem cells for wound healing. J Cell Physiol. 2019;234(6):7903–7914. doi: 10.1002/jcp.27922.
- Zahorec P, Koller J, Danisovic L, et al. Mesenchymal stem cells for chronic wounds therapy. Cell Tissue Bank. 2015;16(1):19–26. doi: 10.1007/s10561-014-9440-2.
- Zhou Y, Zhao B, Zhang X-L, et al. Combined topical and systemic administration with human adipose-derived mesenchymal stem cells (hADSC) and hADSC-derived exosomes markedly promoted cutaneous wound healing and regeneration. Stem Cell Res Ther. 2021;12(1):257. doi: 10.1186/s13287-021-02287-9.
- Liew FF, Chew BC, Ooi DJ. Wound healing properties of exosomes—a review and modelling of combinatorial analysis strategies. Curr Mol Med. 2022;22(2):165–191. doi: 10.2174/1566524021666210405131238.
- Zeng Q-L, Liu D-W. Mesenchymal stem cell-derived exosomes: an emerging therapeutic strategy for normal and chronic wound healing. World J Clin Cases. 2021;9(22):6218–6233. doi: 10.12998/wjcc.v9.i22.6218.
- Joo HS, Suh JH, Lee HJ, et al. Current knowledge and future perspectives on mesenchymal stem cell-derived exosomes as a new therapeutic agent. Int J Mol Sci. 2020;21:727. doi: 10.3390/ijms21030727.
- Lou G, Chen L, Xia C, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39(1):9. doi: 10.1186/s13046-019-1512-5.
- Zhang Y, Bai X, Shen K, et al. Exosomes derived from adipose mesenchymal stem cells promote diabetic chronic wound healing through SIRT3/SOD2. Cells. 2022;11:2568. doi: 10.3390/cells11162568.
- Szlasa W, Zendran I, Zalesińska A, et al. Lipid composition of the cancer cell membrane. J Bioenerg Biomembr. 2020;52(5):321–342. doi: 10.1007/s10863-020-09846-4.
- Syarina PNA, Karthivashan G, Abas F, et al. Wound healing potential of Spirulina platensis extracts on human dermal fibroblast cells. EXCLI J. 2015;14:385.
- Antimisiaris SG, Mourtas S, Marazioti A. Exosomes and exosome-inspired vesicles for targeted drug delivery. Pharmaceutics. 2018;10:218. doi: 10.3390/pharmaceutics10040218.
- Dang XT, Kavishka JM, Zhang DX, et al. Extracellular vesicles as an efficient and versatile system for drug delivery. Cells. 2020;9:2191. doi: 10.3390/cells9102191.