Abstract
Decellularization is a process to harvest the decellularized extra cellular matrix (dECM) that helps develop 3D scaffolds which mimic the native tissue composition. The decellularized tissues retain the structural and functional properties of the extracellular matrix (ECM) by an efficient decellularization process that retains tissue-specific biochemical and biophysical cues for tissue regeneration. In this study, we report an injection-based decellularization method, without perfusion setup. This study also compares the efficiency of the proposed protocol in the two animal models viz rat and mice. This method harvests rat and mice liver dECM using ethylenediamine tetra acetic acid (EDTA) and sodium dodecyl sulphate (SDS) within 08 h and 02 h respectively and preserved significant amount of ECM proteins. We reported that the harvested mice decellularized extracellular matrix (mdECM) and rat decellularized extracellular matrix (rdECM) had significant reduction in their DNA content (∼97%) and retained structural architecture resembling their native tissue counterparts. The total protein content retained in mdECM was ∼39% while that retained in rdECM was ∼65%. It was also found that the sGAG (sulphated glycosaminoglycan) content showed a no List of Figures.
1. Introduction
The human liver is the second largest internal organ and is vital for survival, as it performs predominant functions including regulation of metabolism and detoxification. Extensive damage due to alcohol, drugs or toxins can damage the liver hepatocytes beyond repair and impede in its regenerative proficiency, despite it being the only organ in the human body to regenerate efficiently. Liver diseases like fibrosis, cirrhosis, chronic viral hepatitis, and fatty liver are a major healthcare concern globally. Several of these diseases are prevalent due to lifestyle changes, leading to increased death rate. In 2022, it was reported that about two million deaths (4% of total death) were accounted due to liver diseases [Citation1].
Liver transplantation is the available clinical intervention for chronic liver failure with limitations like poor donor availability, low engraftment rate with increased chances of graft rejection. Other available treatment options like the hepatocyte transplantation [Citation2], bioartificial liver systems [Citation3] have shown moderate success but are not long-term solutions. Advancement in material science and the intervention of latest technology has provided great opportunities for using a variety of biomaterials that can yield viable solutions for hepatic regeneration.
To mimic the native tissue microenvironment, biomaterials are the promising options for fabrication of 3D scaffolds. Scientists have explored many natural biomaterials such as collagen, fibrin, elastin etc., which are components of ECM that are mandatory for cell-matrix communication. ECM is a 3D network of multiple components that are tissue specific and aid in maintaining tissue structure and promote cell signalling. The dECM as a biomaterial is popular owing to its biocompatibility and presence of ECM components that triggers cell signalling. The presence of dECM in 3D scaffolds mimics the tissue microenvironment in terms of structural and functional characteristics resembling the native tissue [Citation4]. The dECM is obtained by various methods of tissue/organ decellularization where all the cellular components are removed to retain maximum ECM content. This process involves rigorous physical, chemical, and enzymatic treatments to eliminate native cellular components while preserving the ECM composition and architecture [Citation5]. Acellular liver scaffolds have been produced from various species, including rats [Citation6–8], pigs [Citation9–11], sheep [Citation12] and humans [Citation13–15]. These scaffolds have been used to culture various hepatic cell lines, primary hepatocytes, and stem cell-derived hepatocyte-like cells [Citation16,Citation17]. Researchers have investigated the efficacy of different reagents, such as sodium dodecyl sulphate (SDS), Triton X-100, peracetic acid, and sodium deoxycholate, in liver decellularization [Citation18,Citation19]. Successful cell removal is typically confirmed by the absence of visible nuclear material in decellularized tissue sections (via DAPI, H&E staining), <200 bp of DNA fragment length and minimal double-stranded DNA (<50 ng/mg tissue) in the resulting scaffold [Citation20]. To assess ECM preservation post-decellularization, researchers often quantify primary liver ECM components, including collagen, elastin, sGAG, and growth factors [Citation21]. Decellularization protocols are designed to optimise the retention of these active components during cellular removal. Studies utilise decellularized liver scaffolds as hydrogels, either solubilised or intact. These hydrogels serve as 3D scaffolds for cell encapsulation, injectable materials for in vivo healing, or bioink for 3D bioprinting applications.
Studies have reported that the use of tissue-specific dECM as a scaffold material has enhanced physiological relevance [Citation22]. Several methods to harvest dECM are being optimised for the purpose of tissue repair and regeneration. However, the efficiency of dECM depends on tissue source and density, decellularization technique and reagents used in the process. The dECM can be used as an additive material for scaffold fabrication in a biomaterial, to coat tissue culture plates, etc [Citation23]. There exist no standard guidelines for obtaining liver dECM from various species. Choudhury et al. summarises the different modes of decellularization in terms of chemicals and devices for various organs [Citation24]. Most whole liver decellularization techniques are done via perfusion, which requires an extensive and expensive set up. Perfusion technique is preferred for decellularizing the liver since the portal vein or the hepatic vein can be cannulated for perfusion with decellularization agents. A study was conducted by a group to determine the best route of perfusion to achieve an efficient whole liver decellularization in rat and hepatic vein was found to be the more efficient route [Citation25]. Diffusion/immersion technique is usually preferred when the organ is not required for recellularization. However, studies report that perfusion technique better preserves the liver ECM architecture and could supply oxygen and nutrients efficiently via the vascular network after recellularization [Citation17].
We propose an effective decellularization technique for harvesting rat and mice liver dECM without the use of a perfusion setup. Ethylenediamine tetra acetic acid (EDTA) and sodium dodecyl sulphate (SDS) are used in this study to remove the cellular components. EDTA is a chelating agent that helps in breaking the cell-matrix adhesions. SDS is an ionic detergent capable of solubilising DNA and cell membrane, thereby helps in removal of cellular and nuclear content [Citation26]. The study also compares the two rodent models – rat and mice to determine the effectiveness of the proposed technique.
2. Materials and methods
2.1. Materials
All chemicals used in this study are of molecular biology grade, i.e., purity ≥98.5%. Sodium dodecyl sulphate, phenol, chloroform, isoamyl alcohol, isopropanol and papain were purchased from Sisco Research Laboratories, India. Ethylenediamine tetra acetic acid and formaldehyde were bought from Qualigens, India. Glutaraldehyde was ordered from Sigma-Aldrich, India. Proteinase K was purchased from Invitrogen, India. Coomassie brilliant blue and protease inhibitor cocktail were obtained from Merck, India. Sodium acetate was purchased from Tokyo Chemical Industry, India. RIPA buffer was bought from Himedia, India. Pierce BCA Protein Assay Kit was purchased from Thermo Fisher Scientific, India, and Blyscan - sulphated glycosaminoglycan (sGAG) assay kit from biocolor, United Kingdom. Wistar rats and Swiss Albino mice were purchased from National Institute of Nutrition, Hyderabad, India.
2.2. Animal ethics approval
All the experimental procedures involving animals were approved by the Institutional Animal Ethics Committee (IAEC), Manipal Academy of Higher Education, Manipal, which is in accordance with CPSCEA guidelines (IAEC/KMC/121/2022). In accordance with ethical standards and guidelines set forth by the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines, rigorous protocols were adhered to in all studies involving laboratory animals.
2.3. Methods
2.3.1. Harvesting liver and decellularization
About 06 Male Wistar rats weighing 150–250 g (9–12 weeks old) and 06 male Swiss Albino mice weighing 25–30 g (7–10 weeks old) were anaesthetised with sodium pentobarbital (50 mg/kg IP). Papaverine (20 mg/kg) was injected into the tail vein to relax the visceral muscles and to euthanize the animal. A transverse incision across the abdomen was made, and ligaments connecting to the liver lobes were carefully cut to expose liver. The liver was excised from the body and placed in a falcon tube with sterile phosphate buffer saline (PBS). The tissues were washed with sterile PBS and stored at −80 °C until further use.
For decellularization, the frozen liver was thawed at 4 °C and washed with sterile PBS and immersed in 0.001 M EDTA for 30 min. The liver was cut into small pieces for ease of handling, and injected with 100 ml distilled water. Consequently, the cut liver pieces received successive injections of SDS at increasing concentrations (0.1%, 0.25%, 0.5%, 0.75%, 1%). Specifically, 100 ml of 0.1% SDS was administered, followed by 100 ml of 0.25% SDS, subsequently 100 ml of 0.5% SDS, 100 ml of 0.75% SDS, and finally 100 ml of 1% SDS, was administered individually. About 8-10 sites of injection points were created on the liver pieces for flushing out cells. Each of the liver pieces were finally washed thrice with 200 ml distilled water and thrice with 200 ml PBS, and they turned translucent upon completion of the process [Citation27].
2.3.2. Histology
In order to perform haematoxylin and eosin (H&E) staining, the native and decellularized tissue samples were fixed in 10% formalin and were processed for H&E staining. Briefly, the fixed samples were embedded in paraffin and sectioned with a microtome to obtain 3 µm sections. Sectioned samples were permeabilized and stained with H&E [Citation28]. The stained samples were observed and imaged using the Olympus CX43 microscope, Japan.
2.3.3. Scanning electron microscopy (SEM)
For SEM imaging, native and decellularized samples were fixed with 2.5% glutaraldehyde at 4 °C for 2 h. The samples were then dehydrated in ascending gradient of ethanol (20% to 90%) for 10 min each and washed with 100% ethanol twice for 10 min each before leaving the samples to air dry overnight [Citation29]. The samples were placed on a carbon coated aluminium stub and was coated with gold using the Quorum SC7620 sputter coater (120s, 10 Pa). The samples were imaged using the Zeiss EVO MA18 SEM, Germany.
2.3.4. DNA quantification
The genomic DNA was isolated from 50 mg of native, mdECM and rdECM by in house phenol-chloroform-isoamyl alcohol extraction method. The tissue samples were homogenised using a Scilogex SC16 PRO homogeniser, USA followed by centrifugation at 5000 rpm at 4 °C for 10 min following which the supernatant was discarded. Lysis buffer (200 µl), Tris-EDTA (TE) buffer (400 µl, 1X) and proteinase K (10 µl) was added to the precipitated tissues in each microcentrifuge tube followed by roto spinning at 25 rpm for 10 min. The samples were placed in a water bath at 55 °C for 2 h, after which phenol (600 µl) was added and vortexed for 20 s, followed by roto spinning at 25 rpm for 10 min. The samples were then centrifuged at 8000 rpm for 10 min at 4 °C. The aqueous phase was pipetted out gently to a new microcentrifuge tube to which phenol (300 µl) and chloroform isoamyl alcohol (24:1, 300 µl) was added. The samples were vortexed for 20 s, followed by roto spinning at 25 rpm for 30 min and centrifuged at 8000 rpm for 10 min at 4 °C. The aqueous phase was pipetted out gently to a new microcentrifuge tube to which equal volumes of phenol and chloroform isoamyl alcohol (500 µl) were added. The samples were vortexed for 20 s, followed by roto spinning at 25 rpm for 30 min and centrifuged at 8000 rpm for 10 min at 4 °C. The aqueous phase was pipetted out gently to a new microcentrifuge tube to which chilled isopropanol (600 µl) and sodium acetate (3 M, 60 µl) was added. The microcentrifuge tubes were gently inverted several times to observe a thread-like structure in case of presence of DNA. If no thread or precipitation was observed, the samples were kept in −20 °C for 30 min before centrifuging at 12,000 rpm for 20 min at 4 °C. The supernatant was discarded, and 70% ethanol was added and centrifuged at 12,000 rpm for 20 min at 4 °C. The supernatant was discarded, and the pellet was left to airdry overnight [Citation30]. The pellet was dissolved in TE buffer according to the pellet size obtained (10 µl − 30 µl). The samples can be stored at −20 °C for further use. The amount of DNA was measured at 260 nm and 280 nm using a Synergy H1 multi-mode microplate reader, USA. Absorbance values were normalised to sample wet weight. A gel electrophoresis with 1% Agarose gel was also performed to visualise the presence of DNA in the samples.
2.3.5. Protein quantification
Proteins were extracted from native and decellularized liver tissues (50 mg) by digesting in 1X RIPA buffer (180 µl) along with protease inhibitor cock-tail (20 µl). The total protein content was quantified by Bicinchoninic acid (BCA) assay. The sample (25 µl) was mixed with working reagent (200 µl), which was provided in the kit on a plate shaker for 30 s. The plate was then incubated at 37 °C for 30 min before measuring absorbance at 562 nm. Protein content in each sample was calculated and adjusted to load 20 µg/µl of sample in each well. Protein sample (20 µg/µl) was mixed with a loading buffer and reducing agent (NuPAGE, Thermo Fisher Scientific). Samples were heated for 10 min at 70 °C. The protein was resolved on a 6% SDS-PAGE gel and was run for 90 min at constant 100 V [Citation31]. The gel was stained with Coomassie blue and was visualised using an Invitrogen iBright cl1500 imaging system, USA.
2.3.6 sGAG quantification
The sGAG content was quantified using Blyscan glycosaminoglycan assay kit. The native and decellularized tissue samples (50 mg) were digested with papain extraction regent (1 ml) at 65 °C for 3 h, and centrifuged at 10,000 g for 10 min. The obtained supernatant was further used according to the manufacturer’s instructions. The absorbance of each sample was measured on a microplate reader at 656 nm [Citation32].
2.3.7. Statistical analysis
All statistical analysis was conducted with the aid of GraphPad Prism version 9.5.1. All quantitative data were reported as mean ± standard deviation (SD). One way ANOVA was applied for DNA and sGAG quantification. Significance was set as p < .05.
3. Results
3.1. Decellularization of rodent liver
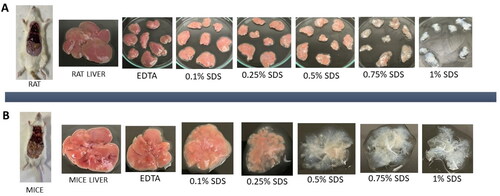
The time taken to complete decellularization varies depending on the weight of the native tissue and the tissue density. Decellularization of rat liver was completed in an average of 8 h and that of mice liver in 2 h by chemical method using EDTA & SDS. Post dissection, the rat liver was cut into small pieces (randomly) to ensure efficient decellularization, while the mice liver was used as a whole for further process due to significantly smaller size of the mice liver tissue. Using this protocol, cellular content in both rat and mice liver was completely removed, which were characterised further to validate for the absence of cellular material. Macroscopic observation demonstrated change in tissue colour gradually from red to pale red and then becomes translucent on injection with gradient increase in SDS concentrations as shown in . The appearance of translucent tissue suggests the removal of various hepatic cells from rat and mice liver.
The weight of rodent’s liver was noted pre and post decellularization process in wet state. It was found that the weight was significantly reduced in mdECM and rdECM when compared to their native liver tissues as represented in . The weight of native mice liver and mdECM was noted as 1.857 ± 0.512 g and 0.255 ± 0.068 g, respectively, while the weight of native rat liver was 7.247 ± 2.175 g and rdECM was1.026 ± 0.378 g. The weight reduction was about 85 ± 3% in both rdECM and mdECM. Although the weight reduction cannot confirm the removal of cells, but similar trend in weight loss can be monitored to ascertain the cellular removal [Citation33].
Table 1. Comparison of weight reduction in rat and mice liver post decellularization.
3.2 Histology
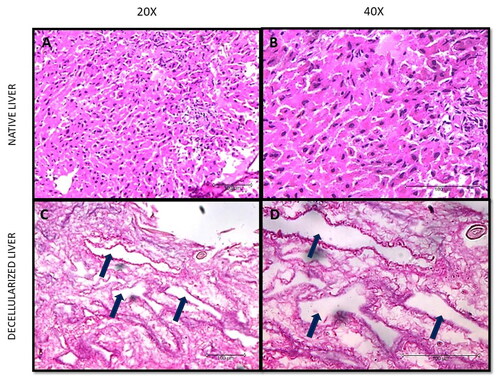
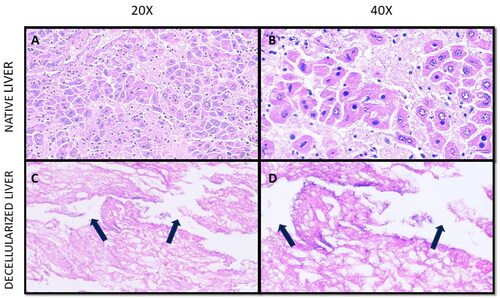
The successful removal of nuclear content was validated by H&E staining as represented in and Citation3. The morphology of the decellularized tissue in rodents was reasonably preserved when compared to the native tissue, where it can be observed that the decellularized tissues did not take up the purple colour of haematoxylin stain. It was also observed from the staining that the ECM matrix was retained and intact in both the species, which took the pink eosin stain. The native tissue clearly took up the haematoxylin (purple) and eosin (pink) stain.
3.3. Scanning electron microscopy
The SEM was performed to assess the architectural changes that could have occurred in the matrix during the process of decellularization. It was observed that the liver micro architecture appeared to be preserved and increased number of pores was found in rdECM () and mdECM (). and B) depicts the rat native liver structure, while and B) reveals the mice native liver structure.
3.4. DNA quantification
The removal of nuclear content in the decellularized tissue was confirmed by DNA quantification as shown in . DNA content in native and decellularized liver was normalised to the initial wet weight of each sample. DNA content was significantly reduced in rdECM and mdECM when compared with their native tissues. The residual DNA content in native mice liver and mdECM was 1036.759 ± 80.328 ng/mg and 27.084 ± 19.821 ng/mg respectively while native rat liver and rdECM was 1157.537 ± 58.216 ng/mg and 29.082 ± 5.509 ng/mg, respectively. Comparison between rat and mice native liver revealed a significant difference in DNA content (1157.537 ± 58.216 ng/mg vs. 1036.759 ± 80.328 ng/mg, p < .05) while their decellularized counterparts showed no significant difference between their residual DNA content (29.082 ±5.509 ng/mg vs. 27.084 ± 19.821 ng/mg, p > .05). The DNA content of all decellularized tissues was significantly different from that of the native tissues. The values are expressed as mean ± SD, with sample size of 04 in each group as represented in . Agarose gel electrophoresis results showed a recognisable dense band of DNA in the native tissue, whereas DNA isolated from decellularized tissues did not show any band, which confirms the elimination of DNA in dECM of both rat and mice.
Table 2. Comparison of residual DNA content in rat and mice liver post decellularization.
3.5 Protein quantification
The SDS PAGE was performed to determine the molecular weight of the protein components present in the dECM of the rodents. demonstrates the SDS PAGE results and the presence of bands in mdECM and rdECM at varying size. These bands confirm the presence of collagen in the rat and mice liver dECM and the same is depicted in the . The α1 and α2 chains of type I collagen were identified at around 120 kDa, while the β chain around 250 kDa. The α1 and α2 chains were found to be the major constituents that form the triple helical structure of collagen molecules. The collagen protein bands were identified at the same level in native and dECM tissue for both the animal models. The total protein content was quantified by BCA assay and is represented in .
Table 3. Comparison of total protein content in rat and mice liver post decellularization.
3.6 sGAG quantification
The sGAG content in native and decellularized organs was normalised to the initial wet weight of each sample. sGAG content reduced significantly in mdECM when compared to the native mice tissue whereas the sGAG content was retained significantly in rdECM when compared to the native rat liver tissue (). The sGAG content in mice native liver and mdECM was noted as 6916.503 ± 027.675 ng/mg and 1391.389 ± 64.575 ng/mg respectively. The native rat liver contained 6962.165 ± 728.785 ng/mg sGAG and rdECM had 5458.577 ± 281.366 ng/mg sGAG. The values are expressed as mean ± SD, with sample size of 04 in each group as represented in .
Table 4. Comparison of sGAG content in rat and mice liver post decellularization.
4. Discussion
The dECM as a biomaterial component offers great promise in tissue engineering as they retain the complex array of proteins like sGAG, collagen, elastin as found in the native tissue, which promotes tissue repair and regeneration [Citation8]. Various studies reported that dECM scaffolds promoted liver regeneration after partial hepatectomy and transplantation [Citation13, Citation34]. In this study, we aim to provide an efficient decellularization protocol without the need for an extensive perfusion setup to harvest the dECM from rat and mice liver. The proposed method is novel since it uses a combination of immersion and injection method. The most prominently used methods so far are perfusion, immersion or stirring methods [Citation35]. The advantage of this proposed injection method is that it does not require any complex setup and hence is low cost. It does not compromise on the efficiency of DNA reduction or protein retention. Most importantly, this protocol reduces the time taken for decellularization from days to hours. Many perfusion-based decellularization protocols have been reported [Citation36–38] and these protocols usually take couple of days for complete decellularization [Citation39], and are used for whole organ recellularization or in cases where the vascular networks need to be preserved. The current study focuses on comparing the two-animal models viz. rat and mice liver for complete decellularization, while retaining ECM proteins. This protocol takes about 8 h to decellularize rat liver, while it takes about 2 h to decellularize mice liver. A few studies have reported rapid decellularization methods via perfusion within 24 h [Citation40–43]. However, these studies mention the time taken to decellularize excluding the washing step. Some of these studies report the washing of detergents from the organ to take more than 10 days. Also, most of these studies state that the ECM proteins were retained and were confirmed by staining methods but total protein retention was not quantified. Reducing the time of decellularization can prevent the loss of prominent ECM proteins. Post decellularization, the translucent tissues confirm the effect of SDS and it is also decided by factors such as tissue density and weight [Citation26], which was also observed in our case. Using this protocol, we observed the minimum weight reduction was ∼80% for each sample and on an average ∼85% of weight reduction in dECM of rodents when compared to their native liver tissue (). Although the average % weight reduction was same between the two groups, the weight after decellularization in rat was ∼1.02 g and that in mice was ∼0.25 g. The % weight reduction is relative to the weight before and after decellularization. We believe that the method of decellularization influences the weight and appearance of the dECM obtained.
The efficiency, quality, and composition of the decellularized tissue was determined by DNA quantification, total protein retention and sGAG estimation post decellularization. The decellularization protocol was effective as the dECM obtained was devoid of most nuclear remnants with negligible residual DNA as shown in , an important criterion to justify maximum decellularization where the residual DNA is less than 50 ng/mg [Citation19]. The DNA content in mdECM and rdECM are 97.39%, 97.49% less than their native counterparts. There was no significant difference between residual DNA content in mdECM and rdECM. The % DNA reduction might be similar in both rodent groups as this is relative to the DNA content before and after decellularization. A significant difference was observed in DNA content between native rat and native mice groups. Although the % weight reduction and % DNA reduction is similar in both the rodents, we hypothesise that the decellularization method used may lead to retention of more proteins in the rdECM when compared to the mdECM due to the difference in weight and density of the rat and mice tissues. The second criteria for efficient decellularization are the DNA fragment length less than 200 bp, which was observed in . The third criteria is the visualisation of tissues through H&E staining, a dense eosin-stained cytoplasm and non-specifically stained proteins present in the ECM while dark purple-stained nuclei were observed in the native tissue ( and B) and ). In contrast, dECM tissue samples showed eosin-stained ECM, with a lack of cell and nuclear fragments, which confirmed efficient cell removal after the decellularization of samples ( and D) and ). It is also observed that the decellularized tissue has pores (pointed by arrows) when compared to the native tissue, which indicates the absence of resident cells. The pores are formed at spots where the cells are removed. Our findings revealed that the decellularization protocol was successful with minimum disruption to native tissue morphology as observed via H&E-stained tissue sections ( and ).
Figure 3. Histological characterisation of native and decellularized tissue. (A) Mice native liver tissue at 20X magnification, (B) Mice native liver tissue at 40X magnification, (C) Mice decellularized liver tissue at 20X magnification, and (D) Mice decellularized liver tissue at 40X magnification.
The scanning electron micrograph images revealed 3D arrangement of the matrix was retained in the decellularized tissues ( and ), while no obliteration of the ECM matrix was observed in decellularized tissues of rat and mice. The same was also corelated with SDS PAGE, wherein the decellularized tissues retained most collagen and the bands appear at similar level as that of native tissue (). Hence, SDS PAGE results suggest that the original collagen structure of the tissue is not damaged during chemical treatment for liver decellularization. Some lower molecular weight proteins were also retained in the dECM between 50 and 100 kD; however, these bands were faint. The total protein content was calculated from the BCA assay and the results were corelating with sGAG quantification. The mdECM (39%) retained less proteins when compared to the rdECM (65%) due to difference in body weight and tissue complexity. The shape of hepatocytes is visible in native samples (pointed by red arrows) while the pores are visible in the decellularized tissues (pointed by yellow arrows). Histology and porosity of decellularized tissues are usually examined by observation of gaps in decellularized tissues, and the presence of cells is noted in the native tissues [Citation44]. It is important for the decellularized tissues to retain most bioactive molecules, since they play a role in enhancing tissue regeneration and improve cell matrix interaction [Citation45]. This ensures that the natural properties of ECM are retained which further help to develop bioengineered scaffolds/tissues.
Figure 4. Scanning Electron Micrograph acquired at 10 kV (A) Rat native liver tissue at 500X magnification, (B) Rat native liver tissue at 1000X magnification, (C) Rat decellularized liver tissue at 500X magnification, and (D) Rat decellularized liver tissue at 1000X magnification.
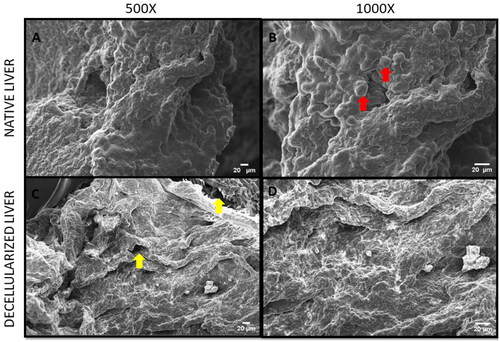
Figure 5. Scanning Electron Micrograph acquired at 10 kV (A) Mice native liver tissue at 500X magnification, (B) Mice native liver tissue at 1000X magnification, (C) Mice decellularized liver tissue at 500X magnification, and (D) Mice decellularized liver tissue at 1000X magnification.
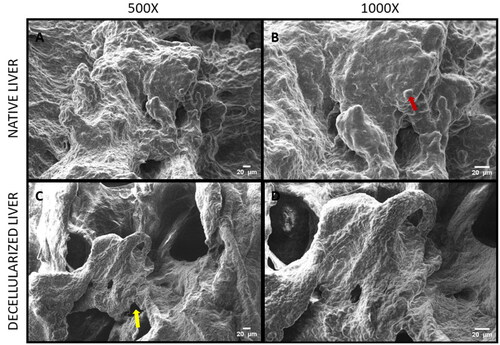
Figure 6. Quantification of DNA in decellularized rat and mice liver. (A) Residual DNA content (*p < .05, ****p < .0001) and (B) DNA gel electrophoresis.
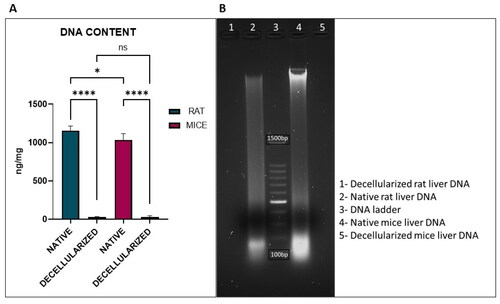
Figure 7. SDS PAGE to determine protein molecular weight distributions in the decellularized liver tissues of rat and mice.
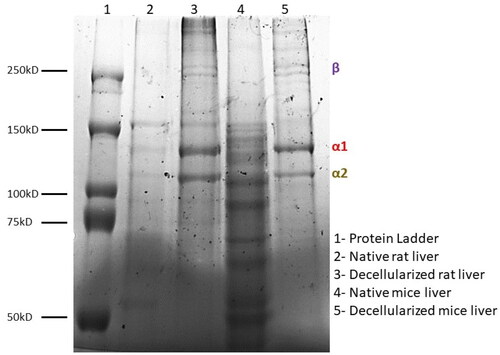
Figure 8. Estimation of sGAG content in decellularized rat and mice liver (**p < 0.05, ***p < .001).
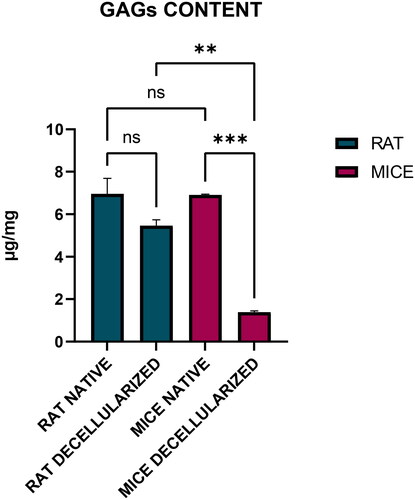
Proteoglycans are a category of biomolecules characterised by the presence of at least one GAG chain as a post-translational modification on a specific core protein [Citation46]. In the context of decellularized tissues, the significance of proteoglycans lies in their contributions to the ECM, which serves as the framework for cellular attachment, signalling, and overall tissue integrity [Citation47]. The sGAG content was quantified in both native and decellularized tissues of rat and mice using the Blyscan kit, which is based on a dye-binding assay and quantifies the measurement of sGAG. The quantification of residual sGAG content in dECM is important to understand their role in signalling pathways and cellular responses [Citation48]. Their functions are also associated with tissue structure, homeostasis, and in regulating signalling pathways which affect cellular processes. It is known that the sGAG are hydrophilic in nature and can retain water in the ECM, which also contributes to resisting compressive forces [Citation49,Citation50]. The results confirmed the retention of ECM constituents, thus preserving the biochemical properties of the native liver tissue. sGAG content was reduced post-decellularization in both rat and mice; however, the reduction was not significant in rat (21.6%), which suggests the use of SDS was not harsh to remove most sGAG from the rat dECM whereas there was a significant reduction of sGAG in mice dECM (79.6%).
This study has examined the liver decellularization process without the need for an external perfusion setup, which can lead to major loss of ECM content. This technique can further be used to develop bio fabrication strategies to produce scaffold with different biomaterials along with dECM as a component [Citation51, Citation52]. The quantification of other ECM contents like elastin, collagen, or fibronectin to evaluate the retention of each ECM component can be analysed which is required to draw further conclusions. The next step in our future research would be to individually quantify and stain other ECM components from the harvested dECM, conduct biocompatibility and cytotoxicity tests by growing hepatocytes on dECM to confirm that all the SDS has been washed off the tissues and also to determine the secretion of ECM components and to examine the efficiency of the process. We also plan to conduct biomechanical tests to the harvested dECM by uniaxial tension and compression tests and determine the swelling ratio. The proposed injection method of decellularization proved to be working efficiently so far but requires further experimentation to draw conclusions on aspects of biocompatibility and quantification of other individual ECM components.
5. Conclusion
The use of decellularized materials has several advantages, as their tissue composition and structure are very similar to those of the native tissues. Biomaterials using dECM as a component for developing scaffolds and hydrogels will provide biological cues to enhance tissue repair and regeneration. The decellularization process used in this study preserved important components of the ECM such as sGAG, collagen and other low molecular weight proteins while removing the nuclear content in mice and rat decellularized livers. However, the adapted technique was much more efficient in rats since it retained most proteins and sGAG when compared to mice. This SDS injection-based method can be preferred over perfusion method since the tissue exposure time to the detergent is drastically reduced leading to maximum protein retention and was also successful in eliminating the nuclear components. The use of dECM as a biomaterial component in scaffolds for liver regeneration looks promising and this can be a prototype for human liver decellularization.
Author contributions
Meghana Kasturi – conception and design, or analysis and interpretation of the data; the drafting of the paper. Kirthanashri S Vasanthan – conception and design, revising it critically for intellectual content, and the final approval of the version to be published.
Acknowledgement
The authors would like to thank Manipal Center for Biotherapeutics Research (MCBR), MAHE, Manipal for the provision of research instruments and infrastructure support.
Disclosure statement
No potential conflict of interest was reported by the author(s)
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Devarbhavi H, Asrani SK, Arab JP, et al. Global burden of liver disease: 2023 update. J Hepatol. 2023; S0168-8278(23):00194–00190.
- Iansante V, Mitry RR, Filippi C, et al. Human hepatocyte transplantation for liver disease: current status and future perspectives. Pediatr Res. 2018; 83(1-2):232–240. doi: 10.1038/pr.2017.284.
- Selden C, Bundy J, Erro E, et al. A clinical-scale BioArtificial liver, developed for GMP, improved clinical parameters of liver function in porcine liver failure. Sci Rep. 2017; 67(1):14518. doi: 10.1038/s41598-017-15021-4.
- Vigier S, Fülöp T. Exploring the extracellular matrix to create biomaterials. Compos Funct Extracell Matrix Human Body. 2016. Travascio, F. (Ed.). InTech. doi: 10.5772/61601
- Allu I, Sahi AK, Koppadi M, et al. Decellularization techniques for tissue engineering: towards replicating native extracellular matrix architecture in liver regeneration. JFB. 2023; 14(10):518. doi: 10.3390/jfb14100518.
- Debnath T, Mallarpu CS, Chelluri LK. Development of bioengineered organ using biological acellular rat liver scaffold and hepatocytes. Organogenesis. 2020; 16(2):61–72. doi: 10.1080/15476278.2020.1742534.
- Acun A, Oganesyan R, Jaramillo M, et al. Human-origin iPSC-based recellularization of decellularized whole rat livers. Bioengineering. 2022; 9(5):219. doi: 10.3390/bioengineering9050219.
- Wang A, Kuriata O, Xu F, et al. A survival model of in vivo partial liver lobe decellularization towards in vivo liver engineering. Tissue Eng Part C Methods. 2020; 26(8):402–417. doi: 10.1089/ten.tec.2019.0194.
- Struecker B, Hillebrandt KH, Voitl R, et al. Porcine liver decellularization under oscillating pressure conditions: a technical refinement to improve the homogeneity of the decellularization process. Tissue Eng Part C Methods. 2015; 21(3):303–313. doi: 10.1089/ten.tec.2014.0321.
- Coronado RE, Somaraki-Cormier M, Natesan S, et al. Decellularization and solubilization of porcine liver for use as a substrate for porcine hepatocyte culture: method optimization and comparison. Cell Transplant. 2017; 26(12):1840–1854. doi: 10.1177/0963689717742157.
- Mattei G, Di Patria V, Tirella A, et al. Mechanostructure and composition of highly reproducible decellularized liver matrices. Acta Biomater. 2014; 10(2):875–882. doi: 10.1016/j.actbio.2013.10.023.
- Kajbafzadeh AM, Javan-Farazmand N, Monajemzadeh M, et al. Determining the optimal decellularization and sterilization protocol for preparing a tissue scaffold of a human-Sized liver tissue. Tissue Eng Part C Methods. 2013; 19(8):642–651. doi: 10.1089/ten.TEC.2012.0334.
- Mazza G, Rombouts K, Rennie Hall A, et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep. 2015; 5(1):13079. doi: 10.1038/srep13079.
- Verstegen MMA, Willemse J, van den Hoek S, et al. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cells Dev. 2017; 26(18):1304–1315. doi: 10.1089/scd.2017.0095.
- Alevra Sarika N, Payen VL, Fléron M, et al. Human liver-Derived extracellular matrix for the culture of distinct human primary liver cells. Cells. 2020; 9(6):1357. doi: 10.3390/cells9061357.
- Antarianto RD, Pragiwaksana A, Septiana WL, et al. Hepatocyte differentiation from iPSCs or MSCs in decellularized liver scaffold: cell–ECM adhesion, spatial distribution, and hepatocyte maturation profile. Organogenesis. 2022; 18(1):2061263. doi: 10.1080/15476278.2022.2061263.
- Khajavi M, Hashemi M, Kalalinia F. Recent advances in optimization of liver decellularization procedures used for liver regeneration. Life Sci. 2021; 281:119801. doi: 10.1016/j.lfs.2021.119801.
- Jeong W, Kim MK, Kang HW. Effect of detergent type on the performance of liver decellularized extracellular matrix-based bio-inks. J Tissue Eng. 2021; 12:2041731421997091. doi: 10.1177/2041731421997091.
- Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011; 32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057.
- Liao J, Guo Q, Xu B, et al. Overview of decellularized materials for tissue repair and organ replacement. Decellularized Materials: Preparations and Biomedical Applications. 2021;1–67.
- Dai Q, Jiang W, Huang F, et al. Recent advances in liver engineering with decellularized scaffold. Front Bioeng Biotechnol. 2022; 10:831477. doi: 10.3389/fbioe.2022.831477.
- Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014; 163(4):268–285. doi: 10.1016/j.trsl.2013.11.003.
- Neishabouri A, Soltani Khaboushan A, Daghigh F, et al. Decellularization in tissue engineering and regenerative medicine: evaluation, modification, and application methods. Front Bioeng Biotechnol. 2022; 10:805299. doi: 10.3389/fbioe.2022.805299.
- Choudhury D, Yee M, Sheng ZL, et al. Decellularization systems and devices: state-of-the-art. Acta Biomater. 2020; 115:51–59. doi: 10.1016/j.actbio.2020.07.060.
- Jambar Nooshin B, Tayebi T, Babajani A, et al. Effects of different perfusing routes through the portal vein, hepatic vein, and biliary duct on whole rat liver decellularization. Cell J Yakhteh. 2023; 25(1):35–44.
- Moffat D, Ye K, Jin S. Decellularization for the retention of tissue niches. J Tissue Eng. 2022; 13:20417314221101151. doi: 10.1177/20417314221101151.
- Antarianto RD, Dewi A, Pragiwaksana A, et al. Decellularization of liver cubes using multiple site syringe injection for generating native liver scaffold: preliminary report. AIP Conf Proc. 2019; 2193(1):040005.
- Alaby Pinheiro Faccioli L, Suhett Dias G, Hoff V, et al. Optimizing the decellularized porcine liver scaffold protocol. Cells Tissues Organs. 2022; 211(4):385–394. doi: 10.1159/000510297.
- Ghim JH, Hussein KH, Park KM, et al. Hepatic cell encapsulation using a decellularized liver scaffold. Biomed Eng Lett. 2015; 5(1):58–64. doi: 10.1007/s13534-015-0176-0.
- Gilbert TW, Freund J, Badylak SF. Quantification of DNA in biologic scaffold materials. J Surg Res. 2009; 152(1):135–139. doi: 10.1016/j.jss.2008.02.013.
- Fernández-Pérez J, Ahearne M. The impact of decellularization methods on extracellular matrix derived hydrogels. Sci Rep. 2019; 9(1):14933. doi: 10.1038/s41598-019-49575-2.
- Wu Q, Bao J, Zhou Y J, et al. Optimizing perfusion-decellularization methods of porcine livers for Clinical-Scale whole-organ bioengineering. Biomed Res Int. 2015; 2015:e785474. doi: 10.1155/2015/785474.
- Topuz B, Aydin HM. Preparation of decellularized optic nerve grafts. Artif Organs. 2022; 46(4):618–632. doi: 10.1111/aor.14098.
- Shimoda H, Yagi H, Higashi H, et al. Decellularized liver scaffolds promote liver regeneration after partial hepatectomy. Sci Rep. 2019; 9(1):12543. doi: 10.1038/s41598-019-48948-x.
- Rabbani M, Zakian N, Alimoradi N. Contribution of physical methods in decellularization of animal tissues. J Med Signals Sens. 2021; 11(1):1–11. doi: 10.4103/jmss.JMSS_2_20.
- Fathi I, Imura T, Inagaki A, et al. Decellularized whole-organ pre-vascularization: a novel approach for organogenesis. Front Bioeng Biotechnol. 2021;971.
- Baptista PM, Siddiqui MM, Lozier G, et al. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53(2):604–617. doi: 10.1002/hep.24067.
- Xu B, Håkansson J, Kuna VK, et al. Assessing rat liver-derived biomatrix for hepatic tissue engineering with human fetal liver stem cells. Adv Tissue Eng Regen Med Open Access. 2016;1(3):00012.
- Willemse J, Verstegen MMA, Vermeulen A, et al. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater Sci Eng C Mater Biol Appl. 2020; 108:110200. doi: 10.1016/j.msec.2019.110200.
- De Kock J, Ceelen L, De Spiegelaere W, et al. Simple and quick method for whole-liver decellularization: a novel in vitro three-dimensional bioengineering tool? Arch Toxicol. 2011; 85(6):607–612. [Database] doi: 10.1007/s00204-011-0706-1.
- Pan MX, Hu PY, Cheng Y, et al. An efficient method for decellularization of the rat liver. J Formosan Med Assoc. 2014; 113(10):680–687. doi: 10.1016/j.jfma.2013.05.003.
- Willemse J, Verstegen MM, Vermeulen A, et al. Fast, robust and effective decellularization of whole human livers using mild detergents and pressure controlled perfusion. Mater Sci Eng C. 2020; 108:110200. doi: 10.1016/j.msec.2019.110200.
- Mazza G, Al-Akkad W, Telese A, Jul., et al. Rapid production of human liver scaffolds for functional tissue engineering by high shear stress oscillation-decellularization. Sci Rep. 2017;17;7(1):5534.
- Behmer Hansen RA, Wang X, Kaw G, et al. Accounting for material changes in decellularized tissue with underutilized methodologies. Biomed Res Int. 2021; 2021:6696295–6696215. doi: 10.1155/2021/6696295.
- Liu C, Pei M, Li Q, et al. Decellularized extracellular matrix mediates tissue construction and regeneration. Front Med. 2022; 16(1):56–82. doi: 10.1007/s11684-021-0900-3.
- Yada T, Koide N, Kimata K. Functions of proteoglycan/glycosaminoglycan in liver. In Extracellular matrix and the liver. Academic Press; 2003; pp. 55–74.
- Silver FH, Kelkar N, Deshmukh T. Molecular basis for mechanical properties of ECMs: proposed role of fibrillar collagen and proteoglycans in tissue biomechanics. Biomolecules. 2021; 11(7):1018. doi: 10.3390/biom11071018.
- He M, Callanan A, Lagaras K, et al. Optimization of SDS exposure on preservation of ECM characteristics in whole organ decellularization of rat kidneys. J Biomed Mater Res. 2017;105(6):1352–1360. doi: 10.1002/jbm.b.33668.
- Perez S, Makshakova O, Angulo J, et al. Glycosaminoglycans: what remains to be deciphered? JACS Au. 2023; 3(3):628–656. doi: 10.1021/jacsau.2c00569.
- Kasturi M, Vasanthan KS. Effect of decellularization using sodium dodecyl sulfate on glycosaminoglycans content in the liver. Regen Med. 2023;18(7):527–530. doi: 10.2217/rme-2023-0050.
- Ma X, Yu C, Wang P, et al. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials. 2018; 1185:310–321. doi: 10.1016/j.biomaterials.2018.09.026.
- Ravichandran A, Murekatete B, Moedder D, et al. Photocrosslinkable liver extracellular matrix hydrogels for the generation of 3D liver microenvironment models. Sci Rep. 2021; 11(1):15566. doi: 10.1038/s41598-021-94990-z.