Abstract
Chinese herbs contain substances that regulate female hormones. Our study confirmed that Zingiberis rhizoma carbonisata contains Zingiberis rhizoma-based carbon dots (ZR-CDs), which exert regulatory effects on serum oestradiol and FSH in mice and show impacts on endometrial growth and follicular development that potentially affect the ability of female fertility. ZR-CDs were characterized to clarify the microstructure, optical features, and functional group characteristics. It shows that ZR-CDs are spherical carbon nanostructures ranging from 0.97 to 2.3 nm in diameter, with fluorescent properties and a surface rich in functional groups. We further investigated the impact of ZR-CDs on oestradiol and FSH in serum, growth, and the development of ovarian and uterine using normal female mice and exogenous oestradiol intervention model. It was observed that ZR-CDs accelerated oestrogen metabolism and attenuated oestradiol-induced endometrial hyperplasia. Simultaneously, ZR-CDs triggered an increase in FSH, even in the presence of high-serum oestradiol that inhibits FSH secretion. Our findings suggest that ZR-CDs could be a potential therapeutic treatment for anovulatory menstruation.
Introduction
Sex hormones are essential for the regulation of female reproduction by affecting the development of the ovaries and uterus and regulating the menstrual cycle. In part, oestrogen is secreted by granulosa cells and its primary functions are to promote folliculogenesis, which increases the expression of gonadotropin receptors, facilitates endometrial regeneration and epithelial cell proliferation, and maintains secondary sexual characteristics [Citation1,Citation2]. Besides, other receptor organs include the vagina, mammary glands and brain [Citation3,Citation4].
Exerting positive and negative feedback on the pituitary and hypothalamus, oestrogen is closely associated with gonadotropin and GnRH secretion, which in turn regulate physiology and reproduction [Citation5]. Follicle-stimulating hormone (FSH) is the primary regulatory hormone on oestrogen secretion in ovarian granulosa cells, affecting the expression of Hsd17b1 and Cyp19a1, the latter of which belongs to aromatase enzymes that serve as a significant rate-limiting enzyme for the conversion of androgens to oestrogens. It is crucial for sustaining follicular development in cyclic females up until the last stage of maturation. Numerous studies have shown that the activities of FSH in the ovary of sexually immature and mature females are identical [Citation6,Citation7]. Normal follicle maturation and ovulation in women depend on the synergistic regulation of several hormones, especially FSH and luteinizing hormone (LH). Therefore, the deficiency of FSH is one of the causes of anovulatory menstruation and thus infertility.
A brand-new class of nanomaterials known as carbon dots (CDs) was originally identified in 2004. To date, CDs have been extensively and intensively investigated in many fields. The use of CDs for drug administration, bio-imaging, nanoprobes, and photocatalysis has received a lot of attention recently because of their advantages such as high water solubility, low toxicity, and excellent biocompatibility [Citation8,Citation9]. Due to the potential existence of phytoestrogens, some parts of Chinese herbal medicine could regulate sex hormones [Citation10,Citation11]. The presence of substances other than phytoestrogens that regulate human hormones is a subject worthy of investigation. Previous studies on the effects of carbon dots in herbal medicines on sex hormones are scarce. Studies have shown that nanocomponents alter female sex hormones [Citation12,Citation13].
Zingiber officinale Roscoe is the processing precursor herb of Zingiberis rhizoma carbonisata (ZRC). They are commonly used in Chinese medicine as haemostatic agents, for the treatment of heavy bleeding that occurs during menstrual cycles, one of the symptoms of anovulatory menstruation [Citation14–16]. In this study, we extracted ZRC-CDs from ZRC and characterized their structures. We further investigated whether ZRC-CDs regulate sex hormones in mice and observed their impacts on ovarian follicle development and uterine growth.
Materials and methods
Chemicals
Zingiberis rhizoma (ZR) was purchased from Beijing Qiancao Herbal Pieces Co., Ltd (Beijing, China). Dialysis membranes of 1000 Da molecular weight cut-off (MWCO) were obtained from Beijing Ruida Henghui Technology Development Co., Ltd (Beijing, China). Soybean oil (for injection used) was purchased from Zhejiang Tianyushan Medicinal Oil Co., Ltd. Oestradiol was purchased from Shanghai yuanye Bio-Technology Co., Ltd. ELISA kits for oestradiol, FSH, and LH (luteinizing hormone) assays were purchased from Jiangsu Kete Biotechnology Co., Ltd (Nanjing, China). All experiments in this study were performed using deionised water (DW).
Preparation of ZR-CDs
The ZR was placed in a crucible and heated in a preheated muffle furnace (TL0612 muffle furnace, Beijing Zhong Ke Aobo Technology Co, Ltd, Beijing, China) at 375 °C for 1 h. After cooling to room temperature, the charcoal drug was removed from the crucible and ground to a fine powder with a mechanical grinder. The ZRC decoction was then produced by boiling fine ZRC powder twice for 1 h while it was soaked in DW. To obtain purified ZR-CDs, the resultant solution was then concentrated, filtered to eliminate any remaining particles, and dialysed with DW for 96 h using a 1000 Da MWCO membrane. DW was used for each step of the ZR-CDs purification process [Citation17]. The full preparation process for the ZR-CDs is illustrated in .
Characterization of ZR-CDs
Transmission electron microscopy (TEM, Tecnai G2 F20, FEI Company, OR, USA) was used to analyse the morphology and microstructure of ZR-CDs at a 200 kV accelerating voltage. By using a high-resolution TEM, the structural specifics and atomic lattice fringes of ZR-CDs were acquired (HRTEM, JEN-1230, Japan Electron Optics Laboratory, Japan). In a typical quartz cuvette, the spectrum characteristics of the ZR-CDs were examined using ultraviolet-visible spectroscopy (UV-vis, CECIL, Cambridge, UK) and fluorescence spectroscopy (F-4500, Tokyo, Japan). Additionally, the organic functional groups in the ZR-CDs were studied using Fourier transform infra-red (FTIR, Thermo, CA, USA) spectra within a spectral window of 400–4000 cm−1. With the use of X-ray photoelectron spectroscopy (XPS, ESCALAB 250 Xi, Thermo Fisher Scientific, Fremont, CA, USA) and a mono X-ray source Al Kl excitation (1486.6 eV), the ZR-CDs’ surface composition and elemental analysis were recorded. By using an X-ray diffractometer (D8-Advanced X-ray diffractometer, Bruker AXS, Karlsruhe, Germany) we gained X-ray diffraction (XRD) patterns.
Fingerprinting of ZR and ZR-CDs by HPLC
After filtering with a 0.22-μm filter membrane, solutions of ZR and ZR-CDs were introduced into the HPLC instrument (10 μl) separately for comparison. An Agilent series 1260 HPLC system outfitted with a thermostatic column, a chromatographic column (ZORBAX SB-C18, 4.6 mm ×250 mm × 5 μm), an autosampler, a degasser, a four-stage pump, and a diode array detector was utilized to specifically identify the chemical differences between ZR and ZR-CDs. Acetonitrile (Solvent A) and water (Solvent B) were used as mobile phases to produce fingerprints of ZR and ZR-CDs according to the following gradient elution conditions. The injection volume was 20 μL, and the column temperature was 30 °C, when the mobile phase’s flow rate was 1 ml/min in the following time intervals: 0–30 min, 35–70% A, 30–50 min, 70–90% A, 50–60 min, 90% A [Citation18]. The detection wavelength was 254 nm.
Animal
Four-week-old female Kunming mice (20.00 ± 2.00 g) were purchased from SPF (Beijing) biotechnology co., LTD. All of the mice were grown in a setting with a 12:12 h cycle for light and dark and a consistent temperature and humidity, feed daily on a common maintenance diet (SPF (Beijing) biotechnology co., LTD). Processes for all animal experiments followed the Guide for the Care and Use of Laboratory Animals approved by the Ethics Committee of Animal Experimentation of Beijing University of Chinese Medicine (BUCM-4-2021052802-2044) and were provided as follows.
Effects in normal mice
Female KM mice were randomly assigned to the control group, ZR-CDs high-dose group, ZR-CDs medium-dose group, and ZR-CDs low-dose group, each group containing 8 mice. The ZR-CDs groups received gavaged doses of ZR-CDs extracts of 12 mg/kg, 6 mg/kg, and 3 mg/kg, respectively, whereas the control group received DW for 7 days. Fasting for 12 h before sampling. Gavage was started at 8:00 am on Day 8 and blood samples were taken from the eyes one-hour post-gavage.
Effects in mice with oestradiol injection
Female KM mice were randomly divided into the control groups, model groups, and ZR-CDs groups, and each group was split into five smaller groups according to different time intervals (0.08 h, 0.25 h, 0.5 h, 1 h, and 2 h), with six mice in each group. After three days of adaptive feeding, the control groups and model groups were gavaged with deionized water while the ZR-CDs groups received 6 mg/kg of ZR-CDs extracts. Before sampling, animals fasted for 12 h (water was permitted). Mice received 40 mg/kg of oestradiol solution (dissolved in soybean oil for injection) intraperitoneally after 1-h post-gavage, except for the control groups. Blood samples were collected at 0.08 h, 0.25 h, 0.5 h, 1 h, and 2 h, respectively.
Effect in mice with 21-day exogenous oestrogen injection
The KM mice were divided into five groups: the control group, the model group, and the ZR-CDs groups (high-dose, medium-dose, and low-dose subgroups). Each group was given the appropriate dose of ZR-CDs, while the model and control groups received DW. Every afternoon for 21 days, the ZR-CDs and model group were injected subcutaneously with 0.6 mg/kg of oestradiol solution (dissolved in soybean oil for injection), while the control group was injected with soybean oil. The last gavage was performed at 8 am on day 22. One hour after the gavage, the eyes of the mice were removed for blood sampling.
ELISA kit and histological analysis
Serum was collected by centrifugation at 3000 rpm for 10 min, and then, ELISA kits were used to assess the levels of serum oestrogen, FSH, and LH. The mice ovaries and uterus were taken out, weighed, and fixed with 10% paraformaldehyde.
Histopathological assessment
The right ovary of the mouse was dehydrated using a gradient method, then fixed in 10% paraformaldehyde solution for 24 h. Haematoxylin and eosin (H&E) were used to stain the 4-μm serial sections before being examined under a light electric microscope.
Statistical analysis
Statistical Product Service Solutions (SPSS, version 22.0) was used to conduct statistical studies. The mean ± SD was used to express regularly distributed data that had equal variances. The least significant difference tests were performed using one-way analysis of variance (ANOVA) to compare multiple comparisons. Non-normally distributed data were expressed using the median (interquartile range). Non-parametric tests were conducted to evaluate within-group differences, and Wilcoxon signed-rank tests were utilized to compare differences between the two groups. Differences were considered statistically significant at p < 0.05 and p < 0.01.
Results
Characterization of ZR-CDs
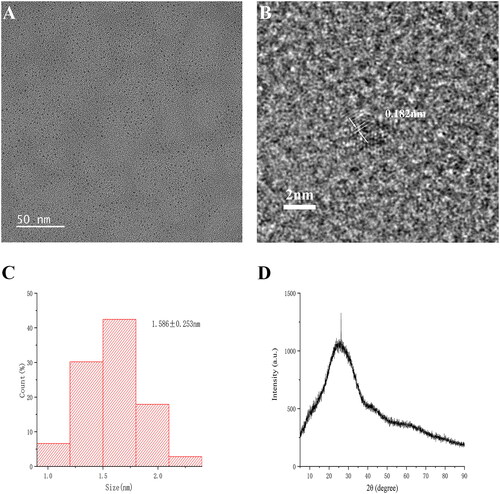
The typical TEM images of ZR-CDs, as seen in , revealed that the average particle diameter of CDs was 1.586 nm (), and they were spherical and well-spaced from each other. The HRTEM photograph illustrated a lattice spacing of 0.182 nm for CDs (). The XRD data showed strong peaks of amorphous carbon structures, which were consistent with TEM results.
Figure 2. The morphology characterisation of ZR-CDs. (A) Transmission electron microscopy image. (B) High-resolution transmission electron microscopy image. (C) Particle size distribution histogram. (D) X-ray diffraction pattern.
The fluorescence spectra of ZR-CDs showed the maximum emission wavelength was located at 470 nm where excitation was at 357 nm (). The surface and functional groups of ZR-CDs were identified by FT-IR spectra. The ZR-CDs showed distinctive peaks at 3438 cm−1, 2913 cm−1, 2850 cm−1, 1635 cm−1, 1382 cm−1, 1054 cm−1, and 611 cm−1 (). The wavelets at 2913 cm−1 and 2850 cm−1 reflected C-H bonding, while the peak at 3438 cm−1 was attributed to O-H bonding. The C=O band was assigned to 1635 cm−1 [Citation19]. 1382 cm−1 showed C-H bonds [Citation20]. The peak at 1054 cm−1 represented C-O, while the band at 1382 cm−1 reflected COO- bonds [Citation21,Citation22].
Figure 3. Optical characterization and HPLC analysis of ZR-CDs. (A) Fluorescence spectra: the maximum excitation and emission spectra. (B) Fourier transforms infra-red spectroscopy spectrum. High-performance liquid chromatography fingerprint of (C) Zingiberis hizome and (D) ZR-CDs, respectively.
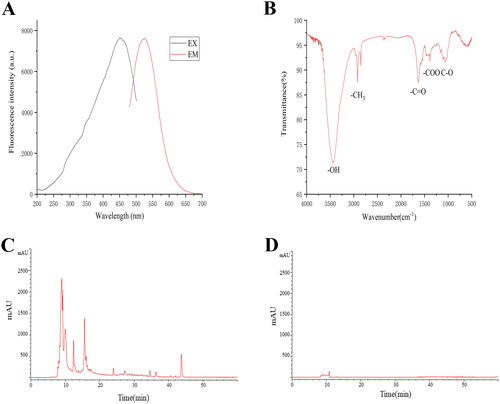
The HPLC results showed () that more soluble substances were present in ZR, while no soluble macromolecule was present in ZR-CDs after 96 h of dialysis with 1000 Da MWCO membrane, demonstrating that ZR-CDs solution as homogeneous and free of micromolecular impurities.
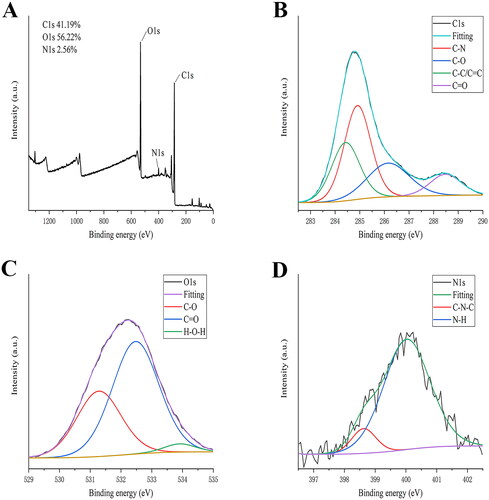
Surface composition and elemental analysis of the prepared ZR-CDs’ were characterized by XPS. Three peaks were detected visibly in the results, corresponding to C1s, O1s, and N1s at 293.45, 540.35, and 408.9 eV, respectively (). Carbon (64.52%), oxygen (32.01%), and nitrogen (2.9%) constituted the bulk of ZR-CDs. Four peaks with binding energies of 284.4, 284.9, 286.2, and 288.5 eV, representing C-C/C=C, C=O, C-O, and C=N bonds, respectively, could be resolved in the high-resolution XPS spectra of C1s (). The O1s spectrum () was divided into three peaks indicating H-O-H, C = O, and C-O, respectively, at 533.8, 532.5, and 531.3 eV. Two peaks in the N1s spectrum () at 398.6 and 400.3 eV were attributed to C-N-C and N-H bonds, respectively [Citation23,Citation24]. Additionally, the XPS data were consistent with the possibility of amino groups on the surface of ZR-CDs.
Effects on serum oestradiol, FSH, and LH in normal female mice
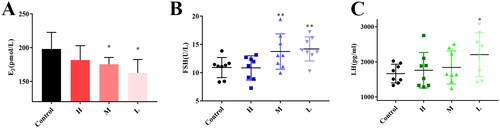
To illustrate the effect of ZR-CDs on sex hormones, we measured serum oestradiol, FSH, and LH in normal female mice. Compared with the control group (200.74 ± 24.81 pmol/L), serum oestradiol levels were significantly lower in the medium-dose (175.45 ± 9.99 pmol/L) and low-dose (162.64 ± 19.86 pmol/L) groups of ZR-CDs (, p <0.05), as well as in the high-dose group (181.74 ± 21.19 pmol/L). In terms of FSH (), compared with the control group (10.90 ± 1.78 U/L), serum FSH was significantly higher (p < 0.05) in the medium-dose and low-dose groups of ZR-CDs, with 13.74 ± 3.13 U/L and 14.19 ± 2.12 U/L, respectively. The serum LH levels of mice all showed an increasing trend after 7 days of gavage, with the mean values of each dose group of ZR-CDs being 1760.50 ± 504.29 pg/ml, 1839.88 ± 469.12 pg/ml, and 2204.88 ± 624.68 pg/ml. As indicated in , the low-dose group was significantly higher than the control group (1664.25 ± 267.78 pg/ml, p < 0.05).
Figure 5. Effect of ZR-CDs on serum oestradiol, FSH, and LH levels in normal female mice. (A) Serum oestradiol levels. (B) Serum FSH levels. (C) Serum LH levels. *p < 0.05 and **p < 0.01, compared with the control group. H: high dose of ZR-CDs, M: medium dose of ZR-CDs, L: low dose of ZR-CDs. N = 8 (each group).
Effects on serum oestrogen of injecting exogenous oestrogen
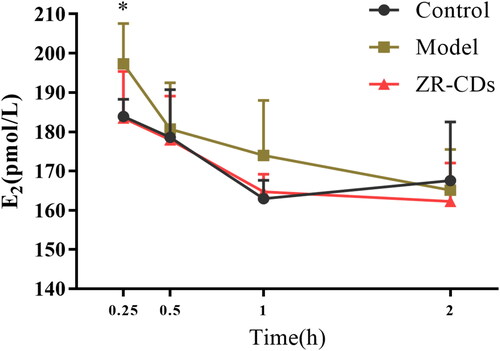
As shown in , serum oestradiol in the ZR-CDs group (6 mg/kg) and the model group were significantly elevated compared to the control group after 0.08 h of intraperitoneal administration of oestradiol solution (p < 0.05). Serum oestradiol in the ZR-CDs group (183.47 ± 11.88 pmol/L), which were significantly lower than those in the model group (197.30 ± 10.30 pmol/L, p < 0.05), had rapidly decreased to a level comparable to that of the control group (183.8 ± 84.38 pmol/L) at 0.25 h. There was no significant difference in the serum oestradiol levels of the mice in any group after 1 h of oestradiol injection.
Serum oestradiol, FSH, and LH responses to exogenous oestradiol injection
The serum oestradiol in the model group (151.38 ± 35.37 pmol/L) and ZR-CDs high-dose group (144.95 ± 38.76 pmol/L) were significantly higher after 21 days of exogenous oestradiol subcutaneous injection than in the control group (113.13 ± 18.47 pmol/L, p < 0.05). Additionally, demonstrated that serum oestradiol levels in the ZR-CDs medium dose group (233.41 ± 71.34 pmol/L) and low dose group (241.43 ± 69.39 pmol/L) mice were significantly higher than those in the model group (p < 0.05). The effects of ZR-CDs on serum oestradiol in normal mice were less similar to our previous findings.
Figure 7. Effect of ZR-CDs on serum oestradiol, FSH, and LH levels in female mice after administration of exogenous oestradiol. (A) Serum oestradiol levels. (B) Serum FSH levels. (C) Serum LH levels. #p < 0.05, compared with the control group *p < 0.05, compared with the model group. H: high dose of ZR-CDs, M: medium dose of ZR-CDs, L: low dose of ZR-CDs. N = 8 (each group).
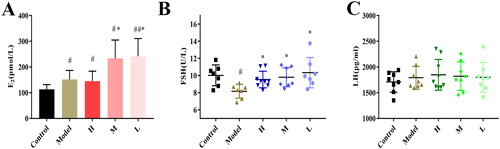
As shown in , after the administration of exogenous oestradiol for 21 days, FSH was dramatically reduced in the model group mice (8.17 ± 0.80 U/L). In contrast, FSH was not significantly different from serum FSH levels in the control group (10.03 ± 1.22 U/L, p < 0.05) and was significantly elevated in the ZR-CDs groups compared to the model group, consisting of high-dose (9.54 ± 0.97 U/L, p < 0.05), medium-dose (9.80 ± 1.11 U/L, p < 0.05), and low-dose (10.34 ± 1.77 U/L, p < 0.05) mice. In terms of LH (), after exogenous administration of oestradiol, there was no statistical difference in serum LH among the model and ZR-CDs groups compared to the control group.
Histopathological assessment of the uterus and ovaries
Except for the mice in the low-dose group of ZR-CDs, which showed an elevated ovarian index, there was no significant change in the ovarian index in any group of mice after 21 days of exogenous oestrogen stimulation, as shown in . HE staining of the uterus of each group revealed that the endometrial thickness of the mice in the model group was significantly greater than that of the control group. The endometrial thickness of the mice in each group dramatically decreased after receiving ZR-CDs and was comparable to the control group ().
Figure 8. Effect of ZR-CDs on the sexual organs of female mice after exogenous oestrogen administration. (A) Ovarian index. (B) Endometrial thickness of the uterus. (C) HE stains of uterus and ovaries, ×50. #p < 0.05, compared with the control group *p < 0.05 and **p < 0.01, compared with model group. H: high dose of ZR-CDs, M: medium dose of ZR-CDs, L: low dose of ZR-CDs. N = 8(each group).
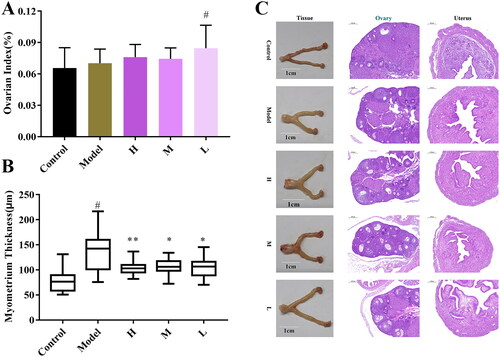
As shown in , whereas the uterus of mice in the model group was short and thick with a whitish tint, the uterus of mice in the control group was consistently extended and yellowish. Compared to the model group, the uterine shape of the mice in the ZR-CDs groups was more elongated. Female mice in the control group had well-developed ovaries with a radial follicle crown and uniform distribution of granulosa cells. Exogenous oestradiol increased the number of primary follicles and primary follicles in the mice while decreasing the number of secondary follicles and mature follicles. The ZR-CDs group showed an increase in the number of mature follicles compared to the model group.
Discussion
Although the mechanisms are still being investigated, there is mounting evidence that carbon nanomaterials may have an impact on the female reproductive system. Since gonadotropin research and even hypothalamic gonadotropin-releasing hormone are upstream of gonadal regulation and are crucial for biological growth and reproduction, the majority of research focuses primarily on gonadotropins [Citation25,Citation26]. According to research, some carbon nanoparticles can affect women’s sex hormone levels, disrupt their menstrual cycle, and even be hazardous to their reproductive organs [Citation27,Citation28]. Gold nanoparticles, for instance, have been found to infiltrate rat ovarian granulosa cells and subcellular organelles through modulating oestrogen accumulation in vitro [Citation29]. Studies have shown that despite titanium dioxide nanostructure administration preferentially lowering LH levels, exposure to nickel nanostructures enhances gonadotropin levels [Citation30,Citation31]. Although the presence of carbon particles disrupts the natural balance of female hormones, especially in the context of occupational exposure. We can therefore exploit their hormonal effects to treat female endocrine dysfunction. Furthermore, ZR-CDs, as a carbon nanoparticle, positively regulated hormone secretion and maintained endocrine homeostasis in mice in the simulated disease conditions in our study.
Chinese herbs play an important role in controlling sex hormones as a rich source of drug-derived precursors. For instance, chemical components such as isoflavones, coumestans, and glycyrrhizinic acid extracted from herbs are effective in treating endocrine disorders by acting on the corresponding tissues or cells of the body, or by regulating sex hormones [Citation32–34]. Based on the above inspiration, it is the objective of this study whether the nanocomponent we isolated from the herb can regulate hormones. The analgesic effects of the ZR-CDs were discovered in an earlier study [Citation35]. Moreover, ZR-CDs were also found to be able to exert considerable effects on sex hormones in our study. We recognized that ZR-CDs are sphere-like nanoparticles with fluorescence properties and abundant functional groups, this is similar to the results obtained in previous studies. However, the ZR-CDs we extracted did not contain small-molecule chemistry, but only pure CDs, which was verified by HPLC analysis results. Therefore, ZR-CDs are one of the components in ZRC that can affect hormone secretion. ZRC has long been employed for the clinical management of gynaecological ailments, despite a lack of established scientific rationale. For instance, conditions such as perimenopausal physiological changes or premature ovarian failure markedly diminish the quality of life among women. Conventional hormone replacement therapies come with inherent drawbacks, including cardiovascular risks. By facilitating the restoration of normal FSH levels, ZR-CDs are poised to emerge as a valuable adjunctive therapy for the treatment of endocrine disorders.
Oestradiol is an important component of the female hormone that controls the growth and development of the ovaries and uterus, maintains secondary sexual characteristics, controls the female menstrual cycle and reproduction, and has effects that last until menopause when oestradiol rapidly declines [Citation36–38]. ZR-CDs exhibited antagonistic effects on oestradiol in female mice, which was confirmed by the serum concentration-time curve after exogenous injection of oestradiol. The serum oestradiol levels peaked rapidly after receiving intraperitoneal injections of high quantities of oestradiol within a short period, followed by quickly reverting to normal levels due to the hepatic action. ZR-CDs hastened oestradiol metabolism throughout the whole procedure. This enables us to observe a significant decrease in oestradiol following ZR-CDs intervention. However, serum oestradiol levels were remarkably elevated after 21 days oestradiol treatment. Hence, ZR-CDs exhibit both agonistic and antagonistic effects on oestradiol. The anterior pituitary gland secretes FSH, which stimulates follicle development and maturation as well as the proliferation and expansion of ovarian granulosa cells [Citation27], whereas LH encourages the conversion of cholesterol to oestrogen [Citation39]. Both serum FSH and LH were significantly elevated as a result of intervention by ZR-CDs. High serum levels of oestradiol impose a negative feedback regulation on the pituitary gland during long-term administration, preventing the release of FSH and lessening its beneficial influence on oestrogen [Citation40]. In addition, the intervention of ZR-CDs did not inhibit FSH secretion and maintained FSH levels within the normal range in the presence of significantly elevated serum oestrogens. These results suggested that the agonistic effect of ZR-CDs on FSH was much stronger than the negative feedback regulation by oestrogen. This may provide some new options for the treatment of patients suffering from anovulatory infertility.
We evaluated morphological and pathological aspects of the ovaries and uterus in intervened mice and clarified that exogenous oestradiol promoted endometrial hyperplasia but had no significant effect on ovarian growth. Abnormal endometrial hyperplasia caused by abnormal oestradiol secretion may lead to a variety of gynaecological diseases, such as uterine bleeding and uterine fibroids [Citation41,Citation42]. Even when serum oestradiol was elevated in mice under the intervention of ZR-CDs, the endometrium of mice did not over-proliferate as a result. In clinical patients, premature ovarian failure is often accompanied by high serum FSH concentrations and low serum oestradiol concentrations [Citation43]. Despite the high serum FSH accompanying the intervention of ZR-CDs, no significant signs of premature failure were observed from the pathological findings of the ovaries.
Anovulation is a significant cause of female infertility that is frequently associated with polycystic ovary syndrome, which the exact mechanism is unclear. Several theories have been proposed in recent research to explain this phenomenon, including inhibition of ovarian aromatase activity, insufficient follicle-stimulating hormone signalling, insulin resistance, and an excess of androgens [Citation44,Citation45]. Clomiphene citrate (CC) is one of the most commonly used drugs in the clinical treatment of anovulation, competing for oestrogen receptors to attenuate negative feedback regulation by oestrogen, but is teratogenic and drug resistant [Citation46]. The results of this study demonstrated that ZR-CDs regulate FSH secretion and antagonize high serum oestradiol-induced endometrial hyperplasia without affecting follicular development and maturation, which is consistent with the treatment of anovulatory uterine bleeding. It, therefore, deserves to be further investigated to determine whether this could be a drug for anovulation or one of the treatment options for polycystic ovary syndrome. Meanwhile, further evidence is needed to elucidate the specific mechanisms and targets of ZR-CDs to increase FSH. The functional group characterization of the surface of ZR-CDs may be the cornerstone of their specific therapeutic action and is the focus of future studies.
The present study was the first to isolate nanocomponents from ZRC that possess the ability to impact gonadotropic hormones, providing evidence for the use of ZRC in the treatment of gynaecological disorders in clinical practice. Meanwhile, distinct from the unfavourable effects of nanoparticles on reproductive organs found in other studies, ZR-CDs exert protective roles on the ovaries and uterus of mice instead. Many studies impute the effects of nanoparticles on hormones to toxic effects and have a negative attitude, whereas the present study confirms ZR-CDs’ positive feedback on FSH, which could potentially serve as a material basis for the development of new medicines.
Conclusion
In conclusion, this study extracted the hormone-regulating nanocomponent ZR-CDs from the herb and demonstrated that promote FSH secretion and affect the metabolism of oestradiol in female mice. Our findings suggest that ZR-CDs have the potential to be developed as an effective drug for the treatment of anovulatory menstruation.
Acknowledgements
The authors appreciate the support of Beijing University of Chinese Medicine and the Classical Prescription Basic Research Team of Beijing University of Chinese Medicine.
Author contributions
Hui Kong, Yan Zhao, and Huihua Qu proposed the idea and layout for the experiments. The study was conducted by Yumin Chen, Xue Bai, Ying Zhang, Yafang Zhao, Yinghui Guo, and Tong Wu. Huagen Ma, Yunbo Yang, Meijun Wang, and Xiaopeng Li evaluated and analysed the data. Both Yumin Chen and Yue Zhang contributed to the draft manuscript preparation. The final manuscript was approved by all authors. All authors meet ICMJE criteria for authorship.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The original data for this study support the findings and conclusions of the article and are available if required.
Additional information
Funding
References
- Smits G, Olatunbosun O, Delbaere A, et al. Ovarian hyperstimulation syndrome due to a mutation in the follicle-stimulating hormone receptor. N Engl J Med. 2003;349(8):760–766. doi: 10.1056/NEJMoa030064.
- Krause WC, Rodriguez R, Gegenhuber B, et al. Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature. 2021;599(7883):131–135. doi: 10.1038/s41586-021-04010-3.
- Groothuis PG, Dassen HH, Romano A, et al. Estrogen and the endometrium: lessons learned from gene expression profiling in rodents and human. Hum Reprod Update. 2007;13(4):405–417. doi: 10.1093/humupd/dmm009.
- Rosenfeld CS, Wagner JS, Roberts RM, et al. Intraovarian actions of oestrogen. Reproduction. 2001;122(2):215–226. doi: 10.1530/rep.0.1220215.
- Deviche P. Editorial - Neuroendocrine control of reproduction. Mol Cell Endocrinol. 2022;551:111662. doi: 10.1016/j.mce.2022.111662.
- Donaubauer EM, Hunzicker-Dunn ME. Extracellular signal-regulated kinase (ERK)-dependent phosphorylation of Y-Box-binding protein 1 (YB-1) enhances gene expression in granulosa cells in response to follicle-stimulating hormone (FSH). J Biol Chem. 2016;291(23):12145–12160. doi: 10.1074/jbc.M115.705368.
- Fitzpatrick SL, Richards JS. Regulation of cytochrome P450 aromatase messenger ribonucleic acid and activity by steroids and gonadotropins in rat granulosa cells. Endocrinology. 1991;129(3):1452–1462. doi: 10.1210/endo-129-3-1452.
- Zhang ML, Zhao Y, Cheng JJ, et al. Novel carbon dots derived from schizonepetae herba carbonisata and investigation of their haemostatic efficacy. Artif Cell Nanomed B. 2018;46(8):1562–1571.
- Yan X, Zhao Y, Luo J, et al. Hemostatic bioactivity of novel pollen typhae carbonisata-derived carbon quantum dots. J Nanobiotechnology. 2017;15(1):60. doi: 10.1186/s12951-017-0296-z.
- Mersereau JE, Levy N, Staub RE, et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol Cell Endocrinol. 2008;283(1-2):49–57. doi: 10.1016/j.mce.2007.11.020.
- Wang JQ, Song CH, Gao DM, et al. Effects of Paeonia lactiflora extract on estrogen receptor β, TPH2, and SERT in rats with PMS anxiety. Biomed Res Int. 2020;2020,8. doi:10.1155/2020/4690504.
- Simon V, Avet C, Grange-Messent V, et al. Carbon black nanoparticles inhibit aromatase expression and estradiol secretion in human granulosa cells through the ERK1/2 pathway. Endocrinology. 2017;158(10):3200–3211. doi: 10.1210/en.2017-00374.
- Wang R, Song B, Wu J, et al. Potential adverse effects of nanoparticles on the reproductive system. Int J Nanomedicine. 2018;13:8487–8506. doi: 10.2147/IJN.S170723.
- Niu YF, Wen P, Li LL, et al. Study on the related substances and channel tropism of baked ginger on warming meridian and hemostasis based on ‘syndrome-efficacy-analysis of biological Samples’. China Journal of Traditional Chinese Medicine and Pharmacy. 2020;35(3):1454–1461.
- Kashefi F, Khajehei M, Alavinia M, et al. Effect of ginger (zingiber officinale) on heavy menstrual bleeding: a placebo-controlled, randomized clinical trial. Phytother Res. 2015;29(1):114–119. doi: 10.1002/ptr.5235.
- Su JB, Zhu QH, Xue XY, et al. Serum metabolism study of the warming channel and hemostatic effect of zingiberis hizome carbonisata. Chinese Traditional and Herbal Drugs. 2015;28(6):1177–1179.
- Zhang M, Cheng J, Hu J, et al. Green phellodendri chinensis cortex-based carbon dots for ameliorating imiquimod-induced psoriasis-like inflammation in mice. J Nanobiotechnology. 2021;19(1):105. doi: 10.1186/s12951-021-00847-y.
- Cheng J, Zhang M, Sun Z, et al. Hemostatic and hepatoprotective bioactivity of junci medulla carbonisata-derived carbon dots. Nanomedicine (Lond). 2019;14(4):431–446. doi: 10.2217/nnm-2018-0285.
- Podstawka E. Structural properties of bombesin-like peptides revealed by surface-enhanced raman scattering on roughened silver electrodes. Biopolymers. 2008;89(11):980–992. doi: 10.1002/bip.21047.
- Jiang J, He Y, Li SY, et al. Amino acids as the source for producing carbon nanodots: microwave assisted one-step synthesis, intrinsic photoluminescence property and intense chemiluminescence enhancement. Chem Commun (Camb). 2012;48(77):9634–9636. doi: 10.1039/c2cc34612e.
- Wei X, Li L, Liu J, et al. Green synthesis of fluorescent carbon dots from gynostemma for bioimaging and antioxidant in zebrafish. ACS Appl Mater Interfaces. 2019;11(10):9832–9840. doi: 10.1021/acsami.9b00074.
- Yang R, Guo XF, Jia LH, et al. Green preparation of carbon dots with mangosteen pulp for the selective detection of Fe3+ ions and cell imaging. Appl Surf Sci. 2017;423:426–432. doi: 10.1016/j.apsusc.2017.05.252.
- Yu BY, Kwak SY. Carbon quantum dots embedded with mesoporous hematite nanospheres as efficient visible light-active photocatalysts. J Mater Chem. 2012;22(17):8345–8353. doi: 10.1039/c2jm16931b.
- Shen J, Shang SM, Chen XY, et al. Highly fluorescent N, S-co-doped carbon dots and their potential applications as antioxidants and sensitive probes for Cr (VI) detection. Sensor Actuat B-Chem. 2017;248:92–100. doi: 10.1016/j.snb.2017.03.123.
- Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433(7023):269–277. doi: 10.1038/nature03206.
- Fox KM, Dias JA, Van Roey P. Three-dimensional structure of human follicle-stimulating hormone. Mol Endocrinol. 2001;15(3):378–389. doi: 10.1210/mend.15.3.0603.
- McGee EA, Perlas E, LaPolt PS, et al. Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol Reprod. 1997;57(5):990–998. doi: 10.1095/biolreprod57.5.990.
- Yoshida S, Hiyoshi K, Ichinose T, et al. Effect of nanoparticles on the male reproductive system of mice. Int J Androl. 2009;32(4):337–342. doi: 10.1111/j.1365-2605.2007.00865.x.
- Stelzer R, Hutz RJ. Gold nanoparticles enter rat ovarian granulosa cells and subcellular organelles, and alter in-vitro estrogen accumulation. J Reprod Dev. 2009;55(6):685–690. doi: 10.1262/jrd.20241.
- Gao G, Ze Y, Li B, et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J Hazard Mater. 2012;243:19–27. doi: 10.1016/j.jhazmat.2012.08.049.
- Garrel G, Racine C, L’Hote D, et al. Anti-Mullerian hormone: a new actor of sexual dimorphism in pituitary gonadotrope activity before puberty. Sci Rep. 2016;6:23790. doi: 10.1038/srep23790.
- Kraus SD. Glycyrrhetinic acid–a triterpene with antioestrogenic and anti-inflammatory activity. J Pharm Pharmacol. 1960;12(1):300–306. doi: 10.1111/j.2042-7158.1960.tb12667.x.
- Ollberding NJ, Lim U, Wilkens LR, et al. Legume, soy, tofu, and isoflavone intake and endometrial cancer risk in postmenopausal women in the multiethnic cohort study. J Natl Cancer Inst. 2012;104(1):67–76. doi: 10.1093/jnci/djr475.
- Basu P, Maier C. Phytoestrogens and breast cancer: in vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed Pharmacother. 2018;107:1648–1666. doi: 10.1016/j.biopha.2018.08.100.
- Zhang ML, Cheng JJ, Zhang Y, et al. Green synthesis of zingiberis hizome-based carbon dots attenuates chemical and thermal stimulus pain in mice. Nanomedicine (Lond). 2020;15(9):851–869. doi: 10.2217/nnm-2019-0369.
- Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids. 2008;73(5):473–487. doi: 10.1016/j.steroids.2008.01.017.
- Estradiol/progesterone (bijuva) for menopausal vasomotor symptoms (reprinted from medical letter on drugs and therapeutics, vol 61, pg 99-101, 2019). Jama-J Am Med Assoc. 2019;322(12):1206–1207.
- Hugon-Rodin J, Amand G, Plu-Bureau G. Safety of estradiol treatment in perimenopausal asymptomatic women. JAMA Psychiatry. 2018;75(5):528–529. doi: 10.1001/jamapsychiatry.2018.0615.
- Casarini L, Santi D, Simoni M, et al. Spare’ luteinizing hormone receptors: facts and fiction. Trends Endocrinol Metab. 2018;29(4):208–217. doi: 10.1016/j.tem.2018.01.007.
- Dewailly D, Robin G, Peigne M, et al. Interactions between androgens, FSH, anti-Mullerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016;22(6):709–724. doi: 10.1093/humupd/dmw027.
- Vandormael-Pournin S, Guigon CJ, Ishaq M, et al. Oocyte-specific inactivation of Omcg1 leads to DNA damage and c-Abl/Tap63-dependent oocyte death associated with dramatic remodeling of ovarian somatic cells. Cell Death Differ. 2015;22(1):108–117. doi: 10.1038/cdd.2014.122.
- Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 2012;13(4):235–251. doi: 10.1007/s11154-012-9221-9.
- Chen XY, Chen WL, Ma M, et al. The potential of follicle-stimulating hormone peptide-modified triptolide-loaded nanoparticles to induce a mouse model of premature ovarian insufficiency. Int J Nanomedicine. 2015;10:2765–2774. doi: 10.2147/IJN.S72593.
- Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994;9(2):188–191. doi: 10.1093/oxfordjournals.humrep.a138480.
- Robinson S, Kiddy D, Gelding SV, et al. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf). 1993;39(3):351–355. doi: 10.1111/j.1365-2265.1993.tb02376.x.
- Gadalla MA, Huang S, Wang R, et al. Effect of clomiphene citrate on endometrial thickness, ovulation, pregnancy and live birth in anovulatory women: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(1):64–76. doi: 10.1002/uog.18933.