ABSTRACT
By December 2021, administration of the third dose of COVID-19 vaccinations coincided with the spread of the Omicron variant in Europe. Questions had been raised on protection against infection conferred by previous vaccination and/or infection. Our study population included 252,433 participants from the COVID-19 vaccination registry in Malta. Data were then matched with the national testing database. We collected vaccination status, vaccine brand, vaccination date, infection history, and age. Using logistic regression, we examined different combinations of vaccine dose, prior infection status and time, and the odds of infection during the period when the Omicron variant was the dominant variant in Malta. Participants infected with Sars-Cov-2 prior to the Omicron wave had a significantly lower odds of being infected with the Omicron variant. Additionally, the more recent the infection and the more recent the vaccination, the lower the odds of infection. Receiving a third dose within 20 weeks of the start of the Omicron wave in Malta offered similar odds of infection as receiving a second dose within the same period. Time since vaccination was a strong determinant against infection, as was previous infection status and the number of doses taken. This finding reinforces the importance of future booster dose provision especially to vulnerable populations.
Introduction
Vaccines are a critical tool to reduce the burden of coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Public health institutions have also questioned the degree to which getting infected by SARS-CoV-2 would affect immunity, and for how long, especially in vaccinated people.
The first infection of COVID-19 in Malta was reported on 7th March 2020. Since then, Malta has gone through several waves of COVID-19, with the highest number of cases reported during the Omicron wave on December 2021 and January 2022. In addition, Malta had one of the fastest vaccination rollouts in the world [Citation1], and within each wave vaccination coverage has increased. To date, COVID-19 vaccine coverage reached over 86% for two doses and over 67% for three doses [Citation2]. Over 50% of those above 65 years of age have taken four doses [Citation3].
Since November 2021, the Omicron SARS-Cov-2 variant spread rapidly around the world. In Malta, it was first detected in early December 2021, reaching over 80% prevalence in the week starting 20th December 2021 [Citation4]. Compared to previous variants (Alpha and Delta) Omicron has been found to be more infectious, but less virulent, including when adjusted for vaccination and previous infection [Citation5–7].
Andeweg et al. studied the effects of COVID-19 vaccination and previous SARS-CoV-2 infections, and found that they offer relatively low protection against Omicron infection [Citation8], with a greater risk of infectivity and breakthrough [Citation9]. Higher protection was observed against Omicron in individuals with both vaccination and previous infection, compared to either one, and protection since infection or vaccination decreased over time, also corraborated through a systematic review and meta-regression carried out by Feikin et al. [Citation10]. This finding was been corroborated by Altarawneh et al., who also concluded that hybrid immunity from prior infection and recent booster vaccination confers strong protection against Omicron infection [Citation11]. Vaccination, they conclude, enhances protection of those with a prior infection.
Objective
This study, carried out on large cohort of people in Malta aims to understand vaccine effectiveness through a combination of vaccination, infection, and time. For ease of reference, the variants mentioned are referred to using WHO labels not their Pango lineage: Alpha (B.1.1.7), Beta (B.1.351), Delta (B.1.617.2) and Omicron (B.1.1.529) [Citation12]. Similarly, we will be using the popular names for vaccines included in this study: Pfizer/Biontech (BNT162b2), Oxford-Oxford-AstraZeneca (ChADOx1 nCoV-19), Janssen (Ad26.COV2) and Moderna (mRNA-1273).
The main objective of our study was to evaluate the association between different combinations and timing of previous SAR-CoV-2 infections and vaccinations, with SAR-CoV-2 infection during the Omicron variant period in Malta, within the vaccinated Maltese population.
Materials and methods
No ethical approval was necessary for this study as data collected were aggregate data from our surveillance systems, and results cannot be traced back to an individual. No funding was provided for this study.
Study design and study subjects
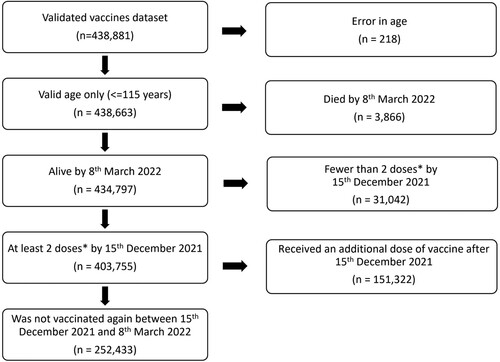
The study is a retrospective cohort study, with study participants being all validated vaccinees in Malta’s COVID-19 vaccination registry between 27th December 2020 and 8th March 2022. The following criteria were used for inclusion of participants in our study (Supplementary Table 3).
Inclusion criteria and data description
The following inclusion criteria applied in this study:
Vaccinations between the 27th of December 2020 and 8th March 2022
Vaccines received: Pfizer/Biontech, Oxford-AstraZeneca, Janssen, and/or Moderna
Individuals alive on 8th March 2022
Received at least 2 doses of vaccines (or 1 dose of Janssen)
Did not receive a third dose between 15th December 2021 and 8th March 2022
The 15th December 2021 date was selected as being the midpoint of the week when Omicron became the dominant (>80% prevalence) Sars-Cov-2 variant in Malta. 80% was selected as the cut-off point following the example set by Andrews et al. [Citation13]. This was further confirmed on a sensitivity analysis to be a suitable date (Supplementary Table 4), since there was little change in results for the dates of December 8th, 15th and December 22nd (making December 15th a suitable midpoint). Thorough quality checks were carried out on the vaccination registry database ensuring congruent data which included 403,755 participants. In this study, the outcome of interest was infection with SARS-Cov-2 between 15th December 2021 and 8th of March 2022, when it was assumed most new infections would be of the Omicron variant, which became the dominant variant (over 80%) of all infections that week.
Reverse Transcriptase Polymerase Chain Reaction (RT–PCR) and Rapid Diagnostic Test (RDT) test results for COVID-19 were obtained through the national database of RT–PCR and RDT tests. Since the RDT tests utilized in Malta were of high specificity and sensitivity (see Appendix 2), they were taken as confirmative of COVID-19 infection if positive. The data included all COVID-19 tests carried out in Malta until 8th March 2022 – following this date, the uptake of self-testing kits at home accelerated and self-reporting of COVID-19 dropped significantly, making any data after 8th March 2022 unreliable for our study. In individuals with repeated infection, an infection “event” was considered to be the first registered COVID-19 infection occurring at least 60 days after the previous infection event, as per literature findings [Citation14]. The first registered event was either a positive RT–PCR test (75% of events) or a positive RDT test (25%).
Participants’ vaccination status was ascertained from the national immunization registry for COVID-19. Four vaccines were considered for this study, as they were main vaccinations provided to the population: Pfizer/Biontech, Moderna, Janssen, and Oxford-AstraZeneca. These were grouped into two groups: mRNA vaccines (Pfizer/Biontech and Moderna) and Adenoviral vaccines (Oxford-AstraZeneca and Janssen). Genomic data at a patient level was scarce, and therefore population level data were used. Two time points were determined: patients admitted before the Omicron variant reached 80% at a national level, namely, the Delta and other variants wave, vs patients admitted after 80% of the national sequences tested positive to Omicron, namely, the Omicron wave. All sequencing data were ascertained by whole genome sequencing.
During analysis, an attempt was made to include severity as an outcome. Unfortunately, data on severity (including symptomatic and hospitalization) was not validated and could not be ascertained to be of sufficient quality to include in a population-level study. Consequently, a decision was made to exclude this parameter from our study.
The vaccination registry system registered 438,881 validated individuals from 27th December 2020 to 8th March 2022. Participants were excluded from the analysis as per the exclusion criteria, leaving a total of 403,755 possible participants (92.0%) in this study. After excluding those who got vaccinated between 15th December 2021 and 8th March 2022, 252,433 participants (57.5%) remained in the study, which is 48.9% of Malta’s residents [Citation15] (). By week 51, 2021, 82.4% of all variants sequenced were of the Omicron variant (Appendix 1), so the midpoint of the prior week (15th December 2021) was selected as a cut-off point.
Statistical analysis
Data were cleaned and analysed using R software. Logistic regressions (R glm function) were fitted with infection during the Omicron wave as the outcome variable, and vaccination programme (number of doses), age (≤40 years, 41 years–70 years, 71 years+), infection pre-Omicron wave (Yes, No) and time (>20/<20 weeks since last vaccination/infection and 15th December 2021) as fixed effects. 20 weeks, or 5 months, were chosen as a cut-off for time as it was used in other studies measuring antibody responses to vaccination and infection [Citation16–18]. Participants who had only taken two doses of vaccination prior to 15th December 2021, with the latest dose taken more than 20 weeks prior with no previous Sars-Cov-2 infection were considered the reference for these analyses.
Results
Demographics
Among 252,433 participants included in this study, the median age was 53 years (IQR 32 years–69 years). Out of the 252,433 study participants, 13,241 (5.2%) tested positive for COVID-19 during the Omicron wave (15th December 2021 to 8th March 2022) (). Of those who tested positive for COVID-19 during the Omicron wave, 537 (4.0%) had tested positive for COVID-19 before the Omicron wave. In our study, COVID-19 infection during the Omicron wave was most common in those aged 40 years and below (n = 7472, 56%), while those aged 71 years and above were the least likely to test positive for COVID-19 during the Omicron wave (n = 1388, 10.0%). The most common vaccination category until 8th March 2022 were participants who had taken 3 doses with the latest dose less than 20 weeks prior to 15th December 2021 and had no previous infection (n = 139,797, 55%). There were no participants registered as having taken 3 doses with the latest dose more than 20 weeks prior to 15th December 2021. Malta started administering the booster dose on 1st September 2021, less than 20 weeks before the Omicron wave.
Table 1. Descriptive table for the cohort included in the study, showing denominators and column percentages.
Stratification by age group
When stratified by age groups () a clear pattern emerges for all age groups, showing that both the number of vaccine doses received and previous infection are factors that contribute to an improvement on the protection against Omicron infection. Specifically, those with recent (<20 weeks) vaccination or recent (<20 weeks) infection or both have significant lower odds of getting infected during the Omicron period than those that had never been infected and were vaccinated with two doses more than 20 weeks before December 15th. For example, for those with 3 doses, with the third dose taken <20 weeks prior to December 15th and who had been infected <20 weeks prior to December 15th we obtained the following results across the three age groups: 40 years and below, OR = 0.14, CI = 0.04,0.33; 41 years–70 years, OR = 0.05, CI = 0.01,0.12; 71+ years, OR = 0.08, CI = 0.001,0.37 as compared to the reference category listed with OR = 1.0 (refer to and compare across age groups for all other combinations). The 71+ years age group had the greatest protection against infection during Omicron (OR = 0.30; CI = 0.28, 0.32, compared to the reference group of 40 years and below, 1.00), with 70% lower odds of getting infected with Omicron (Supplementary Table 1).
Table 2. Univariate logistic regression, stratified by age groups.
Stratification by vaccination groups
Multiple logistic regression was carried out for vaccination groups ( and ), adjusting for agegroup since vaccine regimens differed significantly between one age group and another. As in the rest of this paper, two adenoviral (AdX) vaccines can refer to either participants who received two Oxford-AstraZeneca vaccines, or one Janssen vaccine. The reference categories here were people who did not get infected prior to 15th December 2021 and who were vaccinated more than 20 weeks prior to 15th December 2021. Note that for the third dose, there were no participants receiving a third dose more than 20 weeks prior to 15th December 2021.
Figure 2. Forest plot showing the Odds Ratios for Omicron infection with different vaccination groups described in Table 3, adjusted for age group. Red and blue indicate mRNA vaccines and Adenoviral vaccines respectively. NB: The “3 Adenoviral” regimen indicates 2 Adenoviral doses equivalent and 1 mRNA booster. Figure Footnote: The reference category is indicated by the vertical line in the graph, showing a 1.00 OR. This refers to the 2 mRNA vaccines, >20 weeks, no infection category (weakest immunity conferred).
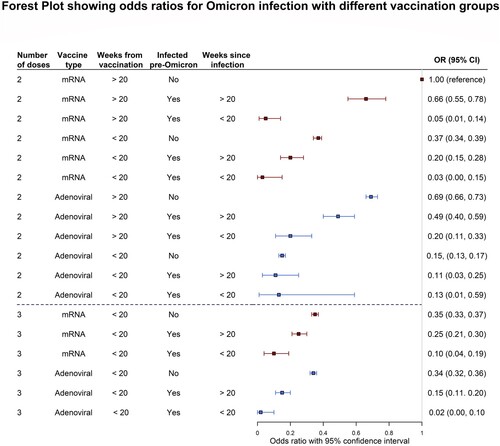
Table 3. Multiple Logistic Regression for vaccination groups adjusted for age groups.
Compared to the reference, those who received a third dose before 15th December 2021 and had no previous infection had 65% (OR = 0.35, CI 0.33, 0.37) lower odds of getting an infection with Omicron up to the 8th of March 2022. Those who received the equivalent of two adenoviral doses however, even if more than 20 weeks prior to 15th December 2021, had 31% (OR = 0.69, CI 0.66:0.73) lower odds of becoming infected with Omicron.
Combined with infection, the protective effect is greatly amplified across all regimens (). For example, those who took two mRNA vaccines and were infected with Omicron more than 20 weeks prior to 15th December 2021 had 34% (OR = 0.66, CI 0.55, 0.78) lower odds while those who took two adenoviral vaccines’ equivalent and were likewise infected more than 20 weeks prior had 51% (OR = 0.49, CI 0.40, 0.59) lower odds. This effect is further amplified with a recent infection with 20 weeks of 15th December 2021 for more recent infections, with 95% (OR = 0.05, CI 0.01, 0.14) and 80% (OR = 0.20, CI 0.11, 0.33) for two mRNA and two adenoviral vaccines’ equivalent respectively. Taking a third dose within 20 weeks of 15th December 2021 further increased this protective effect.
Discussion
This study highlights the role of time, vaccination and infection against infection with the Omicron variant. The outcomes of this study inform public health institutions and policymakers on current and future vaccination strategies for COVID-19. These insights should prove increasingly important as the world enters a scenario where COVID-19 is increasingly being treated as an endemic disease [Citation19,Citation20], when additional booster doses being offered to the more vulnerable members of society.
A matter of time
The outcomes of this study demonstrate that being vaccinated with three doses of vaccine offers greater protection against Omicron infection overall than two doses of vaccine. Receiving a third dose within 20 weeks of the start of the Omicron wave in Malta with no previous infection offered similar odds of infection as receiving a second dose within the same period, with no previous infection (). This applies for both mRNA and adenoviral regimens. This indicates that for those not previously infected, it is more a matter of timing since the last vaccination, rather than a matter of the number of doses, that has the stronger protective effect against Omicron. Being previously infected with COVID-19 also confers additional protection against infection during the Omicron period. However, the most impactful factor in conferring protection against infection is the timing of the most recent vaccination or infection (more or less than 20 weeks until 15th December 2021). For both infection and vaccination, less than 20 weeks until the start of the Omicron period conferred better protection. This finding matches the literature, which refers to waning immunity over time [Citation21–24]. Hybrid immunity (infection and vaccination) also provides the higher protection than vaccination on its own, again matching literature on the subject [Citation25].
Age matters
Academic literature shows strong evidence of increasing age being a significant risk factor for infection increased severity of COVID-19 infection [Citation26,Citation27]. However, in this analysis belonging to an older age group compared to a younger age group lowered the odds of being infected during the Omicron period (). This seemingly contradictory result is most likely due to the greater vaccine booster uptake by older (and more vulnerable) people in Malta, priming their immunity during the Omicron wave [Citation2]. Younger people were less likely to take up a third dose; by 15th December 2021, only 20.5% of all those 40 years and below (n = 19,251/93,769), had been vaccinated with a third dose. This can be contrasted to the 94.1% (n = 51,095/55,116) of all those aged 71+ years who had taken a third dose by that date (Supplementary Table 2). This might explain why the youngest age group had the higher odds of being infected by Omicron.
Additionally, when looking within age groups one finds that for those aged 41–70 years the odds ratios are lower than for the two other groups. This difference in odds ratios within one group and another could fit with the narrative of younger people exhibiting lower cautious behaviour compared to older people, while older people having a lower immunity overall, as the lowering of odds changes significantly within each group.
For those aged 71+ years, the results in the “2 doses” section are not statistically significant. The wide confidence ratios for the “2 doses” section points to low power (and numbers), which contrasts with the much tighter confidence intervals in the “3 doses” section. This points to Malta’s very successful uptake of the third booster dose by the 15th of December 2021. Additionally, this finding suggests that unless given a booster dose, people aged 71+ years do not have associated lower odds of infection, even when compared to those that had COVID-19 before. This finding is an important one and underlines the importance of providing additional boosters over time to the elderly and vulnerable segments of the population as an effective public health measure.
Vaccination groups
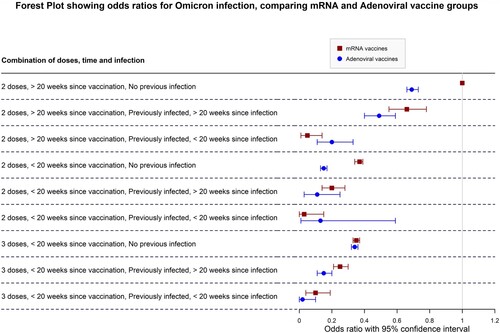
Different vaccination regimens also seem to confer different protective effects against infection during the Omicron period, even after adjusting for age groups. While the magnitude of protection down different combination categories follows a similar pattern throughout, for two doses, adenoviral vaccines seem to offer greater protection against infection overall when compared to mRNA vaccine regimens if the last dose taken was more than 20 weeks prior to 15th December 2021. This may point to the different mode of action of adenoviral vaccines compared to mRNA vaccines, the former possibly offering better lasting protection overall compared to mRNA vaccine. This finding might also point to a longer-lasting protective effect of adenoviral vaccines in general compared to mRNA vaccines. This distinction disappears however with the third booster dose, with odds ratios of 65% lower odds for those with three mRNA vaccines and 67% (OR = 0.33, CI 0.31, 0.36) for those with two adenoviral and one mRNA vaccine. There is also considerable overlap of odds ratios between the two categories (), indicating that this difference may not be significant except for those who took 2 doses and did not have a previous infection. For the three-dose category there is effectively no significant difference between both vaccine regimens, indicating that both are highly protective against Omicron, with recent infection improving the odds against infection. However, the confidence intervals for adenoviral vaccines in the 3rd dose categories suggest that perhaps mixing of vaccinations may provide a better protective effect against Omicron infection. This matches available literature on heterologous vaccine regiment effectiveness compared to homologous vaccination [Citation28–30]. Unfortunately, those receiving other mixed vaccine regimens (for example, 1 mRNA + 2 adenoviral vaccines) were too few, limiting power so that we were not able to achieve any significant interpretations and findings, and have been excluded from and the forest plots.
Figure 3. Forest plot comparing adenoviral to mRNA vaccine groups., adjusted for age group. NB: The “3 Adenoviral” regimen indicates 2 Adenoviral doses equivalent and 1 mRNA booster. Figure Footnote: The reference category is indicated by the vertical line in the graph, showing a 1.00 OR. This refers to the 2 mRNA vaccines, >20 weeks, no infection category (weakest immunity conferred).
Hybrid immunity
The strongest finding in this study is the protective effect of a combination of vaccination and previous infection against Omicron infection. The results of this study strongly affirm these findings, with lower odds ratios for those who had been previously infected with SARS-CoV-2 and had been vaccinated compared to those who received vaccination alone, for both two- and three-dose regimen. This is a subject of intense discussion in the literature, with evidence worldwide strongly supporting the greater immune response from vaccination and infection, as opposed to either one alone [Citation11,Citation31–33]. As aforementioned, the more recent the infection and/or vaccination, the greater the protective effect conferred against infection with the Omicron variant.
Strengths and limitations
This study has several limitations. First, it is a cohort taken from Malta’s vaccination registry system, meaning that findings here are not related to unvaccinated individuals. As people have been getting with infected with COVID-19 and over 87% of the population are vaccinated, it becomes irrelevant to compare to an unvaccinated, uninfected group. Consequently, odds ratios here are not representative of vaccine efficacy, but are rather compared to the so-called “weakest immunity” scenario: people who received the equivalent of two doses of vaccine more than 20 weeks prior to the start of the Omicron period in Malta, with no previous infection. Here, therefore, we observe the protective effect against infection of one sub-category over another during the Omicron period.
Second, this dataset did not include sex, which could be an important confounder for immunity: a 2021 metanalysis by Bignucolo et al. found vaccine efficacy to be higher in men than in women [Citation34]. Third, this study does not examine subvariants of Omicron prevalent in Malta up until 8th March 2022. However, it is safe to assume that the two variants present at the time were the BA.1 and BA.2 subtypes. Fourth, 25% of participants had a positive RDT test as a first indicator of an infection event and were included in this study as infected participants even if they were not confirmed as RT–PCR positive (although in most cases they were confirmed). However, RDT tests used in Malta were of very high specificity and sensitivity (Appendix 2). In addition, if these RDTs present a lower sensitivity than shown in Appendix 2, it would be safe to assume that this lower sensitivity would not be associated with the exposure – leading to non-differential misclassification. If the misclassification is non-differential, we would observe an increase in the width of the confidence interval, which would be corrected by our large sample size. Fifth, our genomic information came from population estimates, not from individual level data. This approach has been used before.
Sixth, in the Andrews et al., study the authors also included a cut-point of 80% for estimating the switch on a dominant variant, and we opted to follow their example [Citation7]. This however left a limitation in the study where some people might have been infected with the Omicron infection prior to the 15th of December. In order to confirm the 15th of December as the ideal date, we carried out sensitivity analysis on the following dates as the cut-off point: 8th December 2021, 15th December 2021 and 22nd December 2021 (Supplementary Table 4). We found little difference in results for the three dates. We therefore opted for the 15th December which also coincides with the midpoint of the week when Omicron became the dominant variant in Malta [Citation4]. Seven, while our study compares the odds of infection between different “immunity” groups, it is not a reflection of antibody levels. Therefore, we caution the reader not to interpret these results as a level of antibody response. Lastly, our study lacks a measure of severity of infection, which would have been interesting given the combination of time, vaccination and previous infection as a measure of this study.
Our study also has several strengths. First, our cohort is a large, mostly homogenous population of over 255,433 participants. This makes the findings robust. We also removed erroneous entries to ensure that the data were as clean and accurate as possible. Second, in Malta until the 8th of March 2022 self-testing kits were not easily available, and all private and public RDT and RT–PCR tests were reported to a centralized database, making the outcomes measured here comprehensive for the Maltese population. Third, Malta has a very high vaccine uptake compared to other countries, which makes such a study comparing the effects of two to three dose vaccinations and infection particularly robust.
Conclusion
The outcomes of this study stress the importance of time in triggering an immune response and protective effect against infection by the Omicron variant. In this light, booster doses remain highly important regardless of infection status, especially when hybrid immunity is shown to be highly effective against subsequent infections by SARS-CoV-2. As most countries prepare to treat COVID-19 as an endemic rather than an epidemic disease, the findings of this study point to the importance of maintaining booster dose uptake by the more vulnerable members of society, especially considering new variants that may develop over time. Given the evidence on waning immunity over time, research on finding the critical best time to administer boosters becomes increasingly important [Citation24,Citation35]. There also remains a significant risk of getting infected with the Omicron (and possibly future) variants for vulnerable segments of the population in spite of vaccination status and previous infection; in this case, avoidance of exposure remains necessary especially during period of high infection incidence of COVID-19.
Supplemental Material
Download Zip (740.5 KB)Acknowledgements
We would like to thank all supporting staff, including public health personnel, laboratory personnel and the vaccine registry team for their support, assistance and provision of data which made this study possible.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Cuschieri S, Agius S, Souness J, et al. The fastest national COVID vaccination in Europe – Malta’s strategies. Health Sci Rev. 2021;1:100001, doi:10.1016/j.hsr.2021.100001.
- ECDC. COVID-19 vaccine tracker | European centre for disease prevention and control. Published; 2022. [cited 2022 Jul 12]. Available from: https://vaccine tracker.ecdc.europa.eu/public/extensions/covid-19/vaccine-tracker.html#uptake-tab.
- Vaccination Registry System M. COVID-19 in Malta – open dataset. Published online July 6, 2022. [cited 2022 Jul 20]. Available from: https://github.com/COVID19-Malta/COVID19-Data/blob/fdcfca23701eb1567577b09aeec1f7d2f41a1da2/COVID-19%20Malta%20-%20Vaccination%20Data.csv.
- GISAID. Hcov-19 variants dashboard. GISAID. Published online November 2022. Available from: https://gisaid.org/hcov-19-variants-dashboard/.
- Lewnard JA, Hong VX, Patel MM, et al. Clinical outcomes associated with Omicron (B.1.1.529) variant and BA.1/BA.1.1 or BA.2 subvariant infection in Southern California. Nat Med. 2022;28:1933–1943. https://doi.org/10.1038/s41591-022-01887-z
- Davies MA, Kassanjee R, Rousseau P, et al. Outcomes of laboratory-confirmed SARS-CoV-2 infection in the Omicron-driven fourth wave compared with previous waves in the Western Cape Province, South Africa. MedRxiv. Published online 2022.
- Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–46. doi:10.1056/NEJMoa2119451
- Andeweg SP, de Gier B, Eggink D, et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1, BA.2 and delta SARS-CoV-2 infections. Nat Commun. 2022;13:4738. doi:10.1101/2022.02.06.22270457
- Chen J, Wang R, Gilby NB, et al. Omicron variant (B. 1.1. 529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model. 2022;62(2):412–422.
- Feikin DR, Higdon MM, Abu-Raddad LJ, et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399(10328):924–944.
- Altarawneh HN, Chemaitelly H, Ayoub H, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387(1):21–34.
- WHO. Tracking SARS-CoV-2 variants. Published 2022. [cited 2022 Jul 18]. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants.
- Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. 2022;386(4):340–350. doi:10.1056/NEJMoa2115481.
- Nevejan L, Cuypers L, Laenen L, et al. Early SARS-CoV-2 reinfections within 60 days highlight the need to consider antigenic variations together with duration of immunity in defining retesting policies. medRxiv. Published online 2022.
- National Statistics Office Malta. Malta: World Population Day. National Statistics Office Malta; 2021:13. [cited 2022 Jun 30]. Available from: https://nso.gov.mt/en/News_Releases/Documents/2021/07/News2021_122.pdf.
- Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi:10.1016/S0140-6736(21)02249-2.
- Firinu D, Perra A, Campagna M, et al. Evaluation of antibody response to BNT162b2 mRNA COVID-19 vaccine in patients affected by immune-mediated inflammatory diseases up to 5 months after vaccination. Clin Exp Med. 2021;22:477–485. doi:10.1007/s10238-021-00771-3.
- El Sahly HM, Baden LR, Essink B, et al. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021;385(19):1774–85. doi:10.1056/NEJMoa2113017
- Katzourakis A. COVID-19: endemic doesn’t mean harmless. Nature. 2022;601(7894):485–485. doi:10.1038/d41586-022-00155-x.
- Al-Tawfiq JA, Chu DT, Hoang VT, et al. From pandemicity to endemicity: the journey of SARS-CoV-2. J Epidemiol Glob Health. 2022;12:147–149. doi:10.1007/s44197-022-00046-4
- Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416.
- Gupta RK, Topol EJ. COVID-19 vaccine breakthrough infections. Science. 2021;374(6575):1561–1562. doi:10.1126/science.abl8487.
- Levin EG, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. New England J Medicine. 2021;385(24):e84.
- Ferdinands JM, Rao S, Dixon BE, et al. Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance – VISION network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(7):255–263. doi:10.15585/mmwr.mm7107e2.
- Pilz S, Theiler-Schwetz V, Trummer C, et al. SARS-CoV-2 reinfections: overview of efficacy and duration of natural and hybrid immunity. Environ Res. 2022;209:112911. doi:10.1016/j.envres.2022.112911.
- Gallo Marin B, Aghagoli G, Lavine K, et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021;31(1):1–10.
- Pijls BG, Jolani S, Atherley A, et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: a meta-analysis of 59 studies. BMJ Open. 2021;11(1):e044640.
- Zinatizadeh MR, Zarandi PK, Zinatizadeh M, et al. Efficacy of mRNA, adenoviral vector, and perfusion protein COVID-19 vaccines. Biomed Pharmacother. 2022;146:112527. doi:10.1016/j.biopha.2021.112527.
- Chiu NC, Chi H, Tu YK, et al. To mix or not to mix? A rapid systematic review of heterologous prime–boost COVID-19 vaccination. Expert Rev Vaccines. 2021;20(10):1211–1220. doi:10.1080/14760584.2021.1971522.
- Rashedi R, Samieefar N, Masoumi N, et al. COVID-19 vaccines mix-and-match: The concept, the efficacy and the doubts. J Med Virol. 2022;94(4):1294–1299. doi:10.1002/jmv.27463.
- Bhattacharya M, Sharma AR, Dhama K, et al. Hybrid immunity against COVID-19 in different countries with a special emphasis on the Indian scenario during the omicron period. Int Immunopharmacol. 2022;108:108766, doi:10.1016/j.intimp.2022.108766.
- Goldberg Y, Mandel M, Bar-On YM, et al. Protection and waning of natural and hybrid COVID-19 immunity. MedRxiv. Published online 2021.
- Epsi NJ, Richard SA, Lindholm DA, et al. Understanding ‘hybrid immunity’: comparison and predictors of humoral immune responses to SARS-CoV-2 infection and COVID-19 vaccines. Clin Infect Dis. Published online 2022.
- Bignucolo A, Scarabel L, Mezzalira S, et al. Sex disparities in efficacy in COVID-19 vaccines: a systematic review and meta-analysis. Vaccines (Basel). 2021;9(8):825. doi:10.3390/vaccines9080825.
- Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. 2022;386(18):1712–1720. doi:10.1056/NEJMoa2201570.
Appendices
Appendix 1. Genetic sequencing of SARS-CoV-2 variants over time in Malta
The red line depicts the Delta sequences whereas the blue line depicts the Omicron sequences.

Appendix 2. Rapid diagnostic tests
The following is a list of all Rapid Diagnostic Tests used in Malta. Note that percentages listed are specifications provided by the manufacturer.