 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
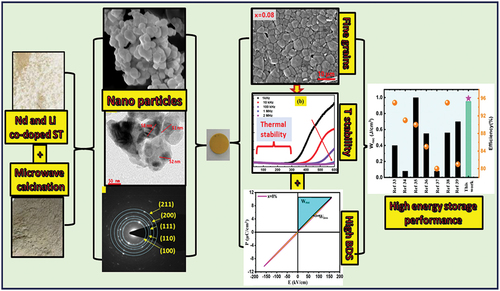
There is an immediate demand for eco-friendly, high-performance, and highly stable energy storage materials for pulse power systems. Ceramics based on SrTiO3 have a high breakdown strength (BDS) and dielectric constant. In this work, we fabricated a polycrystalline of Sr(1-x)(Nd, Li)xTiO3 ceramics via microwave-assisted heating of the starting materials. X-ray diffraction analysis reveals a pure perovskite phase without any secondary phase. Scanning electron microscopy images exhibit dense grain morphology with a decrease in grain size as the dopant concentration increases. The frequency dependences of the dielectric properties were studied in the frequency range of 100 Hz-1 MHz at room temperature. The polarization-electric field hysteresis loops were examined to ascertain the effect of co-doping on the energy-storage capability of SrTiO3 ceramics. After increasing the co-dopants from 0 to 8%, the energy density increased nine times (from 0.11 J/cm3 to 0.952 J/cm3), and the energy storage efficiency increased from 80.71% to 95.98%, respectively. In addition, the samples demonstrate excellent thermal stability, and their energy storage properties are stable up to 80°C. We may infer from this discovery that the bulk Nd3+ and Li+ co-doped SrTiO3 materials are good candidates for high-energy-density capacitor applications.
GRAPHICAL ABSTRACT
1. Introduction
The fast expansion of the global economy has resulted in a worldwide uptick in the demand for electrical power. The capability to store and release energy is growing in complexity and significance. To attain efficient, versatile, and environmentally friendly energy, there is a pressing need for more robust and high-performance energy storage systems [Citation1,Citation2]. Dielectric capacitors are unique among energy storage devices in that they may release their stored energy quickly and produce a high-intensity pulsed current or voltage. As a result, its potential use in pulsed power electronics appears encouraging [Citation3,Citation4]. However, their limited usefulness is mainly due to their low energy density. As a result, capacitors need to increase their energy density while their size decreases.
The polarization-electric field (P-E) hysteresis loop, as illustrated in Eqs. (1)-(3) may be used to analyze the energy storage qualities of dielectric capacitors [Citation5–7].
Bulk ceramics may be used in energy storage development, including linear dielectrics, relaxor ferroelectrics, and antiferroelectric [Citation8–11]. According to the preceding calculations (Equationequtions 1(1)
(1) -Equation3
(3)
(3) ), the best materials for high energy storage density have a high breakdown strength (Eb), a high Pmax, and a low Pr. In these calculations, Pr represents the residual polarization, Pmax represents the maximum polarization, and E denotes the applied electric field. The linear dielectric SrTiO3 (ST) ceramic exhibits the potential for energy-storage applications among the dielectric materials by having a high Eb and small Pr. Unfortunately, the low Pmax indicates that, due to the absence of spontaneous polarization, the material typically displays a low Wrec.
Therefore, implementing a higher Eb value proves to be a more productive approach. Several multiscale variables influence the parameter Eb, including atomic scale doping, nanoscale interface phenomena, microscale grain size variations, macroscale thickness, and porosity characteristics [Citation12–15]. Adding a suitable quantity of Bi3+ to SrTiO3 has increased Eb by reducing both the sintering -temperature and the grain size [Citation16]. The incorporation of Zr4+ into Sr0.5Ca0.5TiO3 ceramics was proposed by Pu et al. to mitigate grain formation [Citation17]. Furthermore, the annealing process in an oxygen-rich environment boosted the barrier effect at grain boundaries. The researchers achieved a high electric field strength of 440 kV/cm [Citation17]. The researcher fabricated Zn and Nb co-doped SrTiO3 ceramics. They observed that co-doping and compositing techniques achieved a high electric breakdown strength (Eb) of 422 kV/cm and an energy storage density of 2.35 J/cm3 [Citation18]. Dy-doped SrTiO3 demonstrates a notable achievement in terms of its recoverable energy density, measuring at 4.00 J/cm3, and exhibits an exceptionally high breakdown strength of 510 kV/cm. These outcomes are achieved by subjecting the material to oxygen treatment, which effectively enhances the resistance of both crystal grains and grain boundaries [Citation19]. A high breakdown strength of up to 354 kV/cm and a relatively high recoverable energy density of 2.13 J/cm3 have been achieved by B. Zhong et al. [Citation20]. There have lately been significant efforts made to enhance the Wrec of ST Chemical alterations (such as doping with metal ions on the A/B sites, creating compounds with complicated endmembers, and adding sintering aids) and using various sintering processes are among them [Citation21–30]. To improve its energy-storage performance, ST may be chemically modified to grain size and boost its breakdown strength. For instance, Liu L. et al. [Citation31] revealed that SrTiO3-based ceramics could achieve both high energy storage capability and ultrafast discharge speed thanks to a synergistic impact of chemical alteration and defect chemistry. An energy storage density of 1.1 J/cm3 and an energy efficiency of 87% has been achieved by doped 5% of Sn in the Ti site of SrTiO3 has been reported by J. Xie et al. [Citation32]. Xu Guo et al. [Citation33] reported a high energy storage density of 4.00 J/cm3 and high energy storage efficiency of 89.49% at a maximum applied field of 400 kV/cm are simultaneously realized in Dy doped SrTiO3 ceramics through defect and interface engineering. compares our study’s energy storage properties, and those of a different lead-free energy storage ceramic revealed recently measured at 160 kV/cm [Citation33–39].
Figure 1. A comparison between the energy storage features of SNLTx ceramics and other lead-free ceramics with prospective energy storage applications measured at 160 kV/cm.

Previous studies have also indicated that the A-sites of ABO3 ceramics tend to form Li+-Al3+ ionic pairs within the same cell along the [001] direction when co-doped with Li+ and Al3+ having small ionic radii. This dramatically enhanced energy storage performance due to the local lattice distortion, as observed in [Citation40]. The effect of the ionic pair is greater than that of single doping ions, thus greatly influencing the physical properties properties of the ABO3 ferroelectric ceramics. Moreover, co-doping can enhance the chemical and thermal stability of ABO3 materials, ensuring that they maintain their energy storage properties over extended periods and under various environmental conditions. Based on the preceding discussion, we present a straightforward and efficient approach to enhance the energy storage capabilities of SrTiO3 ceramics by introducing ionic pairs with small ionic radii. The primary objective in addressing this issue is to increase the breakdown strength, resulting in better energy storage performance. Recently, Nd3+ and Li+ ions were incorporated into ST to create SNLT ceramics, resulting in a significant increase in the breakdown strength of ST from approximately 70 kV/cm to 160 kV/cm, which may be attributed to the reduction in grain size. A comprehensive analysis that included structural characterization and electrical properties was conducted to investigate the mechanism behind the enhanced energy storage properties.
2. Experimental
A polycrystalline of Sr(1-x)(Nd, Li)xTiO3; (0%≤x ≤ 8%) abbreviated as (SNLTx) has been fabricated using the solid-state reaction method. The SrCO3, Li2CO3, Nd2O3, and TiO2 (Sigma-Aldrich, 99.99% purity) were used to obtain Nd3+ and Li+ co-doped ST powders.
Stoichiometries ratios of the precursors were taken and ball-milled using high energy ball milling meshing for 4 h by adding acetone as balling media and subsequently irradiated with microwaves in MWSS for 30 minutes duration. The target temperature was 1000°C, and the dwell time was 30 minutes, while heating and cooling rates were 50°C/min. Structural characterization of various calcined samples has been detected using an X-ray diffractometer (PAN analytical X’pert Pro). The calcined powders were re-milled once again for 4 h duration, dried in an oven for 2 hours, mixed with 1 wt% Poly Vinyl Alcohol (PVA) as a binder, and compacted into disc-shaped pellets with a diameter of 10 mm. The green pellets were heated to 500°C at a heating rate of 2°C/min for binder evaporation, then sintered in conventional furnaces at 1300°C for 4 hours with heating and cooling rates of 5°C/min. Phase identification is performed by the usual method of comparative peak matching with the standard (JCPDS Card No. 35–0734) [Citation41,Citation42]. The samples’ crystallite size (D.P.) could be estimated as [Citation43].
Where K is Scherrer’s constant (0.89), and λ is the wavelength of the X-ray radiation (1.5406 nm). High-resolution transmission electron microscopy (JEM-2100F) was used to measure the crystallite size of the SNLT8% samples. X-ray photoelectron spectroscopy (XPS) (JPS-9200) was used to identify the chemical state of each element used to fabricate the SNLT8% sample. The morphology of the sintered ceramics was observed by FE-SEM (JSM‐7600F). The frequency-dependent dielectric constant measurements were carried out using an impedance analyzer, Agilent E4294A (100 Hz to 1 MHz). The frequency-dependent dielectric constant measurement was carried out for the sample SNLT8% in the temperature range of 30–600°C and frequency range of 1 kHz to 2 MHz using Agilent E4294A. The samples were polished, and the thickness was decreased to 0.40 mm to investigate the P – E hysteresis loops. The ferroelectric study was carried out at room temperature and a frequency of 10 Hz using Precision Premier II Radians. The energy storage properties are theoretically estimated by integrating the P-E hysteresis loop.
3. Result and discussion
3.1. Structural analysis
shows the X-ray diffraction patterns of the prepared SNLTx ceramics. It is observed that the prepared samples contain only a cubic structure with the space group Pm3m (JCPDS Card No. 35–0734) [Citation41,Citation42]. No distinct second phase is detected. shows that the intensity of the (111) peaks is decreased with the increase of the co-substitution concentration. The introduction of Nd3+ and Li+ into Sr2+ can cause a slight shift in the peaks toward higher 2θ, as shown in the right graph in . This shift indicates a decrease in the lattice parameter and unit cell volume, as shown in . This variation can be explained considering that Sr2+ ions are co-doped by Nd3+ and Li+ ions with a smaller radius. By taking the coordination number into account, the ionic radii of Nd3+ (IR = 1.27 Å) and Li+ (IR = 0.73 Å) are smaller compared to that of
Figure 2. (a) X-ray diffraction patterns, (b) an enlargement view of the (001) and (111) plans to show the peak shifting, (c) high-resolution scanning transmission electron microscopy (HR-TEM) image shows the particle size of the calcined powder, and (d) selected area electron diffraction (SAED) for the SNLT8% sample.

Figure 3. (a) shows SNLT4% ceramic XRD pattern Rietveld refinement. They are refined with Fullprof. Experimental data appear as black circles, whereas software-calculated intensities are portrayed as solid red lines. Allowed Bragg diffraction peaks are the green vertical lines. The blue curves at the graph’s bottom end show the difference between the experimental arrangement’s intensities and the refining program’s corresponding intensities. (b) the charge density maps of SNLT4%. The center contour and the region enclosed inside it correspond to the Ti4+ ion. Additionally, the four atoms surrounding the core ion, positioned at the midpoint of each side of the cube or pseudo-cube, represent the O2- anions, (c) variation of the unit cell constant and unit cell volume with co-dopant concentrations. (d) variation of crystallite size in nm and tolerance factor with co-dopant concentrations.

Sr2+(1.44 Å) [Citation44], so that a clear shifting in peaks toward higher 2θ angle is taken place, which lead to a decreasing in lattice parameter as shown in . The decrease in the lattice parameter (a) due to co-doping a small ionic radius in place of a large ionic radius has been reported in the literature [Citation45]. The refinement analysis is conducted as shown in to prove all the observations mentioned above. The unit cell volume decreased with increased Nd3+ and Li+ content. This decrease indicates the A-O (A=Sr/Nd, Li) bonds fall with an increase in the Nd3+ and Li+ content. This may affect the rotational mode of the TiO6 octahedron [Citation46], decreasing the unit cell volume of ST crystal. Additionally, the peak broadening is increased with increased co-doping content, indicating a decrease in crystallite size.
Moreover, the electron density distribution of the TiO6 octahedron is shown in . , d) displays the correlation between the lattice parameter, crystallite size, and co-dopant concentrations. The crystallite size and crystalline structure of the x = 0.08 sample were studied using high-resolution transmission electron microscopy (HR-TEM), as shown in . of the SAED patterns shows the superimposition of the bright spots with a Debye ring pattern, indicating that the produced samples are nanocrystalline [Citation47], and the crystallite size acquired from TEM is in good agreement with that obtained from XRD.
The variation of Nd3+ and Li+ co-substituted ST lattice parameters can also be explained in terms of tolerance factor t. The formula gives the tolerance factor of strontium titanate [Citation48].
R.A. is the radius of the A-site ion, R.B. is the radius of the B-site ion, and R.O. is the ionic radius of oxygen [Citation48]. For Nd3+ and Li+ co-doped SrTiO3 ceramics, the Eq (5) becomes.
Where RO = 1.4Å, RA = RSr = 1.44Å, RBi = 1.1 Å, RLi = 0.73Å and RTi = 0.605Å [Citation44]. The values of this tolerance factor were calculated and reported in . The successive decrease in tolerance factor is noticed with increasing Nd3+ and Li+ concentrations. It is close to 1, indicating that the lattice is highly packed and may consider a solid solution of Nd3+ and Li+ co-doped ST stabilizes the average cubic symmetry in the ceramics. So, in the case of Nd3+ and Li+ co-doping for Sr ions occupying the A sites. The increased concentration rat of Nd3+ and Li+ confirmed that the smaller ions of Nd3+ and Li+ co-doped for larger Sr host ions.
Table 1. The lattice parameter, unit cell volume, crystallite size, and tolerance factor of SNLTx ceramics as a function of Nd3+ and Li+ concentration.
3.2. Morphological analysis
The SEM micrographs of SNLTx; 0%≤x ≤ 8%) ceramics are shown in . The images showed that all samples possessed well-densified grains of different sizes and shapes. In general, the grain size of the pure sample is larger than that of the co-doped samples. The average grain size is found to be 10.218 μm, 8.040 μm, 6.844 μm, and 3.46 μm for pure x = 0, 0.02, 0.04, and 0.08 respectively. This size reduction is due to the ionic radius difference between ionic radii of Nd3+ (1.25 Å) and Li+ (0.73Å) and compared to that of Sr2+(1.44 Å) [Citation44]. It is also observed that the grain distribution becomes uniform with the increase of the Nd3+ and Li+ additives due to the accumulation of Nd3+ and Li+ at grain boundaries, which disturbs the grain growth process. Incorporating Nd3+ and Li+ dopants into the Sr3+ site of SrTiO3 is a frequently used technique for altering the material’s characteristics. However, it should be noted that this approach may not result in a direct reduction in grain size. Grain size is primarily influenced by factors such as the size of dopants during the sintering process, the duration of sintering, and the presence of an A-site deficit for charge compensation. Nevertheless, introducing dopants might indirectly influence the size of grains by altering the microstructure and thermodynamic characteristics of the material. Here are a few key considerations to consider while reducing grain size in SrTiO3 by incorporating Nd3+ and Li+ dopants: The ionic radii of Nd3+ and Li+ vary from those of strontium in dopant size. The introduction of Nd3+ and Li+ ions as substitutes for Sr2+ ions have the potential to induce lattice strain, potentially influencing the grain growth process. The presence of smaller dopants has the potential to impede the process of grain expansion, resulting in the formation of smaller grains. On the other hand, the Sr-site deficiency, the presence of Nd3+ in the SrTiO3 lattice, a dopant in Sr2+, leads to the formation of defects, which are compensated by vacancies of Sr ions
. Consequently, a rise in the degree of A-site deficiency results in a reduces the grain size [Citation49]. The ionic compensation process, as described by the Kroger-Vink notation, may be represented as follows [Citation50]:
Figure 4. Scanning electron microscopy (SEM) image of SNLTx ceramics sintered at (1300°C). The scale bar represents 10 micrometers, and the image was acquired at 10000x magnification. The images exhibit a uniform and dense distribution with different grain sizes.

Also, the replacement of monovalent Li+ in place of divalent Sr2+ resulting in the formation of excess hole carriers in the neutral ST:
Thus, the energy needed to form compensating defects determines the solubility at SrTiO3 two lattice sites. In contrast, dopants at grain boundaries don’t require energy to concentrate. Consequently, Nd3+ and Li+ is may abundant near or at grain boundaries, preventing abnormal grain growth during sintering and promoting fine grain formation in SrTiO3 [Citation51]. As seen in the coming sections, the small grains significantly improved energy storage performance. To determine its constituent makeup, an EDS mapping investigation was performed on 8% Nd and Li co-doped SrTiO3 ceramic material. displays the results of the measurements. The chosen area showed no signs of segregation in the distribution of five major elements (Sr, Ti, Nd, Li, and O). This result demonstrates that the 8% co-doping concentration of Nd3+ and Li+ ions was sufficient to completely dissolve and integrate the doped ions into the SrTiO3 lattice.
3.3. XPS analysis
The chemical states and elements contained in the SNLT8% sample were also identified by XPS studies. displays the survey spectrum and high-resolution spectra of Sr 3d, Nd 3d, Li 1s, Ti 2p, and O 1s. At the same time, the adventitious carbon tap used on the XPS sample holder during the XPS measurement was blamed for the peak for C 1s. The survey spectrum () confirmed the presence of Sr, Nd, Li, Ti, and O in the investigated sample. The ranges of the Sr 3d occupied state () show the formation of a doublet signal with binding energies of 131.88 eV and 133.7 eV, which correspond to the Sr 3d5/2 and Sr 3d3/2 orbitals, respectively. This indicates that strontium was present in the matrix as Sr2+ [Citation52]. Two asymmetric peaks in the XPS spectra of Nd 3d () have binding energies of 981.14 eV and 1003.05 eV, respectively, and may be attributed to the Nd 3d5/2 and Nd 3d3/2 states. These peaks are followed by shoulders, separated from the corresponding core peak by around 4.1 eV, and may be considered two satellite peaks. This observable spin-orbit splitting indicates that Nd3+ is present in the sample [Citation53]. In the Li 1s spectra, a peak associated with Li2CO3 [Citation54] can be detected at around 60.01 eV (). The XPS spectra of Ti 2p in show two sets of peaks, Ti2p3/2 and Ti2p1/2, at binding energies of 456.71 eV and 462.34 eV, respectively, demonstrating that the core valence state of Ti in the SNLT8% lattice is + 4 [Citation55]. The shoulder peak, which occurs at 455.41 eV, is caused by the Ti3+. The development of oxygen vacancies and titanium 3+ sites in the SrTiO3 lattice might indicate that oxygen is not sufficiently active to oxidize titanium [Citation56] entirely. shows the O1s peak divided into two distinct peaks; the first peak is 528.67 eV, while the second is 530.21 eV. The peak with lower binding energy is caused by the Ti-O bond [Citation57]. The peak at lower binding energy denotes an oxygen lattice. The peak at higher binding energy shows a non-oxygen lattice, primarily related to oxygen vacancies [Citation58].
Figure 6. (a) an XPS survey is shown for the SNLT8% sample with all the significant peaks indicated, (b) high-resolution XPS spectra of Sr 3d, (c) high-resolution XPS spectra of Nd 3d, (d) high-resolution XPS spectra of Li 1s, (e) high-resolution XPS spectra of Ti 2p, and (f) high-resolution XPS spectra of O 1s.

3.4. Permittivity analysis
depicts the frequency dependence of the dielectric constant and the dielectric loss tangent of SNLTx ceramics at room temperature and the frequency range of (100 Hz-1 MHz). The magnitude of the dielectric constant is shown to decline with an increase in frequency and become almost constant at higher frequencies, as seen in . As the dipoles can track the alternating electric field, the dielectric constant is most prominent at low frequencies. The dielectric constant and dielectric loss decrease with an increase in frequency, as is typical with polar dielectric materials in general [Citation59]. Dipolar, electronic, ionic, and interfacial polarizations, all of which have varying relaxation durations, contribute to the overall polarization of the dielectric material [Citation60]. All polarization types react quickly to changes in electric field strength at lower frequencies. This will result in a higher measured dielectric constant [Citation61].
Figure 7. (a) frequency dependence of permittivity and loss tangent at room temperature for the SNLTx ceramics., (b, and c) temperature dependence of dielectric constant and loss tangent for the sample with x=0.08 measured at different frequencies namely 1 kHz, 10 kHz, 100 kHz, 1 MHz, and 2 MHz.

Since there is less time for the interfacial dipoles to align themselves in the direction of the alternating field at higher frequencies, the various polarization contributions begin to relax. Therefore, when the frequency rises, the dielectric constant decreases because the net polarization of the material decreases [Citation62]. In addition, it can be shown that higher concentrations of Nd3+ and Li+ result in a higher dielectric constant. It is interesting to note that the loss tangent of the co-dopant samples is less than that of the pure sample. This difference diminishes approximately as the amount of co-doping increases. This feature helps develop the potential use of this material as an energy storage application.
depicts the dielectric characteristics of 8% Nd and Li co-doped ST ceramics as a function of temperature measured at five distinct frequencies. The graph shows a series of permittivity peaks with temperature-dependent frequency dispersion. Relaxation occurs due to a shift in dipole orientation, which is temperature and frequency dependent. The loss of oxygen from the crystal lattice during the sintering process might cause oxygen vacancies, according to a defect study in doped SrTiO3 ceramics [Citation63–65]. Different defects, such as ,
,
and
., may occur in Nd and Li co-doped SrTiO3. Under the influence of Coulomb forces, positively charged defects may combine with negatively charged defects, generating defects dipoles such as:
Because oxygen vacancies Vo are the most common flaws in these samples, the collection of peaks that arise is likewise connected to oxygen vacancies. Furthermore, the substitution of monovalent Li+ for divalent Ba2+ results in the creation of extra hole carriers in neutral BT.
It is possible. The behavior of samples is shown in to be dielectric constant dependent on temperature and frequency. It can be observed that the dielectric constant (e) values are more significant at lower frequencies and lower at higher frequencies, which is comparable to the feature reported in the literature [Citation66,Citation67]. Dielectric polarization in materials is caused by the contribution of many forms of polarization, including (1) electronic polarization, (2) ionic polarization, (3) dipolar polarization, and (4) space charge or interfacial polarization. Electronic and ionic polarizations at higher frequencies contribute to the dielectric constant, while space charge and dipolar polarizations contribute at lower frequencies [Citation68]. indicate that the dielectric constant values rise with increasing temperature at all frequencies. This result concludes that the rising dielectric constant with temperature is caused by the growing contribution of space charge polarization [Citation68]. shows a collection of relaxation peaks in the loss tangent. These peaks are associated with the displacement of defective dipoles due to an applied external electric field [Citation69]. The relaxation behavior may be ascribed to minor position adjustments of the Under the influence of an external electric field, i.e. the relaxation peaks in can be attributed to small displacements of
Or/and
from their locations. The observed change in dielectric constant with frequency is comparable to the reported variation in dielectric loss with frequency.
3.5. Energy storage analysis
The polarization-electric field (P-E) hysteresis loops were carried out to examine the energy storage capability of SNLTx ceramics. depicts the energy storage capabilities of SNLTx ceramics. shows the examined ceramics with high Pmax, low Pr, and high breakdown voltage. The maximum polarization (9.56 µC/cm2) observed for the sample SNLT8% at a maximum applied electric field of 160.41 kV/cm is around three times more than that of the pure ST sample (3.54 µC/cm2). This could be because the average grain size of co-doping samples is smaller than that of pure ones, as shown in SEM images. Hence, the energy storage density and efficiency rise with increased Nd3+ and Li+ concentrations.
Figure 8. Room temperature P–E hysteresis loop with their schematic diagram for calculating the energy storage properties in SNLTx ceramics. The region colored in cyan corresponds to the recovered energy density (Wrec) or the energy density discharged during the discharge process. The area highlighted in yellow corresponds to the energy dissipation (Wloss) that transpires throughout the charge-discharge cycle. The sum of the energy gained (wrec) and the energy lost (Wloss) corresponds to the total energy density (Wtotal) accumulated during the charging procedure.

Additionally, the co-doping-induced dens microstructure may be responsible for increasing the polarization as co-dopants increase. The area between the polarization axis and the discharge curve was integrated using the P-E loop, as illustrated in , and based on EquationEqs (1)(1)
(1) -(Equation3
(3)
(3) ) to get the energy storage density and efficiency. The obtained parameters Wrec and ɳ are plotted as a function of Nd3+ and Li+ concentration, as shown in . The energy storage densities of SNLTx may be affected by several parameters, including polarization and breakdown strength. The breakdown strength of the co-dopant sample is linked to the grain size effect [Citation70,Citation71]. Therefore, the variance in breakdown strength is primarily responsible for the discrepancy in energy storage density across the Nd3+ and Li+ co-doped ST lattice. Interestingly, the research obtained a high energy efficiency of 96% and a high energy storage density of 0.952 J/cm3.
Figure 9. (a) P-E loops of the SNLT8% ceramic measured at different temperatures at 160 kV/cm and 10 Hz frequency. (b) the variation of Wrec and η values with co-dopants concentrations. (c) the energy storage density Wrec and energy storage efficiency η values were calculated from with different temperatures.

Finally, we explain the stability of our ideal SNLT8% samples at various temperature conditions. Temperature stability is a well-known essential condition for energy storage technologies. The P-E loops were measured at different temperatures ranging from 25°C to 80°C, at f = 10 Hz and Eb = 160 kV/cm, as shown in . The Pmax stays constant throughout the investigated temperature range, as observed. The necessary Wrec and data are shown in . The results show that Wrec and (0.952 J/cm3 and 96%) are unchanged. The composition’s high breakdown strength, low dielectric loss, and possible energy storage density with x = 8% show that the Nd3+ and Li+ co-doped SrTiO3 ceramics have essential properties for energy storage applications.
4. Conclusion
The polycrystalline Nd3+ and Li+ co-substituted SrTiO3 samples were successfully prepared by heating the beginning materials with microwave assistance. The sample particles were sintered at 1300°C for four hours to produce ceramics with relative densities that exceeded 96% of their theoretical values. The lattice parameter and grain size decreased as the co-doping concentration increased. Dielectric properties were investigated at ambient temperature and in the frequency range 100 Hz-1 MHz. Both polarization-electric field hysteresis loops were analyzed to determine the effect of co-doping on the energy-storage capacity of SrTiO3 ceramics. After increasing the co-dopants from 0% to 8%, the energy density increased ninefold (from 0.11 J/cm3 to 0.952 J/cm3), and the energy storage efficiency increased from 80.71% to 95.98%. Moreover, the samples exhibit exceptional thermal stability, and their energy storage properties are stable up to 80°C. This discovery suggests that bulk Nd3+ and Li+ co-doped SrTiO3 materials are excellent candidates for applications requiring capacitors with a high energy density.
Authorship contribution statement
Mahmoud Alkathy: Writing, Correction, formal analysis, and original draft preparation.
Srinivas Pattipaka: Analysis and writing the original draft.
Mansour K. Gatasheh: Investigator.
H. Kassim: Synthetization, Methodology, and Formal Analysis.
Mohamed Saad Daoud: Investigator
Prof Eiras: Writing, Correction, supervision, and approval of the final version.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data generated or analyzed during this study are included in this article.
Additional information
Funding
References
- Liu C, Li F, Ma LP, et al. Advanced materials for energy storage. Adv Mater. 2010;22(8):E28–E62. doi: 10.1002/adma.200903328
- Qi H, Xie AW, Zuo RZ. Local structure engineered lead-free ferroic dielectrics for superior energy-storage capacitors: a review. Energy Storage Mater. 2022;45:541–567. doi: 10.1016/j.ensm.2021.11.043
- Yuan QB, Chen M, Zhan SL. et al. Ceramic-based dielectrics for electrostatic energy storage applications: fundamental aspects, recent progress, and remaining challenges. Chem Eng J. 2022;446:136315. doi: 10.1016/j.cej.2022.136315
- Zhao PY, Wang HX, Wu LW, et al. High-performance relaxor ferroelectric materials for energy storage applications. Adv Energy Mater. 2019;9(17):1803048. doi: 10.1002/aenm.201803048
- Liu L, Liu Y, Hao J, et al. Multi-scale collaborative optimization of SrTiO3-based energy storage ceramics with high performance and excellent stability. J Nano Energy. 2023;109:108275. doi: 10.1016/j.nanoen.2023.108275
- Alkathy MS, Rahaman A, Mastelaro VR. et al. Enhanced energy-storage density of BaTi0.95Zr0.05O3 via generation of defect dipoles upon lithium-doping. Mater Chem Phys. 2023;294:127032. doi: 10.1016/j.matchemphys.2022.127032
- Alkathy M, Rahaman A, Mastelaro VR. et al. Achieving high energy storage density simultaneously with large efficiency and excellent thermal stability by defect dipole, and microstructural engineering in modified-BaTiO3 ceramics. J Alloy Compd. 2023;934:167887. doi: 10.1016/j.jallcom.2022.167887
- Li TY, Chen PF, Li F, et al. Energy storage performance of Na0.5Bi0.5TiO3- SrTiO3 lead-free relaxors modified by AgNb0.85Ta0.15O3. Chem Eng J. 2021;406:127151. doi: 10.1016/j.cej.2020.127151
- Luo NN, Han K, Zhuo FP, et al. Design for high energy storage density and temperature-insensitive lead-free antiferroelectric ceramics. Mater Chem C. 2019;7(17):4999–5008. doi: 10.1039/C8TC06549G
- Wang G, Lu ZL, Li Y, et al. Electroceramics for high-energy density capacitors: current status and future perspectives. Chem Rev. 2021;121(10):6124–6172. doi: 10.1021/acs.chemrev.0c01264
- Li D, Lin Y, Zhang M. Achieved ultrahigh energy storage properties and outstanding charge–discharge performances in (Na0.5Bi0.5)0.7Sr0.3TiO3-based ceramics by introducing a linear additive. Chem Eng J. 2020;392:123729. doi: 10.1016/j.cej.2019.123729
- Wang G, Lu Z, Li Y, et al. Electroceramics for high-energy-density capacitors: current status and future perspectives. Chem Rev. 2021;121(10):6124–6172. doi: 10.1021/acs.chemrev.0c01264
- Wang X, Zhang Y, Song X, et al. Glass additive in barium titanate ceramics and its influence on electrical breakdown strength in relation with energy storage properties. J Eur Ceram. 2012;32(3):559–567. doi: 10.1016/j.jeurceramsoc.2011.09.024
- Jan A, Liu H, Hao H, et al. Enhanced dielectric breakdown strength and ultra-fast discharge performance of novel SrTiO3 based ceramics system. J Alloy Compd. 2020;830:154611. doi: 10.1016/j.jallcom.2020.154611
- Huang J, Zhang Y, Ma T, et al. Correlation between dielectric breakdown strength and interface polarization in barium strontium titanate glass ceramics Appl. Phys Lett. 2010;96(4):042902. doi: 10.1063/1.3293456
- Zhu X, Shi P, Kang R, et al. Enhanced energy storage density of Sr0.7BixTiO3 lead-free relaxor ceramics via A-site defect and grain size tuning Chem. Eng J. 2021;420:129808. doi: 10.1016/j.cej.2021.129808
- Pu Y, Wang W, Guo X, et al. Enhancing the energy storage properties of Ca0.5Sr0.5TiO3-based lead-free linear dielectric ceramics with excellent stability through regulating grain boundary defects. J Mater Chem C. 2019;7(45):14384–14393. doi: 10.1039/C9TC04738G
- Pan W, Cao M, Jan A, et al. High breakdown strength and energy storage performance in (nb, zn) modified SrTiO 3 ceramics via synergy manipulation. J Mater Chem C. 2020;8(6):2019–2027. doi: 10.1039/C9TC06256D
- Guo X, Pu Y, Wang W, et al. Ultrahigh energy storage performance and fast charge-discharge capability in Dy-modified SrTiO3 linear ceramics with high optical transmissivity by defect and interface engineering Ceram. Int. 2020;46(13):21719–21727. doi: 10.1016/j.ceramint.2020.05.280
- Zhong B, Zuo C, Yang C, et al. Bifunctional europium-doped SrTiO3 ceramics with energy storage and photoluminescence. J Alloys Compd. 2022;901:163556. doi: 10.1016/j.jallcom.2021.163556
- Zhou S, Yongping P, Zhang X, et al. High energy density, temperature stable lead-free ceramics by introducing high entropy perovskite oxide. J Chem Eng. 2022;427:131684. doi: 10.1016/j.cej.2021.131684
- Chen L, Deng S, Liu H, et al. Giant energy-storage density with ultrahigh efficiency in lead-free relaxors via high-entropy design. Nat Commun. 2022 2;13(1):3089. doi: 10.1038/s41467-022-30821-7
- Huang YH, Wu YJ, Qiu WJ, et al. Enhanced energy storage density of Ba0.4Sr0.6TiO3–MgO composite prepared by spark plasma sintering. J Eur Ceram Soc. 2015;35(5):1469–1476. doi: 10.1016/j.jeurceramsoc.2014.11.022
- Huang YH, Wu YJ, Liu B, et al. From core–shell Ba 0.4 Sr 0.6 TiO 3 @SiO 2 particles to dense ceramics with high energy storage performance by spark plasma sintering. J Mater Chem A. 2018;6(10):4477–4484. doi: 10.1039/C7TA10821D
- Yang HB, Yan F, L Y, et al. Enhanced energy storage properties of Ba0.4Sr0.6TiO3 lead-free ceramics with Bi2O3-B2O3-SiO2 glass addition. J Eur Ceram Soc. 2018;38(4):1367–1373. doi: 10.1016/j.jeurceramsoc.2017.11.058
- Pan WG, Cao MH, Jan A, et al. High breakdown strength and energy storage performance in (nb, zn) modified SrTiO3 ceramics via synergy manipulation. J Mater Chem C. 2020;8(6):2019–2027. doi: 10.1039/C9TC06256D
- Kong X, Yang LT, Cheng ZX, et al. Bi-modified SrTiO3-based ceramics for high-temperature energy storage applications. J Am Ceram Soc. 2020;103(3):1722–1731. doi: 10.1111/jace.16844
- Liu LL, Chu BK, Li P, et al. Achieving high energy storage performance and ultrafast discharge speed in SrTiO3-based ceramics via a synergistic effect of chemical modification and defect chemistry. Chem Eng J. 2022;429:1322548. doi: 10.1016/j.cej.2021.132548
- Wu YJ, Huang YH, Wang N, et al. Effects of phase constitution and microstructure on energy storage properties of barium strontium titanate ceramics. J Eur Ceram Soc. 2017;37(5):2099–2104. doi: 10.1016/j.jeurceramsoc.2016.12.052
- Liu BB, Wang XH, Zhang RX, et al. Energy storage properties of ultrafine-grained Ba0.4Sr0.6TiO3-based ceramics sintered at low temperature. J Alloy Compd. 2017;691:619–623. doi: 10.1016/j.jallcom.2016.08.317
- Liu L, Chu B, Li P. et al. Achieving high energy storage performance and ultrafast discharge speed in SrTiO3-based ceramics via a synergistic effect of chemical modification and defect chemistry. Chem Eng J. 2022;429:132548. doi: 10.1016/j.cej.2021.132548
- Xie J, Hao H, Liu H, et al. Dielectric relaxation behavior and energy storage properties of sn modified SrTiO 3 based ceramics. Int. 2016;42(11):12796–12801. doi: 10.1016/j.ceramint.2016.05.042
- Guo X, Yongping P, Wang W, et al. Ultrahigh energy storage performance and fast charge-discharge capability in Dy-modified SrTiO3 linear ceramics with high optical transmissivity by defect and interface engineering. Ceram Int. 2020;46(13):21719–21727. doi: 10.1016/j.ceramint.2020.05.280
- Yao Z, Luo Q, Zhang G, et al. Improved energy-storage performance and breakdown enhancement mechanism of Mg-doped SrTiO3 bulk ceramics for high energy density capacitor applications. J Mater Sci Mater Electron. 2017;28(15):11491–11499. doi: 10.1007/s10854-017-6945-z
- Yang H, Yan F, Lin Y, et al. Enhanced recoverable energy storage density and high efficiency of SrTiO3-based lead-free ceramics. Appl Phys Lett. 2017;111(25):253903. doi: 10.1063/1.5000980
- Shi WJ, Zhang LY, Yang YL, et al. Improved energy storage performance of bismuth sodium titanate-based lead-free relaxor ferroelectric ceramics via Bi-containing complex ions doping. Rare Met. 2023;42(5):1472–1482. doi: 10.1007/s12598-022-02176-x
- Tang L, Pan Z, Zhao J, et al. Significantly enhanced energy storage capability of BNT-based ceramics via optimized sintering aids. J Alloy Compd. 2023;935:168124. doi: 10.1016/j.jallcom.2022.168124
- Cui C, Pu Y, Shi R. High-energy storage performance in lead-free (0.8-x)SrTiO3-0.2Na0.5Bi0.5TiO3-xBaTiO3 relaxor ferroelectric ceramics. J Alloy Compd. 2018;740:1180–1187. doi: 10.1016/j.jallcom.2018.01.106
- Alkathy MS, Zabotto FL, Milton FP, et al. Achieving high energy storage performance and breakdown strength in modified strontium titanate ceramics. J Mater Sci Mater Electron. 2022;33(19):15483–15494. doi: 10.1007/s10854-022-08455-8
- Zhang T, Zhao Y, Li W. et al. High energy storage density at low electric field of ABO3 antiferroelectric films with ionic pair doping. Energy Storage Mater. 2019;18:238–245. doi: 10.1016/j.ensm.2018.09.011
- Le MV, Vo NQ, Le QC, et al. Manipulating the structure and characterization of Sr1−xLaxTiO3nanotubes toward the photodegradation of 2-naphthol under artificial solar light. Catalysts. 2021;11(5):564. doi: 10.3390/catal11050564
- Da Silva LF, Avansi W, Moreira ML, et al. Relationship between crystal shape, photoluminescence, and local structure in SrTiO3 synthesized by microwave-assisted hydrothermal method. J Nanomater. 2012;2012:1–6. doi: 10.1155/2012/890397
- Holzwarth U, Gibson N. The Scherrer equation versus the ’Debye-Scherrer equation Nat. Nanotechnol. 2011;6(9):534. doi: 10.1038/nnano.2011.145
- Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1967;32(5):751–767. doi: 10.1107/S0567739476001551
- Pradhan J, Mallick HK, Sahoo MPK, et al. Enhanced optical and dielectric properties of rare-earth co-doped SrTiO3 ceramics. J Mater Sci Mater Electron. 2021;32(10):13837–13849. doi: 10.1007/s10854-021-05959-7
- Zhou E, Raulot J-M, Hong X, et al. “Structural, electronic, and optical properties of rare-earth-doped SrTiO3 perovskite: a first-principles study. Phys B Condens Matter. 2022;643:414160. doi: 10.1016/j.physb.2022.414160
- Wang X-H, Chen R-Z, Gui Z-L, et al. The grain size effect on dielectric properties of BaTiO3 based ceramics. Mater Sci Eng B. 2003;99(1–3):199–202. doi: 10.1016/S0921-5107(02)00520-2
- Reaney M, Colla EL, Setter N. Dielectric and structural characteristics of Ba-and Sr-based complex perovskites as a function of tolerance factor. Jpn J Appl Phys. 1994;33:3984. doi: 10.1143/JJAP.33.3984
- Gargar Z, Tachafine A, Fasquelle D, et al. Grain size effects on dielectric properties of yttrium doped BaTiO3 ceramics. Phase Transitions. 2023;1–2. doi: 10.1080/01411594.2023.2253957
- Ghosh SK, Ganguly M, Rout SK, et al. Order-disorder correlation on local structure and photo-electrical properties of La3+ ion modified BZT ceramics. Eur Phys J Plus. 2015;130(4):68. doi: 10.1140/epjp/i2015-15068-6
- Yin Q, Zhu B, Zeng H. Microstructure, property and processing of functional ceramics. Springer Science & Business Media: 2010. doi:10.1007/978-3-642-01694-3
- Paulista LO, Albero J, Martins RJ, et al. Turning carbon dioxide and ethane into ethanol by Solar-Driven Heterogeneous Photocatalysis over RuO2-and NiO-co-doped SrTiO3. Catalysts. 2021;11(4):461. doi: 10.3390/catal11040461
- Quiñonero J, Pastor FJ, Orts JM, et al. Photoelectrochemical behavior and computational insights for pristine and doped NdFeO3 thin-film photocathodes. ACS Appl Mater Interfacesquery. 2021;13(12):14150–14159. doi: 10.1021/acsami.0c21792
- Norah H, Brecht Put WMMK, Vereecken PM, et al. Plasma-assisted and thermal atomic layer deposition of electrochemically active Li 2 CO 3. RSC Adv. 2017;7(66):41359–41368. doi: 10.1039/C7RA07722J
- Pan L, Wang S, Zou J-J, et al. Ti3+-defected and V-doped TiO2 quantum dots loaded on MCM-41. Chem Comm. 2014;50(8):988–990. doi: 10.1039/C3CC47752E
- Xie W, Rui L, Qingyu X. Enhanced photocatalytic activity of se-doped TiO2 under visible light irradiation. Sci Rep. 2018;8(1):8752. doi: 10.1038/s41598-018-27135-4
- Xia Y, Jiang Y, Li F. et al. Effect of calcined atmosphere on the photocatalytic activity of P-doped TiO2. Appl Surface Sci. 2014;289:306–315. doi: 10.1016/j.apsusc.2013.10.157
- Young-Jin K, Ho Han M, Lim C, et al. Unveiling the role of ni in ru-ni oxide for oxygen evolution: lattice oxygen participation enhanced by structural distortion. J Energy Chem. 2023;77:54–61. doi: 10.1016/j.jechem.2022.09.032
- Song Y, Shen Y, Liu H, et al. Improving the dielectric constants and breakdown strength of polymer composites: effects of the shape of the BaTiO 3 nanoinclusions, surface modification and polymer matrix. J Mater Chem. 2012;22(32):16491–16498. doi: 10.1039/c2jm32579a
- Demirezen S, Çetinkaya HG, Altındal Ş. Doping rate, interface states and polarization effects on dielectric properties, electric Modulus, and A.C. Conductivity in PCBM/NiO: ZnO/p-si structures in Wide frequency range. Silicon. 2022;14(14):8517–8527. doi: 10.1007/s12633-021-01640-0
- Wang Z, Zhou W, Dong L. et al. Dielectric spectroscopy characterization of relaxation process in Ni/epoxy composites. J Alloy Compd. 2016;682:738–745. doi: 10.1016/j.jallcom.2016.05.025
- Yun W, Forbess MJ, Seraji S, et al. Oxygen-vacancy-related dielectric relaxation in SrBi2Ta1.8V0.2O9 ferroelectrics. J Appl Phys. 2001;89(10):5647–5652. doi: 10.1063/1.1366657
- Chen A, Zhi Y, Cross LE. Oxygen-vacancy-related low-frequency dielectric relaxation and electrical conduction in B i: SrTiO3. Phys Rev B. 2001;62(1):228. doi: 10.1103/PhysRevB.62.228
- Li MD, Tang XG, Zeng SM, et al. Oxygen-vacancy-related dielectric relaxation behaviors and impedance spectroscopy of Bi (Mg1/2Ti1/2)O3 modified BaTiO3 ferroelectric ceramics. J Materiomics. 2018;4(3):194–201. doi: 10.1016/j.jmat.2018.03.001
- Zhi Y, Ang Chen PMV, Mantas PQ, et al. Dielectric relaxation behaviour of Bi: SrTiO3: III. Dielectric properties in the temperature range of 300–600 K. J Eur Ceram Soc. 1998;18(11):1629–1635. doi: 10.1016/S0955-2219(98)00029-6
- Das BP, Choudhary RNP, Mahapatra PK. Effect of europium (eu) on structural, dielectric and electrical properties of Pb(SnTi)O3 ferroelectric ceramics. Mater Sci Eng B. 2003;104(1–2):96–105. doi: 10.1016/S0921-5107(03)00311-8
- Gupta V, Bamzai KK, Kotru PN, et al. Dielectric properties, ac conductivity and thermal behaviour of flux grown cadmium titanate crystals. Mater Sci Eng B. 2006;130(1–3):163–172. doi: 10.1016/j.mseb.2006.03.006
- Jonscher AK. Dielectric relaxation in Solids Chelsea dielectric Press. J. Phys D: Appl Phys. 1999;32(14):R57.
- Song Z, Liu H, Hao H, et al. The effect of grain boundary on the energy storage properties of (Ba0.4Sr0.6M)TiO3 paraelectric ceramics by varying grain sizes. IEEE Trans Ultrason Ferroelectr FrEq Contr. 2015;62:609.
- Lee HY, Cho KH, Nam HD. Grain size and temperature dependence of electrical breakdown in BaTiO3 ceramic. Ferroelectrics. 2006;334(1):165. doi: 10.1080/00150190600694415
- Alkathy MS, Eiras JA, James Raju KC. Energy storage enhancement and bandgap narrowing of lanthanum and sodium co-substituted BaTiO3 ceramics. Ferroelectrics. 2021;570(1):153–161. doi: 10.1080/00150193.2020.1839266

