ABSTRACT
Prospective cohort study to investigate the potential exposure to the Middle East Respiratory Syndrome-Coronavirus (MERS-CoV) following Hajj pilgrims is still very limited. Here, we report the antibody seroconversion study results obtained from successive three years cohort studies (2016–2018) involving the Malaysian Hajj pilgrims returning from the Middle East. A cohort study of Hajj pilgrims from Malaysia enrolled 2,863 participants from 2016–2018, all of whom consented to provide paired blood samples for both pre- and post-Hajj travel to the Middle East. ELISAs and micro-neutralization assays were performed to detect the presence of MERS-CoV IgG antibodies. Sociodemographic data, symptoms experienced during Hajj, and history of exposure to camels or camel products were recorded using structured pre- and post-Hajj questionnaires. A 4-fold increase in anti-MERS-CoV IgG between paired pre-Hajj and post-Hajj serum samples in twelve participants was observed. None of the twelve ELISA-positive sera had detectable levels of virus-neutralizing antibodies. All reportedly had mild symptoms of respiratory symptoms at a certain point during the pilgrimage, implying mild or asymptomatic infections. No association between post-Hajj serum positivity and a history of exposure to camels or camel products was obtained. Findings from the study suggest that serologic conversion to MERS-CoV occurred in at least 0.6% of the Hajj pilgrims returning from the Middle East. Since all the seroconvertants had mild to no symptoms during the sampling period, it highlights the likelihood of occurrence of only low infectivity spillover infections among the Hajj pilgrims.
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic, caused by the Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) virus, is so far responsible for more than 6.5 million deaths [Citation1] and billions of dollars in economic losses worldwide [Citation2]. Other than SARS-CoV-2, the coronavirus family also includes six other human coronaviruses, including the Middle East Respiratory Syndrome- Coronavirus (MERS-CoV) and Severe Acute Respiratory Syndrome- Coronavirus- 1 (SARS-CoV-1), both of which have caused deadly outbreaks during the past 20 years [Citation3]. While there have been no additional reported cases of SARS-CoV-1 reported anywhere in the world since 2004, MERS-CoV continues to cause sporadic cases in the Middle East region [Citation4]. With a case fatality rate that can be as high as 35%, the highest amongst these coronaviruses, MERS-CoV, remains on the World Health Organization’s (WHO) R&D Blueprint list of priority diseases as one of the major threats to global health security [Citation5].
To date, cases of MERS-CoV have been reported in 27 countries, including the following Middle Eastern countries: Jordan, Saudi Arabia, Qatar, the United Arab Emirates, Oman, Kuwait, Yemen, Lebanon, and Iran. Other countries that have reported MERS-CoV cases include the United States, France, Italy, Germany, Greece, the United Kingdom and the Republic of Korea (South Korea) – all of the primary cases involved a history of travel to the Middle East [Citation6]. The outbreak in the Republic of Korea in 2015 is the largest MERS-CoV outbreak outside of the Middle East, which saw 185 confirmed cases and reported 36 deaths, resulting from a single exported case with a travel history in the Middle East [Citation7].
As demonstrated with other coronaviruses, high population mobility and large mass gatherings have the potential to facilitate MERS-CoV spread [Citation8]. Kingdom of Saudi Arabia (KSA) hosts the annual Hajj pilgrimage, which attracts over two million pilgrims from more than 180 countries in one of the largest annual religious mass gatherings worldwide [Citation9]. Similar to other mass gathering events, there are concerns that such an event involving mass movement of people in highly congested and crowded areas with known endemic infections could result in rapid global transmission of the infection beyond the region [Citation10,Citation11]. Previous attempts to detect MERS-CoV infection among returning Hajj pilgrims from the Middle East using RT–PCR in several countries did not confirm any positive MERS-CoV cases [Citation12–24], suggesting that the risk of contracting the infection from Hajj pilgrimage returnees is potentially low. Most of these earlier studies, however, were cross-sectional or “snapshot” studies to sample only those that are potentially having an acute infection during the sampling upon returning from the Middle East, thus, would miss those that were exposed much earlier during/at the height of the pilgrimage itself and among those with mild or asymptomatic infection. In the present study, we conducted a prospective cohort study involving paired serological samplings for MERS-CoV IgG antibodies of pilgrims from Malaysia who went on the Hajj pilgrimage between 2016 - 2018.
Materials & methods
Study design and population
A multiyear prospective cohort study was conducted amongst Malaysian pilgrims who performed Hajj between 2016 and 2018, were aged 18 years and above, and were residing around the Greater Kuala Lumpur metropolitan area (including Putrajaya and Selangor). The study protocol was based on MERCURIAL, a longitudinal seroconversion surveillance study by Johari et al. [Citation25]. This study received IRB ethical approval from the Medical Research and Ethics Committee (MREC), Ministry of Health, Malaysia (NMRR-15-1640-25391).
Pre-Hajj enrolment
Pre-Hajj enrolment and serum sampling were conducted during routine health screenings of the prospective Malaysian Hajj pilgrims at six healthcare institutions around the Greater Kuala Lumpur metropolitan area [Citation25]. Participants in the study were provided with an overview of the study's scope, objectives, and procedures. Before any screening or enrolment procedures, all eligible study participants provided written informed consent. The participants’ privacy rights were preserved at all times. A pre-Hajj questionnaire was administered to obtain demographic data, general health information, and travel history. Each participant provided a 5 mL pre-Hajj blood sample (baseline sample) collected in a sterile serum-separating tube. Each participant's serum sample tube and pre-Hajj questionnaire were labelled with a unique identification number. Serum samples were handled according to standard biosafety measures and transported to the laboratory on the same day and stored at −20°C before analysis.
Post-Hajj follow-up
The post-Hajj follow-up and serum sampling from the enrolled volunteers were conducted upon arrival in the arrival lounge of the Kuala Lumpur International Airport (KLIA), Malaysia. The Hajj pilgrims were required to pass through a thermal scanner upon disembarkment. Participants having a body temperature of 38°C or higher were directed to a separate screening booth operated by the Ministry of Health Malaysia for further evaluation. Meanwhile, healthy study participants were directed to the designated study stations. A post-Hajj questionnaire was distributed to collect information from the study volunteers who experienced any form of respiratory symptoms during the pilgrimage. In addition, data was also gathered regarding visits to camel farms, as well as exposure to camels and untreated camel products. A second blood sample (5 mL follow-up sample) was obtained from the enrolled volunteers in a sterile serum-separating tube and labelled with the patient's name, identification number, and collection date. Serum samples were packaged and transported via proper cold-chain procedures in insulated boxes with ice packs to the TIDREC laboratory on the same day that they were collected. All serum samples were stored at −20°C until needed for the laboratory investigation.
Serologic assay
Paired pre- and post-Hajj serum samples were tested for the presence of MERS-CoV-specific antibodies using the commercially available anti-MERS-CoV IgG rS1 ELISA kit (EUROIMMUN, Lübeck, Germany) according to the manufacturer’s guidelines. Similar kits were used by several other earlier studies [Citation26–29]. The preliminary cut-off value was calculated as mean optical density (OD) + 3 SDs of at least 1,000 seronegative serum samples. Serum samples with OD values greater than the cut-off point (≥ 0.3) were classified as seropositive. A four-fold (4) increase in MERS-CoV-specific IgG titres in post-Hajj serum samples from previously seronegative patients suggested seroconversion, implying recent MERS-CoV infection.
Microneutralization assay
A microneutralization test developed in-house by the Naval Medical Research Center (NMRC, Silver Spring, Maryland) was used to detect the presence of MERS-CoV neutralizing antibodies. The live, non-patented MERS-CoV strain Jordan-N3/2012 used in the in-house test was previously obtained by NMRC as part of the collaboration with it’s overseas laboratory in Cairo, Egypt (NAMRU-3), the World Health Organization (WHO) Eastern Mediterranean Regional Office, and the Jordanian Ministry of Health. Briefly, positive anti-MERS-CoV IgG serum samples were heat-inactivated and serially diluted. Samples were then mixed with live MERS-CoV before incubation with Vero cells. Cells were fixed and analyzed via quantitative ELISA after a 2-day incubation period. Viral neutralization for the dilutions was considered when the ELISA OD values were at least 50% of the OD values of the virus control. The microneutralization assays were conducted entirely within TIDREC’s biosafety level 3 (BSL-3) containment facility.
Managing missing data/values
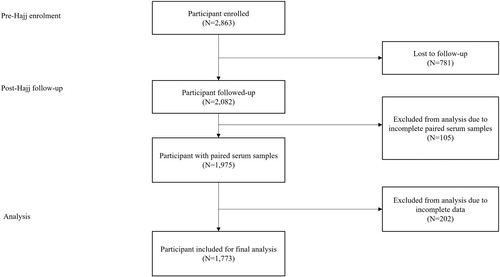
The listwise deletion method was applied to eliminate participants with missing pre- or post-Hajj survey data. Thus, of the 2,863 Malaysian pilgrims enrolled in this study, only 2,082 participants completed the pre- and post-Hajj surveys and thus were included in the data analysis. Paired pre- and post-Hajj serum samples were obtained from 1,975 participants; however, demographic data related to a history of camel exposure were available for only 1,773 (89.8%) of the total serum samples. A maximum of 10.2% (N = 202) of missing data were detected at random (). The variables were not deleted, but the missing values were re-coded as “−1” into the same variables and excluded from IBM SPSS Software analysis.
Statistical analysis
Data analysis was performed using IBM SPSS Software version 25. Background characteristics of the participants and seroconversion rate were analyzed using univariate analysis. Results were presented in frequency (n) and percentage (%). 95% confidence intervals (CI) were calculated for seropositivity. The Chi-square Test of Independence, Fisher’s exact test, and logistic regression analysis were applied to find the association between post-Hajj seropositivity and history of camel exposure. P-values <0.05 was considered statistically significant.
Results
Demographic characteristics
The demographic characteristics of the Malaysian Hajj pilgrims during the 2016–2018 Hajj seasons were summarized in . A total of 2,082 pre-Hajj and post-Hajj demographic data were analyzed. The mean age of all participants was 50 years (range: 18–84 years) with the majority being between the ages of 51 and 60 (33.2%, N = 692). The study population contained slightly more females (54.5%, N = 1,134) than males (45.5%, N = 948). Hypertension (26.8%, N = 559) and diabetes (15.6%, N = 325) are the two most frequent underlying diseases reported by the participants. Asthma and chronic heart disease accounted for 5.9% (N = 123) and 2.4% (N = 51), respectively. Other health conditions such as chronic lung disease, chronic kidney disease, cancer and weakened immune system accounted for less than 1%.
Table 1. Demographic characterictics of Malaysian pilgrims during the 2016–2018 Hajj seasons.
Common respiratory symptoms among the pilgrims
The most common presenting respiratory symptom among the pilgrims was cough (91.7%, N = 1,910), followed by sore throat (78.1%, N = 1,626) and runny/stuffy nose (73.6%, N = 1533). Fever was reported among 1,110 (53.3%) participants while muscle/body aches, headache, and fatigue were reported among 532 (25.6%), 475 (22.8%), and 262 (12.6%) participants, respectively. A total of 183 (8.8%) pilgrims experienced diarrhea, 164 (7.9%) had shortness of breath, and 95 (4.6%) had vomiting ().
Table 2. Common presenting respiratory symptoms among Malaysian pilgrims during the 2016–2018 Hajj seasons.
Detection of MERS-CoV IgG antibody via ELISA
A total of 1,975 paired pre- and post-Hajj serum samples were obtained (). Specifically, 318, 905, and 752 paired serum samples were retrieved for the years 2016, 2017, and 2018, respectively. Out of the 1,975 tested serum samples, 50 (2.5%) pre-Hajj samples and 61 (3.1%) post-Hajj samples were found positive for anti-MERS-CoV IgG antibodies with OD values ≥ 0.3 cut-off point. Higher seropositivity was observed among samples collected from volunteers in 2018, with 20 out of 752 pre-Hajj samples (2.7%, 95% CI: 0.36–0.90) and 24 out of 752 post-Hajj samples (3.2%, 95% CI: 2.07–4.58) testing positive for MERS-CoV antibodies using ELISA. The 95% CI values represent the range of ELISA OD ratios observed among the seronegative and seropositive samples, where the cut-off value for seropositivity is set at 0.3. Out of the 61-post Hajj ELISA positive sera, 12 paired serum samples (0.6%) were found to have at least a 4-fold increase in the rS1-MERS-CoV specific IgG titre.
Table 3. Evaluation of pre- and post-Hajj IgG antibody for MERS-CoV using rS1 Enzyme-Linked Immunosorbent Assay (ELISA).
History of camel exposure
The incidence of exposure to camel and camel products was estimated using a self-reported post-Hajj questionnaire, and the association with MERS-CoV IgG seropositivity was further determined. A total of 20 (1.0%) individuals reported visiting a camel farm, eight (0.5%) had physical contact with camels, twelve (0.7%) consumed camel milk, nine (0.5%) consumed camel urine, and 46 (2.6%) consumed camel meat; however, all the exposed individuals had IgG titres below the selected 0.3 cut-off point, suggesting no exposure to MERS-CoV. Most of the seropositive individuals with an IgG titre of greater than 0.3 had no history of exposure to camel, except for 3 (0.2%) seropositive individuals who consumed camel meat (). The Chi-Square test, Fisher’s exact test, and logistic regression analysis suggested no association between post-Hajj serum positivity and a history of camel exposure (p > 0.05). The majority of the pilgrims did not visit any camel farm (99.0%), had no direct physical contact with camels (99.5%), and did not consume camel products such as milk (99.3%), urine (99.5%) or meat (97.2%). MERS-CoV IgG negative was greater than 90% among these individuals. Seronegativity was also prominent among individuals who reported exposure to camels.
Table 4. The association between post-Hajj serum seropositivity across history of camel exposure.
Detection of MERS-CoV neutralizing antibody
An in-house microneutralization assay was performed to confirm MERS-CoV seropositivity in the seropositive population who had at least a 4-fold increase in IgG titre. Out of the twelve rS1-ELISA positive sera, no samples were confirmed to have positive neutralizing antibodies against MERS-CoV (). Demographic characteristics showed that nine out of twelve individuals (75%) were above 50 years old. None of the 12 individuals had previously tested MERS-CoV ELISA positive during pre-Hajj screening, and none had a history of camel exposure during Hajj. Five seroconverted individuals stated that they had a medical history of diabetes and hypertension. All twelve seroconverted individuals described experiencing respiratory symptoms such as fever, cough, sore throat, runny and stuffy nose, as well as muscle and body aches at a certain point during their pilgrimage, but not on their return.
Table 5. Case findings of Twelve Seroconverted Hajj Pilgrims.
Discussion
Two imported cases in 2014 and 2018, were the only cases of confirmed MERS-CoV infection among Malaysians who travelled to the Middle East [Citation30,Citation31]. This is despite an estimated of over 400,000 Malaysians who travelled to the Middle East annually including those who performed the Umrah and Hajj pilgrimages [Citation32]. These would imply that exposure to MERS-CoV is likely low, especially among Malaysian Hajj pilgrims. The strict movement control of Malaysian Hajj pilgrims, in compliance with the guidance against visiting camel farms and consuming unpasteurized camel milk, urine, and milk could have contributed to the low incidence. Here, a prospective MERS-CoV seroconversion cohort surveillance study among Malaysian Hajj pilgrims was undertaken. This represents the first such study looking at seroconversion using paired blood sampling, pre- and post-travel. The MERS-CoV IgG seroprevalence in the 1,975 paired pre- and post-Hajj serum samples were assayed using the rS1-ELISA IgG serology test which specifically detects the recombinant spike protein (S) of MERS-CoV. A similar assay was previously used in several earlier studies where the performance of the assay was validated and standardized for both predicting neutralization activity in MERS-CoV-infected patients, and diagnosing MERS-CoV infection in epidemiologic surveillance purposes [Citation26–29]. Our findings utilizing the assay provided evidence for the presence of MERS-CoV S1-specific binding antibodies in 2.5% of the pre-Hajj samples and 3.1% post-Hajj samples. It is not presently known why 2.5% of the pre-Hajj samples were positive. Among the possibilities include that these pilgrims had previously been exposed to MERS-CoV or a yet-to-be-identified antigen cross-reacting MERS-CoV-liked virus. None of these pre-Hajj positive volunteers, however, had a four-fold rise in antibody titres against MERS-CoV upon their return from the pilgrimage.
Findings from our study suggest a 0.6% seroconversion rate or 600:100,000 was obtained for the study cohorts. However, since none of the twelve seroconverted volunteers had specific neutralizing antibodies against MERS-CoV, it raised the possibility that perhaps they had only mild subclinical or asymptomatic MERS-CoV infections which could have resulted in temporary and only weak neutralizing antibody responses that rapidly declined, resulting in the negative neutralization assay. Whereas, the general IgG lingers much longer to give elevated total IgG levels. In an earlier study, Muller et al. [Citation27] screened 10,009 individuals using the recombinant ELISA for MERS-CoV-specific IgG and found 1.52% anti-MERS-CoV antibodies in the serum samples. Subsequent tests using recombinant immunofluorescence assays (rIFA) and plaque reduction neutralization testing (PRNT) confirmed less than 0.2% seropositivity among these samples. In comparison, a recent study done by Degnah et al. using 7,461 archived serum samples obtained from healthy individuals of eighteen different nationalities in KSA between 2011 and 2016 detected 2.3% of MERS-CoV S1-specific antibodies via ELISA. Subsequently, only 9.8% of these ELISA-positive samples showed a detectable amount of MERS-CoV neutralizing antibody [Citation33]. Similar results were reported in other previous studies from Kenya [Citation34,Citation35], Pakistan [Citation36] and Morocco [Citation37]. These observations are consistent with our observation above that neutralizing immune response is not always present in MERS-CoV IgG-positive samples.
Respiratory symptoms are a common occurrence among Hajj pilgrims, as documented by a systematic review involving 61 papers conducted by Benkouiten et al. in 2019 [Citation38]. Findings from our study showed that the majority of our study participants reported they had acute respiratory symptoms including cough (91.7%), sore throat (78.1%), and runny/stuffy nose (73.6%), with about half of them (53.3%) had fever at some point during their pilgrimage. Despite the high proportion, the prevalence of the symptoms is comparable to French Hajj pilgrims in 2013; cough (86.8%), sore throat (82.9%), runny/stuffy nose (72.1%), and feverishness (49.6%) [Citation39]. The etiological agent causing the infections in our cohorts, however, is not known, since none were hospitalized with a serious infection, hence, no laboratory investigation for the identification of the causative agent was performed. Several other studies, however, have reported detecting hRVs and hCoV OC43 as among the most frequently detected viruses during Hajj, followed by influenza A [Citation14,Citation20,Citation40]. Co-infections with multiple viruses in symptomatic pilgrims were also relatively common during the Hajj season [Citation40]. This raised the possibility that some of the pilgrims could have been exposed to MERS-CoV but developed only mild symptoms, or was asymptomatic during the time they were performing the pilgrimage rituals. The low infectivity hence was insufficient to evoke adequate neutralizing antibody responses. This assertion is consistent with findings from recent studies that not all individuals with a history of MERS-CoV infection, especially those with mild or asymptomatic infections, could elicit detectable neutralizing antibodies, in comparison to the severe infections that in general would generate a much more robust polyclonal immune reaction [Citation41–43]. A similar observation was also reported in COVID-19 patients in several studies [Citation44–46].
The absence of exposure to camel or camel products among the seroconverted pilgrims suggests the unlikely hood of brief contact with camels as the source of the exposure to MERS-CoV among the pilgrims. This finding opens the possibility that the pilgrims could have been exposed to MERS-CoV from another sub-clinically infected individual during the conduct of the pilgrimage rituals. The human-to-human transmission while rare has been reported involving household members or healthcare staff that come into contact with MERS-CoV patients [Citation47–50]. The 2015 MERS-CoV outbreak in South Korea, highlights the contribution of human-to-human transmission in the spread of MERS-CoV [Citation51–53].
Limitations and future recommendations
There were several limitations to this study. The primary operational limitation was the timing of both the pre- and post-Hajj blood samplings. The pre-Hajj blood sampling was conducted during routine health screenings of the prospective Malaysian Hajj pilgrims, a few months before the Hajj pilgrimage. This was initially chosen because of logistics and field practicalities but could raise the possibility of the Hajj pilgrims acquiring MERS-CoV infection elsewhere during the period before their travel to the Middle East for the Hajj pilgrimage. Similarly, the timing of post-Hajj blood sampling at the airport, which was initially chosen due to the same reasons as above, could fall within the viral incubation window period, during which an increase in antibody levels may not yet be detectable by serological assays, and it was not possible to do a follow-up post-arrival, which could result in an underestimation of the true seroconversion rate. A four-week post-Hajj follow-up could be implemented in future cohorts to obtain better information of disease exposure throughout the entire pilgrimage period. Additionally, the difficulty of follow-up with study participants while they were in the Middle East may result in missing data points that pertain to the precise exposure time and any confounding variables. In future studies, pilgrims could be provided with a daily symptom tracker card to track respiratory issues they experience throughout their pilgrimage. The study could also benefit if the participants’ body temperatures could be constantly monitored in real-time with the use of a wearable temperature sensor, to allow for a more precise and thorough data collection.
The primary methodological concern identified in this work was the sensitivity and specificity of the microneutralization assay. Despite the use of a 50% endpoint, the current study showed undetectable neutralizing antibodies, implying insufficient sensitivity of the assay. The high specificity of the microneutralization assay may lead to reduced sensitivity, as the rS1-ELISA may lead to the detection of S1-binding antibodies rather than the neutralizing antibodies [Citation54]. A recent study showed that using the S1 protein, once the protein folding is properly optimized, as a specific antigen for MERS-CoV serology, a highly specific one-step diagnostic approach without false positives could be obtained. This will omit the need for a confirmatory assay such as the microneutralization assay, which has the potential for underestimation of the true prevalence of MERS-CoV in epidemiological studies [Citation55]. Additionally, another recent study demonstrated the presence of MERS-CoV-specific CD4 + and CD8+ T-cell responses following MERS-CoV infection irrespective of disease severity [Citation56,Citation57]. Therefore, rather than a neutralization assay, T-cell assays could be employed to confirm serologic findings, particularly in mild and asymptomatic cases [Citation57,Citation58]. However, in the present study, the lack of access to the assays and the need to use a higher volume of blood samples limits our capability to further investigate MERS-CoV-specific memory CD4 + and CD8+ T-cell responses.
Conclusion
Despite the persistent circulation of MERS-CoV in KSA until today and the conditions of overcrowding and intense human-to-human contacts during Hajj, the absence of positive MERS-CoV cases related to Hajj is puzzling. Findings from the present study highlight the possible occurrence of MERS-CoV seroconversion among Malaysian Hajj pilgrims, indicating the presence of an undetected pool of individuals with mild or no symptoms that may not be captured by the current surveillance system. The lack of contact between the seroconverted pilgrims and camels or camel products points to the need to undertake further investigation to determine the possible role of asymptomatic human-to-human transmission of MERS-CoV among the Hajj pilgrims.
Authors’ contribution
SAB, BLP, TH, NR, RMZ, JAGR, and JJ were involved in designing the study protocol. JJ, VT, HYL BTT, SSS, CSK, SKL, JAJ, SSN, HY, NCK were involved in the conduct of the study and data analysis. JJ, SAB, and RDH were responsible for preparing the manuscript. All authors were involved in the critical revision of the manuscript for intellectual content.
Supplemental Material
Download MS Word (33 KB)Acknowledgments
The authors acknowledge the support from the Ministry of Higher Education, Malaysia for niche area research under the Higher Institution Centre of Excellence (HICoE) program (Project MO002-2019). The authors also would like to thank the Director General of Health Malaysia for his permission to publish this article. The views expressed in this article reflect the results of research conducted by the author and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the United States Government. The study protocol was approved by the Ministry of Health Malaysia Institutional Review Board in compliance with all applicable federal regulations governing the protection of human subjects. R.D.H., B.L.P., T.H., and J.A.G.R. are military service members or federal/contracted employees of the United States government. This work was prepared as part of their official duties. Title 17 U.S.C. 105 provides that ‘copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. 101 defines U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person's official duties.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. [Cited 2022 Oct 31]. Available from: https://covid19.who.int/.
- Cutler DM, Summers LH. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324(15):1495–1496. doi:10.1001/jama.2020.19759.
- Zhu Z, Lian X, Su X, et al. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res. 2020;21:224. doi:10.1186/s12931-020-01479-w.
- European Centre for Disease Prevention and Control. Geographical distribution of confirmed MERS-COV cases by country of infection and year, from April 2012 - 3 October 2022. (2022, October 7). [Cited 2022 Oct 31]. Available from: https://www.ecdc.europa.eu/en/publications-data/geographical-distribution-confirmed-mers-cov-cases-country-infection-and-year-12.
- World Health Organization. Prioritizing diseases for research and development in emergency contexts. [Cited 2022 Oct 31]. Available from: https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts.
- Al-Tawfiq JA, Zumla A, Memish ZA. Coronaviruses: severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus in travelers. Curr Opin Infect Dis. 2014 Oct;27(5):411–417. doi:10.1097/QCO.0000000000000089.
- Kim KH, Tandi TE, Choi JW, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015: epidemiology, characteristics and public health implications. J Hosp Infect. 2017;95(2):207–213. doi:10.1016/j.jhin.2016.10.008.
- Al-Tawfiq JA, El-Kafrawy SA, McCloskey B, et al. COVID-19 and other respiratory tract infections at mass gathering religious and sporting events. Curr Opin Pulm Med. 2022;28(3):192–198. doi:10.1097/MCP.0000000000000859.
- Ahmed QA, Arabi YM, Memish ZA. Health risks at the Hajj. The Lancet 2006. 9515;367:1008–1015. doi:10.1016/s0140-6736(06)68429-8.
- Al-Tawfiq JA, Zumla A, Memish ZA. Travel implications of emerging coronaviruses: SARS and MERS-CoV. Travel Med Infect Dis. 2014;12(5):422–428. doi:10.1016/j.tmaid.2014.06.007.
- Hoang VT, Gautret P, Memish ZA, et al. Hajj and Umrah mass gatherings and COVID-19 infection. Curr Trop Med Rep. 2020;7:133–140. doi:10.1007/s40475-020-00218-x.
- Aberle JH, Popow-Kraupp T, Kreidl P, et al. Influenza A and B viruses but not MERS-CoV in Hajj pilgrims, Austria, 2014. Emerg Infect Dis. 2015;21:726–727. doi:10.3201/eid2104.141745.
- Amin M, Bakhtiar A, Subarjo M, et al. Screening for Middle East respiratory syndrome coronavirus among febrile Indonesian Hajj pilgrims: a study on 28,197 returning pilgrims. J Infect Prev. 2018;19:236–239. doi:10.1177/1757177418765634.
- Annan A, Owusu M, Marfo KS, et al. High prevalence of common respiratory viruses and no evidence of Middle East respiratory syndrome coronavirus in Hajj pilgrims returning to Ghana, 2013. Trop Med Int Health. 2015;20:807–812. doi:10.1111/tmi.12482.
- Atabani SF, Wilson S, Overton-Lewis C, et al. Active screening and surveillance in the United Kingdom for Middle East respiratory syndrome coronavirus in returning travellers and pilgrims from the Middle East: a prospective descriptive study for the period 2013-2015. Int J Infect Dis. 2016;47:10–14. doi:10.1016/j.ijid.2016.04.016.
- Barasheed O, Rashid H, Alfelali M, et al. Viral respiratory infections among Hajj pilgrims in 2013. Virol Sin. 2014;29:364–371. doi:10.1007/s12250-014-3507-x.
- Benkouiten S, Charrel R, Belhouchat K, et al. Respiratory viruses and bacteria among pilgrims during the 2013 Hajj. Emerg Infect Dis. 2014;20:1821–1827. doi:10.3201/eid2011.140600.
- Gautret P, Charrel R, Belhouchat K, et al. Lack of nasal carriage of novel corona virus (HCoV-EMC) in French Hajj pilgrims returning from the Hajj 2012, despite a high rate of respiratory symptoms. Clin Microbiol Infect. 2013;19:E315–E317. doi:10.1111/1469-0691.12174.
- Gautret P, Charrel R, Benkouiten S, et al. Lack of MERS coronavirus but prevalence of influenza virus in French pilgrims after 2013 Hajj. Emerg Infect Dis. 2014;20:726–728. doi:10.3201/eid2004.131708.
- Hashem AM, Al-Subhi TL, Badroon NA, et al. MERS-cov, influenza and other respiratory viruses among symptomatic pilgrims during 2014 Hajj season. J Med Virol. 2019;91:911–917. doi:10.1002/jmv.25424.
- Koul PA, Mir H, Saha S, et al. Influenza not MERS CoV among returning Hajj and Umrah pilgrims with respiratory illness, kashmir, North India, 2014-15. Travel Med Infect Dis. 2017;15:45–47. doi:10.1016/j.tmaid.2016.12.002.
- Ma X, Liu F, Liu L, et al. No MERS-CoV but positive influenza viruses in returning Hajj pilgrims, China, 2013-2015. BMC Infect Dis. 2017;17:715. doi:10.1186/s12879-017-2791-0.
- Memish ZA, Almasri M, Turkestani A, et al. Etiology of severe community-acquired pneumonia during the 2013 Hajj-part of the MERS-CoV surveillance program. Int J Infect Dis. 2014;25:186–190. doi:10.1016/j.ijid.2014.06.003.
- Refaey S, Amin MM, Roguski K, et al. Cross-sectional survey and surveillance for influenza viruses and MERS-CoV among Egyptian pilgrims returning from Hajj during 2012-2015. Influenza Other Respir Viruses. 2017;11:57–60. doi:10.1111/irv.12429.
- Johari J, Hontz RD, Pike BL, et al. Multiyear prospective cohort study to evaluate the risk potential of MERS-COV infection among Malaysian Hajj pilgrims (mercurial): A study protocol. BMJ Open. 2021;11; doi:10.1136/bmjopen-2021-050901.
- Ko JH, Müller MA, Seok H, et al. Suggested new breakpoints of anti-MERS-CoV antibody ELISA titers: performance analysis of serologic tests. Eur J Clin Microbiol Infect Dis. 2017 Nov;36(11):2179–2186. doi:10.1007/s10096-017-3043-3. Epub 2017 Jul 11.
- Müller MA, Meyer B, Corman VM, et al. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi arabia: a nationwide, cross-sectional, serological study. Lancet Infect Dis. 2015 May;15(5):559–564. doi:10.1016/S1473-3099(15)70090-3. Epub 2015 Apr 8. Erratum in: Lancet Infect Dis. 2015 Jun;15(6):629.
- Alshukairi AN, Zheng J, Zhao J, et al. High prevalence of MERS-CoV infection in camel workers in Saudi arabia. mBio. 2018 Oct 30;9(5):e01985–18. doi:10.1128/mBio.01985-18.
- Song YJ, Yang JS, Yoon HJ, et al. Asymptomatic Middle East respiratory syndrome coronavirus infection using a serologic survey in Korea. Epidemiol Health. 2018 Apr 15;40:e2018014), doi:10.4178/epih.e2018014.
- Premila Devi J, Noraini W, Norhayati R, et al. Laboratory-confirmed case of Middle East respiratory syndrome coronavirus (MERS-CoV) infection in Malaysia: preparedness and response, April 2014. Euro Surveill. 2014 May 8;19(18):20797), doi:10.2807/1560-7917.es2014.19.18.20797.
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-COV) – Malaysia. [Cited 2022 Oct 31]. Available from: https://www.who.int/emergencies/disease-outbreak-news/item/08-january-2018-mers-cov-malaysia-en.
- Ghapa N. Consumer protecton measures for Umrah package travellers in Malaysia. PalArch's Journal of Archaeology of Egypt / Egyptology. [Cited 2022 Oct 31]. Available from: https://archives.palarch.nl/index.php/jae/article/view/290.
- Degnah AA, Al-amri SS, Hassan AM, et al. Seroprevalence of MERS-cov in healthy adults in western Saudi arabia, 2011–2016. J Infect Public Health. 2020;13:697–703. doi:10.1016/j.jiph.2020.01.001.
- Liljander A, Meyer B, Jores J, et al. MERS-CoV Antibodies in humans, Africa, 2013–2014. Emerg Infect Dis. 2016;22(6):1086–1089. doi:10.3201/eid2206.160064.
- Munyua P, Lattwein E, Corman VM, et al. No serologic evidence of Middle East respiratory syndrome coronavirus infection among camel farmers exposed to highly seropositive camel herds: A household linked study, Kenya, 2013. Am J Trop Med Hyg. 2017;96(6):1318–1324. doi:10.4269/ajtmh.16-0880.
- Zohaib A, Saqib M, Athar MA, et al. Countrywide survey for MERS-coronavirus antibodies in dromedaries and humans in Pakistan. Virol Sin. 2018;33(5):410–417. doi:10.1007/s12250-018-0051-0.
- Abbad A, Perera RA, Anga L, et al. Middle East respiratory syndrome coronavirus (MERS-CoV) neutralising antibodies in a high-risk human population, Morocco, November 2017 to January 2018. Euro Surveill. 2019 Nov;24(48):1900244. doi:10.2807/1560-7917.ES.2019.24.48.1900244.
- Benkouiten S, Al-Tawfiq JA, Memish ZA, et al. Clinical respiratory infections and pneumonia during the Hajj pilgrimage: A systematic review. Travel Med Infect Dis. 2019;28:15–26. doi:10.1016/j.tmaid.2018.12.002.
- Benkouiten S, Charrel R, Belhouchat K, et al. Respiratory viruses and bacteria among pilgrims during the 2013 Hajj. Emerg Infect Dis. 2014;20(11):1821–1827. doi:10.3201/eid2011.140600.
- Al-Abdallat MM, Rha B, Alqasrawi S, et al. Acute respiratory infections among returning Hajj pilgrims—Jordan, 2014. J Clin Virol. 2017;89:34–37. doi:10.1016/j.jcv.2017.01.010.
- Choe PG, Perera RAPM, Park WB, et al. MERS-CoV Antibody responses 1 year after symptom onset, South Korea, 2015. Emerg Infect Dis. 2017 Jul;23(7):1079–1084. doi:10.3201/eid2307.170310. Epub 2017 Jul 15.
- Alshukairi AN, Khalid I, Ahmed WA, et al. Antibody response and disease severity in healthcare worker MERS survivors. Emerg Infect Dis. 2016;22(6). doi:10.3201/eid2206.160010.
- Ko JH, Müller MA, Seok H, et al. Serologic responses of 42 MERS-coronavirus-infected patients according to the disease severity. Diagn Microbiol Infect Dis. 2017 Oct;89(2):106–111. doi:10.1016/j.diagmicrobio.2017.07.006. Epub 2017 Jul 14.
- Jeewandara C, Jayathilaka D, Gomes L, et al. SARS-CoV-2 neutralizing antibodies in patients with varying severity of acute COVID-19 illness. Sci Rep. 2021 Jan 21;11(1):2062), doi:10.1038/s41598-021-81629-2.
- Marchi S, Viviani S, Remarque EJ, et al. Characterization of antibody response in asymptomatic and symptomatic SARS-CoV-2 infection. PLoS One. 2021 Jul 2;16(7):e0253977), doi:10.1371/journal.pone.0253977.
- Maciola AK, La Raja M, Pacenti M, et al. Neutralizing antibody responses to SARS-CoV-2 in recovered COVID-19 patients are variable and correlate with disease severity and receptor-binding domain recognition. Front Immunol. 2022 Jan 31;13:830710), doi:10.3389/fimmu.2022.830710.
- Assiri A, McGeer A, Perl TM, et al. KSA MERS-CoV investigation team. hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013 Aug 1;369(5):407–416. doi:10.1056/NEJMoa1306742. Epub 2013 Jun 19. Erratum in: N Engl J Med. 2013 Aug 29;369(9):886.
- Al-Abdallat MM, Payne DC, Alqasrawi S, et al. Gerber SI; Jordan MERS-CoV investigation team. hospital-associated outbreak of Middle East respiratory syndrome coronavirus: a serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014 Nov 1;59(9):1225–1233. doi:10.1093/cid/ciu359. Epub 2014 May 14.
- Park HY, Lee EJ, Ryu YW, et al. Epidemiological investigation of MERS-CoV spread in a single hospital in South Korea, May to June 2015. Euro Surveill. 2015 Jun 25;20(25):1–6. doi:10.2807/1560-7917.es2015.20.25.21169.
- Hui DS, Azhar EI, Kim YJ, et al. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect Dis. 2018 Aug;18(8):e217–e227. doi:10.1016/S1473-3099(18)30127-0. Epub 2018 Apr 18.
- Lee JY, Kim YJ, Chung EH, et al. The clinical and virological features of the first imported case causing MERS-CoV outbreak in South Korea, 2015. BMC Infect Dis. 2017 Jul 14;17(1):498), doi:10.1186/s12879-017-2576-5.
- Majumder MS, Brownstein JS, Finkelstein SN, et al. Nosocomial amplification of MERS-coronavirus in South Korea, 2015. Trans R Soc Trop Med Hyg. 2017 Jun 1;111(6):261–269. doi:10.1093/trstmh/trx046.
- Korea Centers for Disease Control and Prevention. Middle East respiratory syndrome coronavirus outbreak in the Republic of Korea, 2015. Osong Public Health Res Perspect. 2015 Aug;6(4):269–278. doi: 10.1016/j.phrp.2015.08.006. Epub 2015 Sep 5. Erratum in: Osong Public Health Res Perspect. 2016 Apr;7(2):138.
- Hemida MG, Perera RA, Al Jassim RA, et al. Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill. 2014;19; doi:10.2807/1560-7917.es2014.19.23.20828.
- Okba NMA, Raj VS, Widjaja I, et al. Sensitive and specific detection of low-level antibody responses in mild Middle East respiratory syndrome coronavirus infections. Emerg Infect Dis. 2019;25:1868–1877. doi:10.3201/eid2510.190051.
- Alhabbab RY, Algaissi A, Mahmoud AB, et al. MERS-CoV infection elicits long-lasting specific antibody, T and B cell immune responses in recovered individuals. Clin Infect Dis. 2022 Jun 8: ciac456. doi:10.1093/cid/ciac456. Epub ahead of print.
- Zhao J, Alshukairi AN, Baharoon SA, et al. Recovery from the Middle East respiratory syndrome is associated with antibody and T-cell responses. Sci Immunol. 2017 Aug 4;2(14):eaan5393. doi:10.1126/sciimmunol.aan5393. Epub 2017 Aug 4.
- Mok CK, Zhu A, Zhao J, et al. T-cell responses to MERS coronavirus infection in people with occupational exposure to dromedary camels in Nigeria: An observational cohort study. Lancet Infect Dis. 2021;21(3):385–395. doi:10.1016/s1473-3099(20)30599-5.