ABSTRACT
Severe COVID-19 appears to be disproportionately more common in children and adolescents since the emergence of Omicron. More evidence regarding vaccine effectiveness (VE) is urgently needed to assist policymakers in making decisions and minimize vaccine hesitancy among the public. This was a case-control study in the pediatric population using data extracted from the electronic health records database in Hong Kong. Individuals aged 3–17 with COVID-19 confirmed by polymerase chain reaction were included in the study. Each case was matched with up to 10 controls based on age, gender, and index date (within 3 calendar days). The VE of BNT162b2 and CoronaVac in preventing COVID-19, hospitalizations, and severe outcomes were estimated using conditional logistic regression adjusted by patients’ comorbidities and medication history during the outbreak from January to August 2022. A total of 36,434 COVID-19 cases, 2231 COVID-19-related hospitalizations, and 1918 severe COVID-19 cases were matched to 109,004, 21,788, and 18,823 controls, respectively. Compared to the unvaccinated group, three doses of BNT162b2 or CoronaVac was associated with reduced risk of infection [VE: BNT162b2: 56.0% (95% CI: 49.6–61.6), CoronaVac: 39.4% (95% CI: 25.6–50.6)], hospitalization [VE: BNT162b2: 58.9% (95% CI: 36.1–73.6), CoronaVac: 51.7% (11.6–73.6)], and severe outcomes [VE: BNT162b2: 60.2% (95% CI: 33.7–76.1), CoronaVac: 42.2% (95% CI: −6.2–68.6)]. Our findings showed that three doses of BNT162b2 or CoronaVac was effective in preventing COVID-19, hospitalizations, and severe outcomes among the pediatric population during Omicron-dominant pandemic, which was further enhanced after a booster dose.
Key points
In this territory-wide case–control study, three doses of BNT162b2 or CoronaVac were associated with reduced risk of infection [Vaccine Effectiveness (VE): BNT162b2: 56.0%, CoronaVac: 39.4%], hospitalization (VE: BNT162b2: 58.9%; CoronaVac: 51.7%), and severe outcomes (VE: BNT162b2: 60.2%; CoronaVac: 42.2%).
Background
In the pre-Delta pandemic, children and adolescents were less likely to experience severe COVID-19 [Citation1]. On the contrary, the emergence of SARS-CoV-2 Omicron variant appears to disproportionately cause more severe symptoms in children and adolescents, including more hospital admissions, convulsion, croup, and multisystem inflammatory syndrome in children (MIS-C) [Citation2,Citation3]. According to the data from the COVID-19-Associated Hospitalization Surveillance Network (COVID-NET), the weekly COVID-19-associated hospitalization rates in younger patients aged 17 or below increased by 4-fold from 1.8 per 100,000 population during the Delta predominance period to 7.1 per 100,000 during Omicron predominance period compared to a 2.5-fold increase in adults from 15.5 to 38.4 per 100,000 [Citation2,Citation4]. Although the side effect profiles of COVID-19 vaccines are now becoming established based on extensive pharmacovigilance studies [Citation5,Citation6], more evidence regarding vaccine effectiveness (VE) is urgently needed, especially during the Omicron era, which will assist policymakers in making decisions and to minimize vaccine hesitancy among the public.
Hong Kong Special Administrative Region (HKSAR), China, started a territory-wide vaccination programme in February 2021 using mRNA vaccine BNT162b2 (Comirnaty, BioNTech/Pfizer/Fosun) and inactivated vaccine CoronaVac (Sinovac Biotech HK Limited). The vaccination programme was extended to adolescents aged 12–15 starting in June 2021 and was further extended to children aged 5–11 in January 2022. The minimum age for the CoronaVac vaccination was further lowered to infants aged 3 years in February 2022 and aged 6 months in August 2022.
The VE of mRNA vaccines has been demonstrated in children and adolescents in several trials conducted in the early phase of the Omicron pandemic when BA.1 and BA.2 were the dominant subvariants [Citation7–10]. CoronaVac is one of only a few approved vaccines for the pediatric population and is widely used in developing countries. It has been demonstrated to have a VE of around 40% against symptomatic COVID-19 and 60% against hospitalization in children based on two observational studies in Brazil and Chile during the Omicron period. However, these studies did not compare the VE of mRNA and inactivated vaccines, and did not consider the effects of booster doses [Citation11,Citation12]. Therefore, we conducted a territory-wide case–control study to examine the effectiveness of BNT162b2 and CoronaVac vaccines in children and adolescents in Hong Kong during the current Omicron-dominated pandemic, which also considered the number of vaccine doses and their effectiveness on a range of clinical outcomes.
Methods
Data sources
Clinical data on COVID-19 vaccinations in the pediatric and adolescent population in Hong Kong were obtained from the electronic health records database of the Hospital Authority (HA), the vaccination records of the Department of Health (DH), and COVID-19-confirmed case records from the Centre of Health Protection (CHP). Anonymized unique patient identifiers were used to integrate these databases. The HA is a statutory administrative organization that manages all public inpatient services and most of the public outpatient services in Hong Kong. The electronic health records database contains data on patient demographics, diagnoses, prescriptions, and laboratory tests, which provides real-time information to support routine clinical management across all clinics and hospitals in the HA. The DH maintains a vaccination records database of all individuals in Hong Kong. The CHP maintains a database of all confirmed COVID-19 cases based on both mandatory and voluntary reporting of positive polymerase chain reaction (PCR) and rapid antigen test (RAT) results in Hong Kong. These population-based databases have been used in studies on the risk of adverse effects of COVID-19 vaccinations and in other COVID-19 pharmacovigilance studies [Citation5,Citation6,Citation13–27].
Study design and population
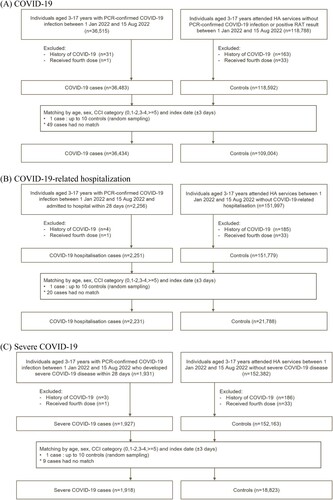
This is a case-control study conducted on children and adolescents aged 3–17 years in Hong Kong. The study period was from 1 January to 15 August, 2022. Individuals with an incident COVID-19-related outcome during the study period were identified as cases. Controls were selected from all other individuals without a COVID-19-related outcome who attended HA services during the study period. The index date was the date of COVID-19-related outcomes for cases and the attendance date for controls. Subjects in the control group who reported a positive RAT result on the online voluntary reporting platform were excluded. The matching procedures were conducted separately for each of the COVID-19-related outcomes. Each case was matched with up to 10 controls according to age, sex, index date (within 3 calendar days), and Charlson Comorbidity Index (0, 1-2, 3-4, ≥5) [Citation28].
Definitions of vaccine exposure
Two COVID-19 vaccines, BNT162b2 and CoronaVac, are provided by the Hong Kong government free of charge in its mass vaccination programme. The BNT162b2 vaccine was first made available to individuals aged ≥16 in March 2021, and was extended to adolescents aged 12–15 in June 2021 and to children aged 5–11 in February 2022. The CoronaVac vaccine was first made available to adolescents aged 12–17 in November 2021 and was extended to children aged 5–11 in January 2022. Details of the full rollout schedule is listed in Supplementary Table 1. Individuals have a choice of the first vaccine dose between BNT162b2 or CoronaVac, but are then restricted to the same vaccine for the second dose. For the booster vaccination, individuals have a choice of either vaccine. In this study, COVID-19 vaccination status was classified into eight mutually exclusive groups based on the type and number of vaccine doses administered: (i) 1 dose BNT162b2, (ii) 1 dose CoronaVac, (iii) 2 doses BNT162b2, (iv) 2 doses CoronaVac, (v) 3 doses (all BNT162b2), (vi) 3 doses (all CoronaVac), (vii) 3 doses (2 doses BNT162b2 and CoronaVac booster), and (viii) 3 doses (2 doses CoronaVac and BNT162b2 booster). Individuals who received a fourth dose or with incomplete vaccination records were excluded from the study.
Definitions of COVID-19 and outcomes
The outcomes investigated in this study were (i) COVID-19 diagnosis; (ii) COVID-19-related hospitalization within 28 days after COVID-19 diagnosis; and (iii) severe COVID-19 defined as any diagnosis of complications or requiring procedures (including ventilatory support) listed in Supplementary Table 2, prescription of tocilizumab, methylprednisolone or intravenous immunoglobulin G, admission to an intensive care unit (ICU), or death within 28 days after COVID-19 diagnosis.
COVID-19 diagnosis was defined as a positive PCR result obtained from the CHP of the HKSAR government and/or HA databases. A positive PCR result is recognized as the gold-standard test for COVID-19 given its high specificity of >99% [Citation29]. The Hong Kong government has implemented extensive PCR testing for SARS-CoV-2 in public hospitals and clinics for those presenting with COVID-like symptoms and for close contacts of confirmed cases. The government has also set up territory-wide community testing centres to screen asymptomatic individuals and provide regular testing for staff at high risk of exposure to SARS-CoV-2, such as those working in nursing homes. A sensitivity analysis was conducted where voluntarily reported positive RAT cases were also included in the definition for COVID-19. Information regarding all-cause mortality was extracted from the Hong Kong Deaths Registry, the official governmental registry covering all registered deaths in Hong Kong.
Statistical analysis
Conditional logistic regressions adjusted for pre-existing asthma, diabetes, epilepsy, and use of immunosuppressants within 90 days were applied to calculate the crude and adjusted odds ratio (OR) with 95% confidence interval [Citation30]. Vaccine effectiveness (VE) was estimated by (1 – adjusted OR) × 100%. Subgroup analyses were conducted and were stratified by age (3–11 and 12–17 years). To evaluate waning VE, additional analyses were conducted at five time-since-vaccination intervals (0–13, 14–60, 61–120, 121–180, and ≥180 days) after receiving each vaccine dose. For each time-since-vaccination interval, only eligible matched pairs, in which both cases and controls were either unvaccinated or fell within the specific time-since vaccination interval, were included to derive the corresponding estimates. Two sensitivity analyses were conducted. First, cases and controls who received their last vaccine dose for more than 90 days were excluded. Second, both PCR and RAT positive cases were recognized in the definition of COVID-19.
All statistical tests were two-sided and P values less than 0.05 were considered statistically significant. Statistical analyses were conducted using R version 4.0.3 (www.R-project.org). Two investigators (VY and FC) conducted the statistical analyses independently for quality assurance. STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement checklists were followed to guide the transparent reporting in this case–control study.
Ethical approval
This study was approved by the Central Institutional Review Board of the Hospital Authority of Hong Kong (CIRB-2021-005-4) and the Department of Health Ethics Committee (LM171/2021).
Results
From 1 January to 15 August 2022, a total of 36,434 COVID-19 cases, 2231 COVID-19-related hospitalizations, and 1918 severe COVID-19 cases were matched to 109,004, 21,788 and 18,823 controls, respectively (). The baseline characteristics of cases and controls are summarized in . During the study period, eight COVID-19-related deaths and 99 cases of COVID-19-related ICU admission or ventilatory support were identified, which are summarized in Supplementary Table 3. Among the eight COVID-19-related deaths (mean [SD] age of 8.75 [5.06] years and 50% male), six (75%) were unvaccinated, one (12.5%) was vaccinated with one dose of BNT162b2, and one (12.5%) was vaccinated with one dose of CoronaVac.
Table 1. Baseline characteristics of cases and controls.
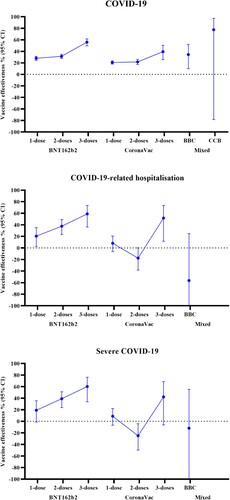
A dose–response relationship was observed in the VE against COVID-19, in which VE increased with more vaccine doses ( and ). In children and adolescents who received two vaccine doses, VE (95% CI) was 31.3% (27.8; 34.7) for BNT162b2 and 21.7% (17.0; 26.2) for CoronaVac. In children and adolescents who received three vaccine doses, VE was observed to be higher at 56.0% (49.6; 61.6) for BNT162b2 and 39.4% (25.6; 50.6) for CoronaVac.
Table 2. Vaccine effectiveness among children and adolescents with different vaccination status.
A similar dose–response relationship was observed for the VE of BNT162b2 against COVID-19-related hospitalizations and severe COVID-19. In children and adolescents who received BNT162b2, VE (95% CI) was 37.6% (23.4; 49.1) against COVID-19-related hospitalization and 39.1% (23.8; 51.2) against severe COVID-19 after two doses, which increased to 58.9% (36.1; 73.6) and 60.2% (33.7; 76.1) after three doses. For CoronaVac, there was a significant reduction in the risk for COVID-19-related hospitalizations and a trend toward a reduction for severe COVID-19 after three doses, with VE (95% CI) of 51.7% (11.6; 73.6) and 42.2% (−6.2; 68.6), respectively. However, there was no observed risk reduction after two doses (adjusted OR [95% CI] for hospitalization: 1.175 [0.998–1.383]; severe COVID-19: 1.249 [1.042–1.497]).
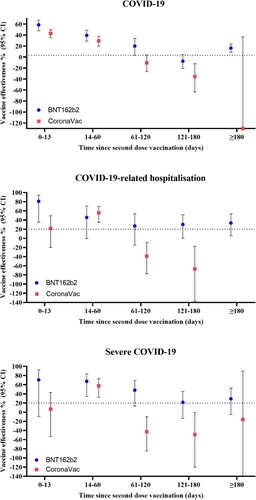
A significant waning of effectiveness against all outcomes was observed for both vaccines over time ( and ). For BNT162b2, VE against COVID-19 and severe COVID-19 peaked at 0–13 days after the second dose, which remained effective up to 61–120 days. For CoronaVac, VE against COVID-19, hospitalization, and severe COVID-19 peaked at 14–60 days, with no significant risk reduction by 61–120 days. However, the trend of waning effectiveness after the third dose was less clear due to the limited number of children and adolescents receiving a third dose for more than 60 days. When waning effectiveness was accounted for by restricting the analyses to those who received their last vaccine dose within 90 days, there was a significant risk reduction in COVID-19-related hospitalizations after two and three doses of CoronaVac (VE [95% CI] two doses: 21.5 [2.1; 37.1], three doses: 50.9% [2.7; 75.2]), and a significant risk reduction against severe COVID-19 with three doses of CoronaVac (VE [95% CI]: 49.4% [1.5; 74.0]) (Supplementary Table 4).
Table 3. Vaccine effectiveness for different time-since-vaccination intervals.
Consistent results were observed between those aged 3–11 and 12–17 years (), and when RAT-positive cases were included in the definition of COVID-19 (Supplementary Table 5).
Table 4. Subgroup analyses by age.
Discussion
Our study evaluated the real-world effectiveness of mRNA (BNT162b2) and inactivated virus (CoronaVac) COVID-19 vaccines among the pediatric population in Hong Kong during the Omicron-dominant pandemic. The results showed a clear dose–response relationship between the number of vaccine doses received and the level of vaccine effectiveness against COVID-19, hospitalizations, and severe symptoms. There was a reduction in VE starting from 60 days after the second dose of both vaccines, which highlights the need for booster doses in children and adolescents.
Our VE estimates during the Omicron predominant wave were generally lower than those in other studies, even after booster doses. For BNT162b2, we observed a VE of 31.3% against infection after two doses and a VE of 56.0% after a third dose compared to the reported VE ranging from 48% to 65% in two-dose BNT162b2 recipients aged 5–11 in the Singapore and Israel studies [Citation7,Citation10]. Our results for the VE against COVID-19-related hospitalizations were also lower than those reported by other studies. In the Singapore study, VE against hospitalization was reported to be higher than 80%. A similar study in the US that stratified individuals based on variants and age groups also reported higher VE estimates against hospitalization (Aged 12-17: 40% vs. 23.1%; Aged 5-11: 68% vs. 45.3%) [Citation8]. For CoronaVac, our results showed VE against infection and hospitalization were 21.7% and −17.5% after two doses, and 39.4% and 51.7% after three doses, whereas VE estimates after two doses were 40% and 60% in the Brazil and Chile studies, respectively [Citation11,Citation12]. One potential explanation for the lower VE levels in our study is the waning protection of both BNT162b2 and CoronaVac, which has been observed in both pediatric and adult populations [Citation9,Citation31]. The VE against infection peaked during the first 60 days after vaccination and then declined thereafter regardless of the number of vaccines doses. Previous studies demonstrated sustained protection against severe outcomes despite waning protection against infection in the general population [Citation31]. However, our results revealed the protection against hospitalization and severe outcomes started to diminish 2 months after vaccination, with more rapid waning in those receiving CoronaVac compared to BNT162b2. Another potential explanation was the introduction of the Vaccine Pass in Hong Kong, which limits unvaccinated individuals from visiting high-risk premises. As a result, the incidence of COVID-19 was underestimated in the unvaccinated group, and thus VE was also underestimated.
The mechanism of immune protection may account for a more rapid reduction in VE against COVID-19 over time, but to a lesser extent against COVID-19-related complications. Although humoral immunity mediated by antibodies blocks SARS-CoV-2 from entering host cells and thus prevents infection [Citation32], specific CD4+ and CD8+ T cells appear to be responsible for limiting disease severity [Citation33]. As a result, despite the rapid reduction of serum antibody titers, memory T cells are more durable and may contribute to the protection from severe disease.
The main strength of this study is that it is one of the first to provide real-world evidence on the effectiveness of both mRNA (BNT162b2) and inactivated virus (CoronaVac) vaccines against different Omicron subvariants among the pediatric population. Our findings highlight the importance of booster doses for reducing the risk of COVID-19 and preventing subsequent COVID-19-related complications including hospitalization and severe outcomes. Nonetheless, the findings of this study need to be interpreted with the following caveats. First, given the limited number of individuals receiving heterologous booster following the primary vaccine course, the VE of a booster dose against several outcomes could not be fully evaluated in this study. Second, a negative case-control study design is not feasible because only positive PCR or RAT results are reported to the DH. Moreover, there is a possibility that people with asymptomatic COVID-19 could be misclassified as controls, leading to a bias in the estimates towards the null. Asymptomatic COVID-19 cases cannot be captured unless nationwide screening is conducted, which at present is not possible in most countries. Furthermore, it is also possible that people with COVID-19 were misclassified due to false negative PCR results, but this is likely to be minimal due to the high specificity, and even less likely for severe cases. Thirdly, although the time-since-vaccination interval was not adjusted in our primary analysis, the assessment of waning vaccine effectiveness was stratified. Lastly, only patients who attended HA services could be included as control in our study. However, the selection bias was minimized during the matching process and with the adjustment of comorbidities during conditional logistic regression.
In conclusion, three doses of either BNT162b2 or CoronaVac vaccine is effective in preventing COVID-19, hospitalizations, and severe outcomes in children and adolescents during the Omicron variant-dominant pandemic. Our findings highlight the need for booster doses to enhance vaccine effectiveness in children and adolescents.
Conflict of interest
FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, outside the submitted work. XL has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region; research and educational grants from Janssen and Pfizer; internal funding from the University of Hong Kong; and consultancy fees from Merck Sharp & Dohme, unrelated to this work. EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, and the Hong Kong Research Grants Council, outside the submitted work. CKHW reports receipt of research funding from the EuroQoL Group Research Foundation, the Hong Kong Research Grants Council, and the Hong Kong Health and Medical Research Fund; outside of the submitted work. EWYC reports honorarium from Hospital Authority; and grants from Research Grants Council (RGC, Hong Kong), Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and Narcotics Division of the Security Bureau of the Hong Kong Special Administrative Region, outside the submitted work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, MSD, and Amgen; and personal fees from PrimeVigilance; outside the submitted work. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, National Institute for Health Research in England, European Commission, and the National Health and Medical Research Council in Australia; has received speaker fees from Janssen and Medice in the previous 3 years; and is an independent non-executive director of Jacobson Medical in Hong Kong. All other authors declare no competing interests. PI has received funding from the Hong Kong Research Grants Council, the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, and Hong Kong Jockey Club Charities Trust.
Data sharing statement
Data will not be available for others as the data custodians have not given permission.
Supplemental Material
Download MS Word (36 KB)Acknowledgement
We gratefully acknowledge the Centre for Health Protection, Department of Health and Hospital Authority for facilitating data access. We would also like to thank Edmund Lane for the professional editing of the manuscript.
Disclosure statement
FTTL has been supported by the RGC Postdoctoral Fellowship under the Hong Kong Research Grants Council and has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, outside the submitted work. XL has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region; research and educational grants from Janssen and Pfizer; internal funding from the University of Hong Kong; and consultancy fees from Merck Sharp & Dohme, unrelated to this work. EYFW has received research grants from the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, and the Hong Kong Research Grants Council, outside the submitted work. CKHW reports receipt of research funding from the EuroQoL Group Research Foundation, the Hong Kong Research Grants Council, and the Hong Kong Health and Medical Research Fund; outside of the submitted work. EWYC reports honorarium from Hospital Authority; and grants from Research Grants Council (RGC, Hong Kong), Research Fund Secretariat of the Food and Health Bureau, National Natural Science Fund of China, Wellcome Trust, Bayer, Bristol-Myers Squibb, Pfizer, Janssen, Amgen, Takeda, and Narcotics Division of the Security Bureau of the Hong Kong Special Administrative Region, outside the submitted work. CSLC has received grants from the Food and Health Bureau of the Hong Kong Government, Hong Kong Research Grant Council, Hong Kong Innovation and Technology Commission, Pfizer, IQVIA, MSD, and Amgen; and personal fees from PrimeVigilance; outside the submitted work. ICKW reports research funding outside the submitted work from Amgen, Bristol-Myers Squibb, Pfizer, Janssen, Bayer, GSK, Novartis, the Hong Kong Research Grants Council, the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, National Institute for Health Research in England, European Commission, and the National Health and Medical Research Council in Australia; has received speaker fees from Janssen and Medice in the previous 3 years; and is an independent non-executive director of Jacobson Medical in Hong Kong. All other authors declare no competing interests. PI has received funding from the Hong Kong Research Grants Council, the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region, and Hong Kong Jockey Club Charities Trust.
Additional information
Funding
References
- Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095.
- Delahoy MJ, Ujamaa D, Whitaker M, et al. Hospitalizations associated with COVID-19 among children and adolescents — COVID-NET, 14 states, March 1, 2020–August 14, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1255–1260.
- Tso WWY, Kwan MYW, Wang YL, et al. Severity of SARS-CoV-2 Omicron BA.2 infection in unvaccinated hospitalized children: comparison to influenza and parainfluenza infections. Emerg Microbes Infect. 2022;11:1742–1750.
- Taylor CA, Whitaker M, Anglin O, et al. COVID-19–associated hospitalizations among adults during SARS-CoV-2 delta and Omicron variant predominance, by race/ethnicity and vaccination status — COVID-NET, 14 states, July 2021–January 2022. Morb Mortal Wkly Rep. 2022;71:466.
- Chua GT, Kwan MYW, Chui CSL, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following comirnaty vaccination. Clin Infect Dis. 2022;75(4):673–681.
- Lai FTT, Li X, Peng K, et al. Carditis after COVID-19 vaccination with a messenger RNA vaccine and an inactivated virus vaccine: a case-control study. Ann Intern Med. 2022;175(3):362–370.
- Cohen-Stavi CJ, Magen O, Barda N, et al. BNT162b2 vaccine effectiveness against Omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:227–236.
- Price AM, Olson SM, Newhams MM, et al. BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899–1909.
- Sacco C, Del Manso M, Mateo-Urdiales A, et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5-11 years in Italy: a retrospective analysis of January-April, 2022. Lancet. 2022;400:97–103.
- Tan SHX, Cook AR, Heng D, et al. Effectiveness of BNT162b2 vaccine against Omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:525–532.
- Florentino PTV, Alves FJO, Cerqueira-Silva T, et al. Vaccine effectiveness of CoronaVac against COVID-19 among children in Brazil during the Omicron period. Nat Commun. 2022;13:4756.
- Jara A, Undurraga EA, Zubizarreta JR, et al. Effectiveness of CoronaVac in children 3-5 years of age during the SARS-CoV-2 Omicron outbreak in Chile. Nat Med. 2022;28:1377–1380.
- Cheng FWT, Fan M, Wong CKH, et al. The effectiveness and safety of mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines among individuals with chronic kidney diseases. Kidney Int. 2022;102(4):922–925
- Chui CSL, Fan M, Wan EYF. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) covid-19 vaccination: a self-controlled case series study. eClinicalMedicine. 2022;50:101504.
- Lai FTT, Chua GT, Chan EWW, et al. Adverse events of special interest following the use of BNT162b2 in adolescents: a population-based retrospective cohort study. Emerg Microbes Infect. 2022;11:885–893.
- Lai FTT, Huang L, Chui CSL, et al. Multimorbidity and adverse events of special interest associated with Covid-19 vaccines in Hong Kong. Nat Commun. 2022;13:411.
- Leung TYM, Chan AYL, Chan EW, et al. Short- and potential long-term adverse health outcomes of COVID-19: a rapid review. Emerg Microbes Infect. 2020;9:2190–2199.
- Li X, Tong X, Yeung WWY, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022;81(4):564–568.
- Li X, Gao L, Tong X, et al. Autoimmune conditions following mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: A descriptive cohort study among 1.1 million vaccinated people in Hong Kong. J Autoimmun. 2022;130:102830.
- Sing CW, Tang CTL, Chui CSL, et al. COVID-19 vaccines and risks of hematological abnormalities: nested case-control and self-controlled case series study. Am J Hematol. 2022;97:470–480.
- Wan EYF, Chui CSL, Lai FTT, et al. Bell’s palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2022;22:64–72.
- Wan EYF, Chui CSL, Wang Y, et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: A self-controlled case series and nested case-control study. Lancet Reg Health West Pac. 2022;21:100393.
- Wan EYF, Wang Y, Chui CSL, et al. Safety of an inactivated, whole-virion COVID-19 vaccine (CoronaVac) in people aged 60 years or older in Hong Kong: a modified self-controlled case series. Lancet Healthy Longev. 2022;3:e491–e500.
- Wong CKH, Lau KTK, Xiong X, et al. Adverse events of special interest and mortality following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines in Hong Kong: A retrospective study. PLoS Med. 2022;19:e1004018.
- Wong CKH, Mak LY, Au ICH, et al. Risk of acute liver injury following the mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines. J Hepatol. 2022;77:1339–1348.
- Xiong X, Wong CKH, Au ICH, et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid. 2022;32(5):505–514.
- Ye X, Ma T, Blais JE, et al. Association between BNT162b2 or CoronaVac COVID-19 vaccines and major adverse cardiovascular events among individuals with cardiovascular disease. Cardiovasc Res. 2022;118(10):2329–2338.
- Huang YQ, Gou R, Diao YS, et al. Charlson comorbidity index helps predict the risk of mortality for patients with type 2 diabetic nephropathy. J Zhejiang Univ Sci B. 2014;15:58–66.
- Miller TE, Garcia Beltran WF, Bard AZ, et al. Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital. FASEB J. 2020;34:13877–13884.
- Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969.
- Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:e83.
- Wherry EJ, Barouch DH. T cell immunity to COVID-19 vaccines. Science. 2022;377:821–822.
- Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-Specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19.