ABSTRACT
It is critical to determine the real-world performance of vaccines against coronavirus disease 2019 (COVID-19) so that appropriate treatments and policies can be implemented. There was a rapid wave of infections by the Omicron variant in Jilin Province (China) during spring 2022. We examined the effectiveness of inactivated vaccines against Omicron using real-world data from this epidemic. This retrospective case-case study of vaccine effectiveness (VE) examined infected patients who were quarantined and treated from April 16 to June 8, 2022 and responded to an electronic questionnaire. Data were analyzed by univariable and multivariable analyses. A total of 2968 cases with SARS-CoV-2 infections (asymptomatic: 1061, mild disease: 1763, pneumonia: 126, severe disease: 18) were enrolled in the study. Multivariable regression indicated that the risk for pneumonia or severe disease was greater in those who were older or had underlying diseases, but was less in those who received COVID-19 vaccines. Relative to no vaccination, VE against the composite of pneumonia and severe disease was significant for those who received 2 doses (60.1%, 95%CI: 40.0%, 73.5%) or 3 doses (68.1%, 95%CI: 44.6%, 81.7%), and VE was similar in the subgroups of males and females. However, VE against the composite of all three classes of symptomatic diseases was not significant overall, nor after stratification by sex. There was no statistical difference in the VE of vaccines from different manufacturers. The inactivated COVID-19 vaccines protected patients against pneumonia and severe disease from Omicron infection, and booster vaccination enhanced this effect.
Background
As of July 27, 2022, there have been over 570,005,017 confirmed cases and more than 6.38 million deaths from COVID-19 worldwide [Citation1]. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has been evolving, and has caused several waves of COVID-19 pandemics since its discovery. The World Health Organization (WHO) classified the Omicron (B.1.1.529) variant of the SARS-CoV-2 as a variant of concern (VOC) on November 26, 2021 [Citation2]. Scientists discovered that Omicron has a large number of mutations in its spike (S) protein, and the appearance of this variant has caused great concern worldwide because of its high transmission rate and number of mutations [Citation3,Citation4]. The current COVID-19 vaccines were developed based on the ancestral strain of the SARS-CoV-2 virus. The protective effect of these current vaccines against the Omicron variant, whose antigenicity is distinct from the ancestral virus, is an issue of great concern. Laboratory data found that vaccinated individuals had a greatly reduced neutralizing antibody response to the Omicron variant relative to the original Delta (B.1.617.2) variant, although boosters increased the response to the Omicron variant [Citation5–8]. The level of neutralizing antibodies correlates with protection against reinfection and with vaccine effectiveness (VE) against infection; therefore, early laboratory findings suggest that the original vaccines had lower VE against the Omicron variant infection[Citation9,Citation10].
Studies of the ChAdOx1 nCoV-19 (AstraZeneca) vaccine and the BNT162b2 (Pfizer) vaccine indicated that primary immunization with two doses of either vaccine only led to limited protection against infection and symptomatic disease in individuals infected with the Omicron variant. Administration of a booster dose of the BNT162b2 vaccine or the mRNA-1273 (Moderna) vaccine after initial vaccination with the ChAdOx1 nCoV-19 vaccine or the BNT162b2 vaccine provided some protection, but this protection declined over time [Citation11,Citation12]. As of 2022, more than 91 countries have used inactivated COVID-19 vaccines[Citation13]. It is important to assess the effectiveness of these inactivated vaccines against the many variants of SARS-CoV-2. The COVID-19 vaccines administered in mainland China were mainly inactivated vaccines, but observational data on the protection provided by these inactivated vaccines against the Omicron variant are currently rare. Thus, there is an urgent need for studies of the real-world effectiveness of inactivated vaccines against the Omicron variant.
In early March 2022, a wave of infections by the Omicron variant rapidly appeared in Jilin Province, China. This was the first large-scale transmission and infection of SARS-CoV-2 faced by Changchun City (Jilin Province, China) following the mass COVID-19 vaccination in 2021. During this epidemic, all residents received daily testing by PCR (the main method) and/or rapid antigen detection kits. All cases that were screened positive and then confirmed positive by PCR testing were isolated or admitted to designated institutions based on symptoms and disease severity. Thus, asymptomatic cases were assigned to shelter hospitals for isolation and observation, and patients with confirmed mild disease, pneumonia, severe disease, or fatal disease were sent to other hospitals. Patients with asymptomatic infections who subsequently developed symptoms were transferred from shelter hospitals to designated hospitals for diagnosis and treatment. During this time, the general population was instructed to take rigorous personal preventive measures, including wearing masks in public places, frequent hand washing, and social distancing. The epidemic in Jilin Province was completely extinguished in early June 2022. During the epidemic in Changchun City, 46,534 people had confirmed infections with SARS-CoV-2. During our study period, the Omicron variant accounted for 100% of all known infections by SARS-CoV-2 in Jilin Province. The present real-world study provides the latest evidence regarding the protection provided by inactivated COVID-19 vaccines against mild disease, pneumonia, and severe disease due to Omicron infections during this epidemic.
Methods
Study design and population
This case-case study examined individuals in Jilin Province who were infected with SARS-CoV-2. All participants were patients non-electively admitted to a shelter hospital (Changchun Infectious Disease Hospital) or one of two designated hospitals (Eastern Division of the First Hospital of Jilin University or Hepatobiliary Hospital of Jilin) for SARS-CoV-2 infection from April 16 to June 8, 2022. All paticipants were asked to complete an electronic questionnaire via their personal mobile phones. Those who could not operate a mobile phone or read (such as some of the elderly and children) completed the electronic questionnaire survey with the assistance of relatives. This questionnaire asked about basic information (age, sex, phone number etc), relevant symptoms (fever, cough, sore throat etc), underlying diseases (hypertension, diabetes, cardiovascular and cerebrovascular diseases, tumors, chronic kidney disease, chronic liver disease, etc.), COVID-19 vaccination status, and the most severe clinical classification (asymptomatic infection, mild disease, pneumonia, and severe disease). The participants were all clinically diagnosed on the first day of hospital admission. During hospitalization, each patient received nucleic acid testing every 1–2 days, and clinicians adjusted the diagnostic classification according to patient progression and other relevant examination results. Patients were required to complete the questionnaire while in the hospital on the day of discharge. The diagnostic classification in our analyses is the most serious condition during the time of hospitalization. Questionnaires were generally completed with the assistance of doctors or nurses.
Individuals were excluded if they were infants or children under the age of 3 (because they were not eligible for the COVID-19 vaccine); if they received non-inactivated COVID-19 vaccines; or if the questionnaire was incorrect or incomplete. All patients provided informed consent and the Institutional Review Board of the First Hospital of Jilin University approved this study (No. 2002-290).
Definitions of infection-related events
Patients were classified by symptoms (asymptomatic, mild, pneumonia, severe disease) as described by the 9th edition of COVID-19 protocols for diagnosis and treatment [Citation14]. Patients in all four groups had positive PCR results. A patient with an asymptomatic infection had no clinical symptoms. A patient with mild disease had symptoms such as low-grade fever, mild fatigue, and disturbance of smell and taste, but no symptoms of pneumonia. A patient with pneumonia had symptoms such as fever, cough, fatigue, and disturbance of smell and taste, with evidence of pneumonia based on imaging. The criteria for severe disease depended on age. In adults, severe disease was defined by shortness of breath (respiratory rate [RR] ≥ 30 times/min); low resting oxygen saturation (≤93%) in normal room air; low arterial partial pressure of oxygen/inhaled oxygen concentration (PaO2/(FiO2 ≤ 300 mmHg [39.9 kPa]), with correction for measurements at altitudes over 1000 m (PaO2/FiO2 × [760/atmospheric pressure (mmHg)]; and progressive worsening of clinical symptoms, with imaging showing that the lung lesions progressed more than 50% within 24–48 h. In children, severe disease was defined by high fever for more than 3 days; shortness of breath excluding the effects of fever and crying (RR ≥ 60 times/min if less than 2 months-old; RR ≥ 50 times/min if 2–12 months-old; RR ≥ 40 times/min if 1–5 years old; and RR ≥ 30 times/min if more than 5 years-old); low resting oxygen saturation (≤93%) in normal room air; need for assisted breathing (nose alar flap, three concave sign); drowsiness and convulsions; and refusal to eat or difficulty in eating, with symptoms of dehydration.
Statistical analysis
IBM SPSS version 25 (IBM Corp., Chicago) was used for all statistical analyses. The χ2 test was used to compare categorical variables. The Shapiro – Wilk test was used to determine the normality of distributions of continuous variables, and a nonparametric test was used to compare continuous variables with non-normal distributions. VE was calculated as (1-OR) × 100%, in which the odds ratio (OR) was from a logistic regression model. Univariate and multivariate logistic regression that adjusted for age, gender, and underlying diseases were used to determine VE. A two-sided P value below 0.05 was considered significant.
Results
Characteristics of participants
We initially examined 3062 persons who participated in the survey. We excluded 39 children under the age of 3 years-old, 16 participants who received the COVID-19 recombinant vaccine or the adenovirus vector vaccine, and 39 participants due to incomplete or unreliable data. Thus, we finally examined 2968 cases with SARS-CoV-2 infections (1472 males [49.6%] and 1496 females [50.4%]). There were 1061 cases with asymptomatic infections, 1763 with mild disease, 126 with pneumonia, and 18 with severe disease (). There were no deaths. The four groups had significant differences in sex ratio, age, underlying diseases, and vaccination status (all P < 0.001). In particular, males accounted for 59.6% of the 1061 asymptomatic patients, but for 44.0% of those with mild disease, 44.4% of those with pneumonia, and 44.4% of those with severe disease. The median age was 43 years-old (interquartile range [IQR]: 30, 56) for those who were asymptomatic, 41 years-old (IQR: 27, 55) for those with mild disease, 54 years-old (IQR: 38, 72) for those with pneumonia, and 70 years-old (IQR: 42, 79) for those with severe disease. Among the 18 patients with severe disease, 12 were more than 60 years-old (66.7%). The percentage of patients with underlying diseases increased as disease severity increased (28.4%, 32.2%, 54.0%, and 100.0%).
Table 1. Characteristics of COVID-19 patients who had asymptomatic infection, mild disease, pneumonia or severe disease
Overall, 2543 of the 2968 patients (85.68%) received at least one vaccine dose (). The percentage of patients who received at least one dose decreased as disease severity increased (86.3%, 86.9%, 68.3% and 50.0%, P < 0.001). We also analyzed the relationship of disease severity with receipt of different numbers of vaccine doses. The percentage of patients receiving no vaccine was highest for those with severe disease (50.0%), followed by pneumonia (31.7%), asymptomatic infections (13.7%), and mild disease (13.1%). The percentage that received two doses was highest for those with mild disease (63.9%), followed asymptomatic infections (58.5%), pneumonia (50.1%), and severe disease (22.2%). The percentage that received three doses was highest for those with asymptomatic infections (22.8%), followed by mild disease (19.5%), severe disease (16.7%), and pneumonia (13.5%). Among all the 2543 subjects vaccinated in this study, 1181 (46.4%) were vaccinated with Sinovac CoronaVac vaccine (including 1, 2 or 3 doses), 1021 (40.2%) were vaccinated with Sinopharm BBIBP-CorV vaccine (including 1, 2 or 3 doses), and 341 (13.4%) were vaccinated with these two mixed vaccines (CoronaVac + BBIBP-CorV).
Vaccine effectiveness against pneumonia and severe disease
Univariate analysis showed that age (OR = 1.04, 95% CI: 1.02, 1.04) and underlying diseases (OR = 3.34, 95%CI: 2.37, 4.71) were significantly associated with an increased risk of pneumonia and severe disease (). Compared with those who were not vaccinated, those who received two vaccine doses (OR = 0.29, 95%CI: 0.20, 0.43) or three vaccine doses (OR = 0.26, 95%CI: 0.15, 0.45) were protected against pneumonia and severe disease. The multivariable regression model showed that the risk for pneumonia or severe disease was greater in those who were older (aOR = 1.03; 95% CI: 1.02, 1.04) or had underlying diseases (aOR = 1.67; 95% CI: 1.12, 2.49), but was less in those who received 2 vaccine doses (aOR = 0.40, 95% CI: 0.27, 0.60) or 3 vaccine doses (aOR = 0.32, 95% CI: 0.18, 0.55).
Table 2. Factors associated with pneumonia or severe disease among SARS-CoV-2 Omicron infection
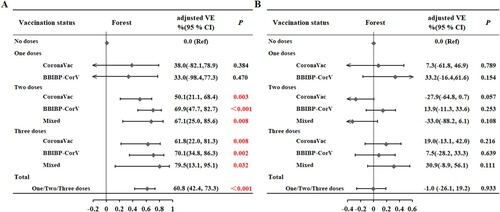
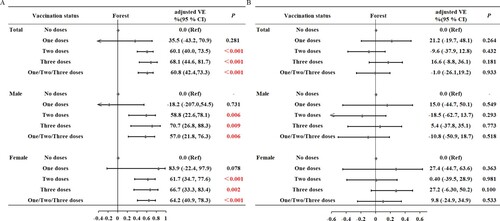
We then performed sex-stratified analysis, with adjustment for age and underlying diseases (A). For males, compared with those who were not vaccinated, the VE against pneumonia and severe disease was 58.8% (95% CI: 22.6%, 78.1%, P = 0.006) for those who received 2 doses, 70.7% (95%CI: 26.8%, 88.3%, P = 0.009) for those who received 3 doses, and 57.0% (95%CI:21.8%, 76.3%, P = 0.006) for those who received any number of doses (1, 2, or 3). For females, compared with those who were not vaccinated, the VE against pneumonia and severe disease was 61.7% (95% CI: 34.7%, 77.6%, P < 0.001) for those who received 2 doses, 66.7% (95% CI: 33.3%, 83.4%, P = 0.002) for those who received 3 doses and 64.2% (95% CI: 40.9%, 78.3%, P < 0.001) for those who received any number of doses (1, 2, or 3).
Figure 1. Effect of the number of doses on vaccine effectiveness (A) against pneumonia or severe disease and (B) against any symptomatic disease (mild disease, pneumonia or severe disease) in males and females.
We then examined the VE of vaccines from different manufacturers (A). In an analysis that adjusted for sex, age, and underlying disease, the VE against pneumonia and severe disease was 50.1% (95% CI: 21.1%, 68.4%) for two doses of CoronaVac and 69.9% (95% CI: 47.7%, 82.7%) for two doses of BBIBP-CorV. The adjusted VE against pneumonia and severe disease was 61.8% (95% CI: 22.0%, 81.3%) for three doses of CoronaVac and 70.1% (95% CI: 34.8%, 86.3%) for three doses of BBIBP-CorV. There was no statistical difference in the VE of vaccines from different manufacturers (P > 0.05). Compared with those who were not vaccinated, the adjusted VE of two mixed doses was 67.1% (95% CI: 25.0%, 85.6%), and the adjusted VE of three mixed doses was 79.5% (95% CI: 13.1%, 95.1%; P < 0.032). In the case of the same dose, there is no statistical difference between the VEs inoculated with vaccines from the same manufacturer and the VEs inoculated with vaccines from two manufacturers (all P > 0.05).
Vaccine effectiveness against any symptomatic disease
Univariate analysis are showed that female sex (OR = 1.87, 95% CI: 1.61, 2.18) and underlying diseases (OR = 1.32, 95% CI: 1.12, 1.55) were significantly associated with an increased risk of symptomatic disease (mild, pneumonia or severe disease) (). Compared with those who were not vaccinated, there was no significant protective effect from one dose (OR = 0.67, 95% CI: 0.45, 1.02), two doses (OR = 1.00, 95% CI: 0.80, 1.24), or three doses (OR = 0.78, 95% CI: 0.60, 1.01) against the composite of any symptomatic disease (mild, pneumonia, or severe disease). Multivariable regression indicated that female sex (aOR = 1.88; 95% CI: 1.61, 2.19) and underlying diseases (aOR = 1.43; 95% CI: 1.18, 1.73) were significantly associated with an increased risk of symptomatic disease. Vaccination status was not significantly associated with the composite of any symptomatic disease (1 dose: aOR = 0.79, 95% CI: 0.52, 1.20; 2 doses: aOR = 1.10, 95% CI: 0.87, 1.38, 3 doses: aOR = 0.83, 95% CI: 0.64, 1.09).
Table 3. Factors associated with symptomatic disease (mild, pneumonia or severe disease) among SARS-CoV-2 Omicron infection
We then performed sex-stratified analysis, with adjustment for age and underlying diseases (B). For males, compared with those who were not vaccinated, the VE against any symptomatic disease was 15.0% (95% CI: −44.7%, 50.1%, P = 0.549) for those who received 1 dose, −18.5% (95% CI: −62.7%, 13.7%, P = 0.293) for those who received 2 doses, 5.40% (95% CI: −37.8%, 35.1%, P = 0.773) for those who received 3 doses, and −10.8% (95% CI: −50.9%, 18.7%, P = 0.518) for those who received any number of doses (1, 2, or 3). For females, compared with those who were not vaccinated, the VE against any symptomatic disease was 27.4% (95% CI: −44.7%, 63.6%, P = 0.363) for those who received 1 dose, 0.40% (95% CI: −39.5%, 28.9%, P = 0.981) for those who received 2 doses, 27.2% (95% CI: −6.30%, 50.2%, P = 0.100) for those who received 3 doses, and 9.8% (95% CI: −24.9%, 34.9%, P = 0.533) for those who received any number of doses (1, 2, or 3). Examination of VE against all 3 symptomatic classifications with stratification by different manufacturers indicated no significant differences (all P > 0.05, B).
Discussion
Current evidence seems to indicate that the Omicron variant of SARS-CoV-2 causes milder disease than previous 2 variants[Citation15–18]. This may be because of the characteristics of the Omicron variant itself or the hybrid cell-mediated immunity induced by vaccination and natural infection. In contrast to these previous studies conducted in other regions or countries, Changchun City had a very low case load prior to 2022, so its population only had negligible herd immunity. Therefore, the results of our study demonstrate the real-world effect of vaccination in a population with very low pre-existing natural immunity. Our data provide real-world evidence that patients who received two or three doses of either inactivated vaccine (CoronaVac or BBIBP-CorV) were protected against pneumonia and severe disease caused by SARS-CoV-2 Omicron. Stratification by sex indicated this protective effect was present in men and women. In addition, analysis of the group of patients who received any number of vaccine doses (1, 2, or 3) indicated there was no protection against the composite outcome of all symptomatic disease (mild, pneumonia, or severe disease) before and after stratification by age, sex, and underlying disease; this is likely because the vast majority of symptomatic patients had mild disease (92.4%), and vaccination provided no protection against mild disease. Our multivariate model showed that old age and underlying diseases were significantly associated with pneumonia and severe disease, consistent with most other studies[Citation19,Citation20]. After adjusting for age, sex, and underlying diseases, two or three COVID-19 vaccine doses protected against pneumonia and severe disease.
In terms of the VE against pneumonia and severe illness, our findings are broadly consistent with other studies. For example, Wu et al. provided evidence that completing primary vaccination within 180 days protected against pneumonia due to Omicron infection [Citation21]. Data from the Chinese Center for Disease Control and Prevention showed that full vaccination and booster vaccination were associated with protection from pneumonia and serious disease caused by the Omicron variant [Citation22]. A study in Hong Kong showed that the inactivated vaccines provided significant protection against severe or fatal disease, and were also protective against mild disease in those who had three doses [Citation23]. An observational study in Hong Kong also showed that two or three doses of BNT162b2 or three doses of CoronaVac provided very high levels of protection against severe disease and death in all age groups [Citation24]. A study of infections by the Omicron variant in South Africa reported VE against hospitalization was 70% [Citation25]. A case – control study in the U.S. showed that full mRNA vaccination significantly reduced the risk of hospitalization (OR = 0.15) and the risk of death or need for invasive mechanical ventilation in patients who were hospitalized (OR = 0.33) [Citation26].
Our analysis of VE in all patients and different patient subgroups showed that vaccination was not significantly protective against the composite outcome of all three symptomatic diseases together (mild disease, pneumonia, and severe disease). This result is consistent with the previous results [Citation12,Citation27]. In particular, Andrews et al. showed that vaccination provided limited protection against symptomatic disease caused by the Omicron variant in patients who received two doses of the ChAdOx1 nCoV-19 vaccine or the BNT162b2 vaccine [Citation12]. This may be because the Omicron variant has many mutations and these vaccines were developed based on the ancestral strain of the SARS-CoV-2 virus. Other studies showed that although the Omicron variant has many mutations that allow it to evade some neutralizing antibodies and memory B cells, memory T cells still provide protection, even against highly mutated Omicron subvariants. In particular, at least 83% of CD4+ (helper) T cell responses and 85% of CD8+ T cell responses remained in patients regardless of vaccination status or SARS-CoV-2 variant [Citation28]. Neutralizing antibodies and memory B cells are only part of the body's adaptive immune response. In a person previously infected by SARS-CoV-2 or vaccinated against COVID-19, T cells do not prevent infection. Instead, they patrol the body and destroy cells that are already infected, thereby preventing virus multiplication and severe disease [Citation28–30]. This can also explain why the inactivated vaccines provided no protection against mild disease, but were protective against pneumonia and severe disease. There is also some evidence that three doses of inactivated vaccines provided significant effectiveness against symptomatic disease [Citation31]. The presence of multiple lines of defense is an important advantage, because an individual still has some protection if the virus overcomes an initial line of defense. From this perspective, it is still necessary to use existing COVID-19 vaccines in a timely manner until new COVID-19 vaccines, which provide broad more targeted protection, are available.
Although we found that vaccination did not provide protection against the overall presence of symptoms, our results showed that people with underlying diseases and women were more likely to have symptoms. It is possible that infected persons with underlying diseases are more prone to symptoms due to their weak physical resistance, and that women are more likely to report mild symptoms than men [Citation32]. Additionally, some reports show that females develop a greater antibody response due to hormonal differences compared to males, which regulates both adaptive and innate immune responses [Citation33,Citation34]. However, levels of sex hormones change with age. After adjusting for age and underlying diseases, we did not find significant differences in VEs against COVID-19 disease between genders. As an observational study, this study cannot reveal the relevant mechanisms. Further basic research is needed to study the difference of COVID-19 symptoms and the VEs between different genders.
In addition, our results showed that the CoronaVac and the BBIBP CorV vaccines had protective effects against pneumonia and severe disease from Omicron, although neither had protective effects against any symptomatic disease, and they had no statistical difference in VE. We also found that mixed vaccination was beneficial in this community setting. In particular, there was no statistical difference in VE when all vaccine doses were from the same manufacturer or when vaccine doses were from different manufacturers, which may be because only a small number of patients received mixed vaccinations. At present, there is only limited knowledge of the effect of mixed vaccination using different inactivated vaccines. However, studies of other heterologous vaccines showed they may be more effective than homologous vaccines. For example, Zhang et al. showed that a third heterologous protein subunit vaccine (two-doses of the BBIBP-CorV vaccine and then one dose of a heterologous protein subunit vaccine) more effectively increased the titer of neutralizing antibodies than three doses of the BBIBP-CorV vaccine [Citation35]. A study in Hong Kong showed that subjects who received two doses of the CoronaVac inactivated vaccine and a third dose of the BNT162b2 vaccine had significantly higher antibody levels and enhanced neutralizing activity of different variants than those who received three doses of the CoronaVac vaccine[Citation36]. The effect of mixed vaccination from different manufacturers needs to be further evaluated in future studies. In terms of safety, Lin et al. reported that use of different brands of inactivated booster vaccines did not increase the risk of adverse reactions, but heterologous boosters increased the risk of adverse reactions in subjects who had two doses of an inactive vaccine [Citation37]. These results thus confirm the safety of using mixed vaccinations of inactive vaccines from different manufacturers.
One of the limitations of this study is that all research participants were infected, and there were no uninfected participants. Therefore, we could not evaluate VE against infections of Omicron variant. Another limitation is that the number of patients with pneumonia and severe disease is small, which is not enough for age stratification analysis. A further large-scale prospective study is needed to more thoroughly evaluate the effectiveness of inactivated COVID-19 vaccines against the Omicron variant.
In conclusion, inactivated COVID-19 vaccines protect infected individuals against pneumonia and severe disease due to the Omicron variant, and booster vaccinations enhance this effect. Our results underline the public health value of full vaccination and booster vaccination to protect individuals from pneumonia and severe disease and to reduce the burden on the healthcare system. Previous studies and the present study demonstrated the need for vaccine boosters, and that the vaccination schedule and types of vaccine combinations need to be further evaluated. In the absence of knowledge about how the SARS-CoV-2 virus will mutate in the future, population-wide vaccination against COVID-19 using the existing vaccines is likely to remain one of the most beneficial public health strategies.
Declaration of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank the participants for their consent to take part in the study and all medical staff who worked in the frontline. We also extend our appreciation to Prof. Songling Zhang for coordinating and guiding the study and Prof. Jing Jiang for advice on study design. We acknowledge Yidu Cloud (https://yiducloud.com.cn/) for creation of the electronic questionnaires and data management.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Junqi Niu: [email protected]], upon reasonable request.
References
- Organization WH. (2022). WHO Coronavirus (COVID-19) Dashboard. 2022: https://covid19.who.int/.
- Organization WH. Classification of Omicron (B.1.1.529): SARS-CoV-2 Variant of Concern. 2021: https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
- Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021;600(7889):367–368.
- Callaway E, Ledford H. How bad is omicron? what scientists know so far. Nature. 2021;600(7888):197–199. Epub 2021/12/04.
- Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. SARS-CoV-2 omicron has extensive but incomplete escape of pfizer BNT162b2 elicited neutralization and requires ACE2 for infection. medRxiv. 2021 Dec 17. Epub 2021/12/16. PMID: 34909788. doi: 10.1101/2021.12.08.21267417. [Preprint].
- Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV; 2, omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467–84 e15.
- Carreno JM, Alshammary H, Tcheou J, et al. Activity of convalescent and vaccine serum against SARS-CoV-2 omicron. Nature. 2022;602(7898):682–688.
- Muik A, Lui BG, Wallisch AK, et al. Neutralization of SARS-CoV-2 omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375(6581):678–680.
- Tseng HF, Ackerson BK, Luo Y, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 omicron and delta variants. Nat Med. 2022;28(5):1063–1071.
- Syed AM, Ciling A, Taha TY, et al. Omicron mutations enhance infectivity and reduce antibody neutralization of SARS-CoV-2 virus-like particles. Proc Natl Acad Sci U S A. 2022;119(31):e2200592119, doi:10.1073/pnas.2200592119.
- Accorsi EK, Britton A, Fleming-Dutra KE, et al. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA. 2022;327(7):639–651. doi:10.1001/jama.2022.0470.
- Andrews N, Stowe J, Kirsebom F, et al. COVID; 19, vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546.
- Tracker CV. Vaccine candidates in clinical trials. 2022: https://covid19.trackvaccines.org/vaccines/.
- China NHCotPsRo. Diagnosis and treatment plan for COVID 19(trial version 9). Clin JC lin Infect Dis. 2022;15(2):81–89. [Chinese].
- Stalcrantz J, Kristoffersen AB, Boas H, et al. Milder disease trajectory among COVID-19 patients hospitalised with the SARS-CoV-2 omicron variant compared with the delta variant in Norway. Scand J Public Health. 2022;50(6):676–682. doi:10.1177/14034948221108548.
- Garrett N, Tapley A, Andriesen J, Seocharan I, Fisher LH, Bunts L, et al. High rate of asymptomatic carriage associated with variant strain omicron. medRxiv. 2022 Jan 14. Epub 2022/01/20. PMID: 35043118. doi:10.1101/2021.12.20.21268130. [Preprint]
- Maslo C, Friedland R, Toubkin M, et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared With previous waves. JAMA. 2022;327(6):583–584. doi:10.1001/jama.2021.24868.
- Organization WH. Severity of disease associated with Omicron variant as compared with Delta variant in hospitalized patients with suspected or confirmed SARS-CoV-2 infection. 7 June 2022:https://www.who.int/publications/i/item/9789240051829.
- Mayr FB, Talisa VB, Castro AD, et al. COVID-19 disease severity in US veterans infected during omicron and delta variant predominant periods. Nat Commun. 2022;13(1):3647), doi:10.1038/s41467-022-31402-4.
- Butt AA, Yan P, Shaikh OS, et al. Rate and risk factors for severe/critical disease Among fully vaccinated persons With breakthrough severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a high-risk national population. Clin Infect Dis. 2022;75(1):e849–ee56. doi:10.1093/cid/ciab1023.
- Wu D, Ye Y, Tang L, et al. A case-case study on the effect of primary and booster immunization with China-produced COVID-19 vaccines on prevention of pneumonia and viral load among vaccinated persons infected by delta and omicron variants. Emerg Microbes Infect. 2022;11(1):1950–1958.
- Li M, Liu Q, Wu D, et al. Association of COVID-19 vaccination and clinical severity of patients infected with delta or omicron variants - China, May 21, 2021-February 28, 2022. China CDC Wkly. 2022;4(14):293–297. doi:10.1080/22221751.2022.2103455.
- Li Ka Shing Faculty of Medicine TUoHK. HKUMed proposes forward planning after Hong Kong’s fifth wave of Omicron BA.2. 2022-3-26. https://www.med.hku.hk/en/news/press/20220322-updates-on-modelling-the-omicron-fifth-wave.
- McMenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–1443.
- Madhi SA, Kwatra G, Myers JE, et al. Population immunity and COVID-19 severity with omicron variant in South Africa. N Engl J Med. 2022;386(14):1314–1326. doi:10.1056/NEJMoa2119658.
- Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043–2054. doi:10.1001/jama.2021.19499.
- Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N Engl J Med. 2022;386(19):1804–1816. doi:10.1056/NEJMoa2200797.
- Tarke A, Coelho CH, Zhang Z, et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from alpha to omicron. Cell. 2022;185(5):847–859. e11. doi:10.1016/j.cell.2022.01.015.
- Wang CY, Hwang KP, Kuo HK, et al. A multitope SARS-CoV-2 vaccine provides long-lasting B cell and T cell immunity against delta and omicron variants. J Clin Invest. 2022;132(10.
- GeurtsvanKessel CH, Geers D, Schmitz KS, et al. Divergent SARS-CoV-2 omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci Immunol. 2022;7(69):eabo2202), doi:10.1126/sciimmunol.abo2202.
- Sheikh A, Kerr S, Woolhouse M, et al. Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design. Lancet Infect Dis. 2022;22(7):959–966. doi:10.1016/S1473-3099(22)00141-4.
- Petersen MS SIK, Eliasen EH, Larsen S, et al. Clinical characteristics of the omicron variant - results from a nationwide symptoms survey in the Faroe Islands. Int J Infect Dis. 2022;122:636–643. doi:10.1016/j.ijid.2022.07.005.
- Abu Jabal K, Ben-Amram H, Beiruti K, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6).
- Notarte KI, Ver AT, Velasco JV, et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 pfizer-BioNTech mRNA vaccination: a systematic review. Crit Rev Clin Lab Sci. 2022;59(6):373–390.
- Ai J, Zhang H, Zhang Y, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11(1):337–343. doi:10.1080/22221751.2021.2022440.
- Mok CKP, Chen C, Yiu K, et al. A randomized clinical trial using CoronaVac or BNT162b2 vaccine as a third dose in adults vaccinated with Two doses of CoronaVac. Am J Respir Crit Care Med. 2022;205(7):844–847. doi:10.1164/rccm.202111-2655LE.
- Lin Z, Wu J, Huang R, et al. Comparison of safety of different vaccine boosters following Two-dose inactivated vaccines: A parallel controlled prospective study. Vaccines (Basel. 2022;10(4). Epub 2022/04/24.