ABSTRACT
SARS-CoV-2 Omicron subvariants have become the predominantly strain in most countries. However, the neutralizing activity of the human serum after Omicron-based vaccine booster against different SARS-CoV-2 variants is poorly understood. Here, we developed an update Omicron vaccine (SCoK-Omicron), based on the RBD-Fc fusion protein vaccine (SCoK) and RBD domain of Omicron BA.1. To assess cross-variant neutralizing activity in adults, 25 volunteers that have received three doses of SCoK and 25 volunteers with two doses of CoronaVac (inactive vaccine) were further boosted with a dose updated vaccine (SCoK-Omicron). The results of pseudovirus neutralization assays demonstrated that the booster potently induced the high-level of neutralizing antibody against SARS-CoV-2 Wild type, Delta and Omicron subvariants in adults. Further assays of single point mutations showed that K444T, L452R, N460K, or F486V was key mutations to cause immune evasion. Together, these data suggest that SCOK-Omicron can be used as a booster vaccine candidate in adults receiving subunit protein or inactivated vaccine in response to the epidemic of COVID-19 Omicron subvariants, and the mutation K444T, L452R, N460K, or F486V needs to be considered in future vaccine design.
SARS-CoV-2 Omicron has spread all over the world and become the predominant strain in most countries. This variant carries more than 15 mutations in the receptor-binding domain (RBD) (Figure S1). Many of these mutations, such as K417Y, G446S, E484A, and Q493R, have been reported to impair neutralizing antibodies [Citation1]. Several studies regarding omicron-based vaccine booster have been published [Citation2–4], but most of studies used animal models. The neutralizing activity of the human serum against different SARS-CoV-2 variants after the Omicron-based vaccine booster is poorly understood.
In the early stages of the pandemic, we designed an RBD-Fc fusion protein vaccine (SCoK) [Citation5], which underwent Phase I/II clinical trials [Citation6]. To meet the challenge of Omicron variants, we developed an updated vaccine, containing RBD of Omicron BA.1 (SCoK-Omicron), which could significantly increase neutralization against SARS-CoV-2 Omicron subvariants in mouse and macaque models [Citation7]. To assess cross-variant neutralizing activity in adults, 25 volunteers that had received three doses of SCoK and 25 volunteers with two doses of CoronaVac (inactive vaccine, Sinovac biotech.) were further boosted with a dose of the updated vaccine (SCoK-Omicron). In addition, as a control, 25 volunteers with three doses of SCoK were boosted with a dose of primary vaccine (SCoK). The detailed vaccination time and other information were shown in Table S1. The volunteers among the three vaccination groups were with balanced sex and age distribution. The sera were collected before and 14 days after booster immunization. To evaluate the immunogenicity of this variant vaccine, the IgG antibody titers were detected, which were significantly increased (more than 15-fold) compared with those before booster immunization both with RBD–Wild type and with RBD-Omicron BA.1 as antigen (Figure S2, P < 0.001). Then, we performed pseudovirus neutralization assays against the sera obtained from the three booster-immunized groups. In the 25 individuals that had received three doses of SCoK, the SCoK-Omicron booster induced detectable neutralizing antibodies against Wild type, Delta, and Omicron BA.1, BA.2, BA.2.12.1, BA.2.75, and BA.3 in 25 (100%) individuals, and Omicron BA.4/BA.5, BF.7, BQ.1, XBB in 22 (88%), 23 (92%), 14 (56%), 23 (92%) individuals ((A), S3A). The geometric mean titers (GMTs) of Wild type, Delta, Omicron BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/BA.5, BF.7, BQ.1, and XBB were 1679, 2245, 1669, 1270, 1399, 1356, 749, 227, 218, 80, and 175, being significantly increased 56.0, 80.2, 66.8, 57.7, 73.6, 64.6, 37.5, 11.9, 11.5, 3.3, and 9.7 folds after SCoK-Omicron booster, respectively (P < 0.01). In contrast, after Wild type RBD–based vaccine SCoK booster, the neutralization titers against all of the tested Omicron subvariants were significantly lower compared with those against the Wild type (P < 0.001, (B)). In the SCoK booster group, the number of individuals with detectable BA.4/BA.5 or XBB-neutralizing antibodies decreased to 15 (60%); the neutralization titers of Delta, Omicron BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/BA.5, BF.7, BQ.1, and XBB were significantly decreased (2.2, 3.9, 4.0, 3.2, 4.8, 3.4, 4.1, 3.0, 1.5, and 8.3 folds, respectively) compared with the SCoK-Omicron booster group (P < 0.001, (D)). These data show that the updated vaccine, SCoK-Omicron, is a better option than SCoK as a booster vaccine candidate against the Omicron subvariants, especially against BA.4/BA.5, and XBB. In the group with two doses of CoronaVac, the GMTs of Wild type, Delta, Omicron BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/BA.5, BF.7, BQ.1, and XBB significantly increased (14.0, 22.3, 13.5, 11.9, 16.6, 6.2, 6.2, 6.4, 5.7, 2.1, and 2.0 folds, respectively) after SCoK-Omicron booster ((C) and S3C). In this group, the number of individuals with detectable neutralizing antibodies remarkably increased to 22 (88%), 19 (76%), 22 (88%), 19 (76%), 19 (76%), 16 (64%), 16 (64%), 17 (68%), 16 (64%), 14 (56%), and 11 (44%) against Wild type, Delta, and Omicron BA.1, BA.2, BA.2.12.1, BA.2.75, BA.3, BA.4/BA.5, BF.7, BQ.1, and XBB, respectively (Figure S3C). These data suggest that SCoK-Omicron can be also used as a booster vaccine candidate in adults who received two doses of inactivated vaccine.
Figure 1. Neutralizing antibodies elicited by an Omicron BA.1–based updated RBD-Fc fusion protein vaccine booster against SARS-CoV-2 variants in adults. A-E, The 50% neutralizing titers (NT50) against SARS-CoV-2 Wild type, Delta, and Omicron subvariants VSV-based pseudoviruses. (A), Individuals who received three doses of RBD-Fc fusion protein vaccine (SCoK) and a booster Omicron BA.1–based updated RBD-Fc fusion protein vaccine (SCoK-Omicron) (n = 25). (B), Individuals who received three doses of RBD-Fc fusion protein vaccine (SCoK) and a booster RBD-Fc fusion protein vaccine (SCoK) (n = 25). (C), Individuals who received two doses of inactivated vaccine (CoronaVac) and a booster Omicron BA.1–based updated RBD-Fc fusion protein vaccine (SCoK) (n = 25). (D), Comparison of two immunization groups that received a booster of the original or updated vaccine (SCoK + SCoK-Omicron vs. SCoK + SCoK). (E, F), The NT50 of two immunization groups (E, SCoK + SCoK-Omicron; F, CoronaVac + SCoK-Omicron) against SARS-CoV-2 Wild type, Delta, and Omicron BA.1 live viruses before and after the booster. (G, I), The NT50 of three immunization groups (G, SCoK + SCoK-Omicron; H, SCoK + SCoK; I, CoronaVac + SCoK-Omicron) against SARS-CoV-2 Wild type, Omicron BA.4/BA.5, and two single-point mutation variants (L452R or F486V) VSV-based pseudoviruses. Data were analyzed by Student's t-test (normal distribution) or one-way ANOVA followed by Dunnett's multiple comparison test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant, P > 0.05. Geometric mean titers (GMT) and positive rates are labelled and annotated above each group of points. The horizontal dotted line indicates the positive seroconversion threshold of NT50 (30 in pseudovirus assay, 16 in live virus assay).
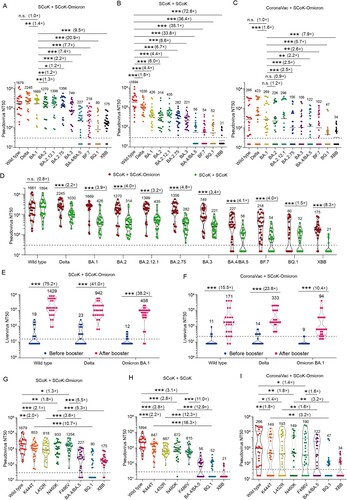
Moreover, the neutralizing antibody titers against SARS-CoV-2 Wild-type, Delta, and Omicron BA.1 live viruses were tested, which were significant positive correlation with the pseudovirus neutralization antibody titers (Figure S4, P < 0.001). In the 25 individuals who had received three doses of SCoK, the GMTs of Wild type, Delta, and Omicron BA.1 were 1429, 942, and 458, being significantly increased 75.2, 41.0, and 38.2 folds after SCoK-Omicron booster, respectively (P < 0.01, (E)). In the 25 individuals who had received two doses of CoronaVac, the GMTs of Wild type, Delta, and Omicron BA.1 were 171, 333, and 94, being significantly increased 15.5, 23.8, and 10.4 folds after SCoK-Omicron booster, respectively (P < 0.01, (F)). These results showed that the SCoK-Omicron booster was able to induce high neutralizing antibody titers against Wild type, Delta, and Omicron subvariants.
Using the pseudovirus neutralization assay, we found that Omicron BA.4/BA.5, BQ.1 and XBB had the lower neutralization titers among Wild type, Delta, and Omicron subvariants ((A–C)), suggesting that BA.4/BA.5, BQ.1 and XBB displays stronger humoral immune evasion than other Omicron subvariants, which is consistent with previous reports [Citation8–10]. To reveal the key amino acid sites associated with immune escape, the NT50s of pseudoviruses with spike-protein mutation (K444T, L452R, N460K, or F486V), which especially appears in Omicron BA.4/BA.5, BQ.1 or XBB, were detected (Figure S1). In the groups that received SCoK and booster SCoK-Omicron, the neutralization titers of K444T, L452R, N460K, or F486V mutation were significantly lower than those of Wild type but significantly higher than those of Omicron BA.4/BA.5, BQ.1 or XBB (P < 0.05, (G)). Similar changes were detected in the neutralization titers of K444T, L452R, N460K, or F486V mutation in the group with SCoK and booster SCoK or the group with CoronaVac and booster SCoK-Omicron ((H, I)). Together, these observations suggest that although K444T, L452R, N460K, or F486V may be key mutations, it has to act in concert with other mutations to cause immune evasion in BA.4/BA.5, BQ.1 or XBB.
Discussion
Notably, vaccination is one of the effective ways to control SARS-CoV-2 transmission and reduce the disease severity. However, the antibody level significantly drops several months after the standard two- or three-dose vaccination, and thus, a third or fourth dose booster has been recommended to boost the antibody response. In this study, we presented the immunogenicity of an Omicron BA.1 RBD–based vaccine SCoK-Omicron in adults who had received subunit or inactivated vaccines; specifically, we demonstrated that such booster immunization elicited potent neutralizing antibodies not only against the SARS-CoV-2 Wild type but also against Delta and Omicron subvariants. Booster vaccination with our updated vaccine SCoK-Omicron represents a rational strategy in response to the epidemic of COVID-19 VOCs, especially Omicron subvariants. In addition, this study has several limitations. First, we just evaluated the neutralizing abilities of sera from adults, who had received subunit or inactivated vaccines. Further study is needed to test the booster vaccine's activity in adults, who had received other kinds of vaccines, like mRNA and adenovirus-based vaccines. Second, vaccine immunogenicity was assessed in this study at days 14 after booster, which did not assess the extended duration of the immune response. Immune responses at later timepoints, including those at least 6 months post booster vaccination, will be evaluated in a follow-up investigation.
Supplemental Material
Download MS Word (630 KB)Acknowledgements
The authors wish to thank Yong Zhang, Shuangli Zhu and Dongmei Yan (National Institute for Viral disease and Prevention, Chinese Center for Disease Control and Prevention) for the experiments about live virus.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data are available in this article, supplementary materials, or available from the corresponding authors.
Additional information
Funding
References
- Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602(7898):657–663.
- Lam JY, Ng YY, Yuen CK, et al. A nasal omicron vaccine booster elicits potent neutralizing antibody response against emerging SARS-CoV-2 variants. Emerg Microbes Infect. 2022;11(1):964–967.
- Muik A, Lui BG, Bacher M, et al. Exposure to BA.4/5 S protein drives neutralization of Omicron BA.1, BA.2, BA.2.12.1, and BA.4/5 in vaccine-experienced humans and mice. Sci Immunol. 2022;7(78):eade9888.
- Scheaffer SM, Lee D, Whitener B, et al. Bivalent SARS-CoV-2 mRNA vaccines increase breadth of neutralization and protect against the BA.5 Omicron variant in mice. Nat Med. 2023;29:247–257. doi:10.1038/s41591-022-02092-8
- Sun S, He L, Zhao Z, et al. Recombinant vaccine containing an RBD-Fc fusion induced protection against SARS-CoV-2 in nonhuman primates and mice. Cell Mol Immunol. 2021;18(4):1070–1073.
- Luo D, Pan H, He P, et al. A randomized, double-blind, placebo-controlled phase 1 and phase 2 clinical trial to evaluate efficacy and safety of a SARS-CoV-2 vaccine SCoK in adults. Clin Transl Med. 2022;12(9):e1016.
- Luo D, Yang X, Li T, et al. An updated RBD-Fc fusion vaccine booster increases neutralization of SARS-CoV-2 Omicron variants. Signal Transduct Target Ther. 2022;7(1):327.
- Cao Y, Yisimayi A, Jian F, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608(7923):593–602.
- Kurhade C, Zou J, Xia H, et al. Low neutralization of SARS-CoV-2 Omicron BA.2.75.2, BQ.1.1 and XBB.1 by parental mRNA vaccine or a BA.5 bivalent booster. Nat Med. 2023;29:344–347. doi:10.1038/s41591-022-02162-x
- Uraki R, Ito M, Furusawa Y, et al. Humoral immune evasion of the omicron subvariants BQ.1.1 and XBB. Lancet Infect Dis. 2023;23(1):30–32.

