ABSTRACT
Dermatophytic pseudomycetoma is a rare invasive infection, involving both immunocompetent and immunocompromised individuals. Since the discovery of inherited immune disorders such as the impairment of CARD9 gene, extended dermatophyte infections are mostly ascribed to any of these host factors. This study is to present and explore the potential causes in a fatal dermatophytic pseudomycetoma patient. We present a chronic and deep pseudomycetoma caused by the common dermatophyte Microsporum canis which ultimately led to the death of the patient. Mycological examination, genetic studies and host immune responses against fungi were performed to explore the potential factors. The patient had decreased lymphocyte counts with significantly reduced CD4+ T cells, although all currently known genetic parameters proved to be normal. Through functional studies, we demonstrated that peripheral blood mononuclear cells from the patient showed severe impairment of adaptive cytokine production upon fungus-specific stimulation, whereas innate immune responses were partially defective. This is, to our knowledge, the first report of fatal dermatophytic pseudomycetoma in a patient with non-HIV CD4 lymphocytopenia, which highlights the importance of screening for immune deficiencies in patients with deep dermatophytosis.
Introduction
Dermatophyte infection in humans, mostly caused by members of the genera Trichophyton, Microsporum, and Epidermophyton, is one of the most frequent fungal infections in humans [Citation1], but has long been considered a minor problem. Infections tend to be mild, mostly limited to the outermost skin and the nails, and appropriate antifungal therapy is generally available [Citation2]. However, in immunocompromised hosts, dermatophytes can also be responsible for extended or invasive forms, which are frequently unrecognized by clinicians. The health impact of these fungi may be due to their wide expansion or to deep penetration including dermal invasion. Four forms of invasive dermatophyte infections are known: Majocchi’s granuloma (also known as nodular granulomatous perifolliculitis), deep dermatophytosis, disseminated dermatophytosis, and dermatophyte (pseudo)mycetoma [Citation3,Citation4]. The invasive infections are chronic, recalcitrant, and eventually destructive, varying from perifollicular papels as in Majocchi’s granuloma [Citation5] to pseudomycetomatous, non-draining hyphal aggregates in the dermis and below [Citation6,Citation7].
The pseudomycetomatous type of dermatophytosis is mainly observed in patients with underlying disorders, particularly following solid organ transplantation (SOT), but some cases were observed in apparently healthy individuals. In the past two decades, several inherited immune disorders have been discovered, of which mutations in the caspase recruitment protein domain 9 (CARD9) is particularly significant for fungi: dermatophytes are one of the main groups emerging with this disorder [Citation8,Citation9]. Lanternier et al. [Citation10] suggested that all dermatophyte infections with this type of clinical course and where the host was reported as otherwise appearing healthy, actually had a non-diagnosed CARD9 disorder.
In this study, we report a case of fatal dermatophyte pseudomycetoma caused by Microsporum canis. All parameters of known genetic disorders were negative. Nevertheless, a significant leucocyte decrease was observed, which probably enhanced the infection and ultimately led to the death of the patient.
Materials and method
Case presentation
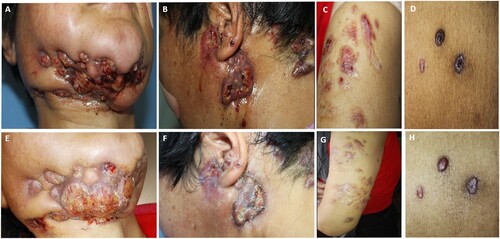
A 51-year-old female farmer from the northwestern region of China presented to our hospital with infiltrated nodules and an abscess in the neck area that had worsened over the past two months ((A,B)). Ten years ago, the patient developed a red papule on the right lower eyelid, likely due to close contact with their home-raised cat, which was left untreated. The patient developed a painless, firm, red nodule on the right mandible which eventually ruptured, resulting in the spread of skin lesions to the neck. The lesions continued to expand to the trunk and extremities, becoming ulcerated, pus-filled, and painful ((C,D)). An empirical diagnosis of sporotrichosis was made at a local hospital, and treatment with itraconazole 400 mg/d was initiated for two years, leading to significant improvement. However, the skin lesions deteriorated after discontinuing oral administration of itraconazole. The local hospital suspected skin tuberculosis and the patient underwent a two-month course of triple anti-tuberculosis therapy (isoniazid, rifampicin, ethambutol), which did not result in significant improvement. The patient was then treated with oral terbinafine 250 mg/d for one year, without significant effect. Ten months later, the cutaneous lesions had worsened and the patient sought admission to our hospital. Upon examination, we observed multiple nodules and masses infiltrating the left upper limb and trunk, as well as a large, hard mass with a sinus in the cheek area extending to the left ear. The surface of the mass exhibited ulceration, exudation, and scabbing (). The histopathological examination and fungal culture of biopsy tissue from the neck lesion revealed dermatophytic pseudomycetoma, which was caused by Microsporum canis. The patient underwent surgical debridement of the mandibular lesion and pus. She was treated with oral terbinafine 500 mg twice a day, and later combined with intravenous fluconazole 400 mg per day during her hospitalization. After discharge, the patient was treated with oral terbinafine (250 mg twice a day) and itraconazole (200 mg twice a day). However, due to the low CD4+ T cell count, a destructive and invasive lesion of the mandible had developed. Despite aggressive treatment, the patient succumbed to cachexia six months later.
Diagnostic examination
Mycology
Samples obtained from the left mandibular lesion tissue and pus from multiple sites were subjected to direct microscopy and fungal culture to detect the presence of fungi. Additionally, a biopsy specimen from the mandibular lesion and pus from multiple sites were cultured on Sabouraud’s Glucose Agar (SGA; BDTM). Skin biopsy tissue was stained with periodic acid–Schiff (PAS). Furthermore, galactomannan (GM), and β-D-glucan (G) tests were performed on blood samples.
Genetics examination
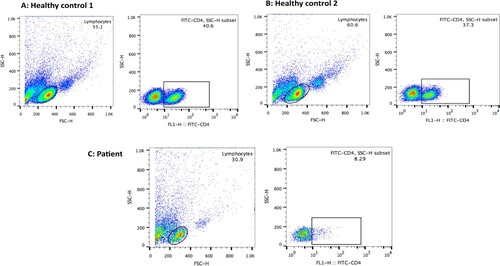
The patient's leukocyte counts showed a significant decrease, with notable changes in lymphocyte subset values. The patient had a total T-lymphocyte count of 57.71% (50–82), a decrease in total B lymphocytes to 3.82% (5–21), a significant decrease in T-helper/regulatory lymphocytes to 1.65% (24–54), an increase in T-suppressor/cytotoxic lymphocytes to 51.77% (14–41), an increase in total NK cells to 39.06% (6–38), and a CD4+/CD8+ ratio of 0.03 (0.7–3.1). The patient's total lymphocyte count was 100.59% (84–99). Flow cytometry analysis of the patient's peripheral blood mononuclear cells (PBMC) revealed a significant decrease in CD4+ T-cells (8.29% vs. normal around 40%). These findings were confirmed in co-culture experiments using flow cytometry.
To further study the host immune responses against fungi, primary peripheral immune cells were isolated from the patient and compared with healthy controls. Peripheral blood mononuclear cells (PBMCs, 1 × 107/mL) were collected from whole blood using Ficoll-Paque Plus (GE Healthcare, Chicago, IL, USA) via density-gradient centrifugation. M. canis isolates were cultured on SGA for 14 days at 28°C to harvest conidia. Heat-killed (HK) conidia for stimulation were prepared for 30 min at 99°C in a water bath. The PBMCs were incubated in 96-well plates at a final concentration of 2.5 × 106/mL in a total volume of 200 mL RPMI 1640 medium with 10% autologous serum per well. The PBMCs were stimulated with different pattern recognition receptor agonists, including lipopolysaccharide (a Toll-like receptor 4 agonist), curdlan (dectin-1 agonists), Pam3CSK4 (a TLR1/2 agonist), and fungal particles (M. canis heat-killed [HK] or viable spores, 107 particles/mL) at 37°C in a 5% CO2 atmosphere. Innate ELISA assays of TNF-α, IL-6, and IL-1β were carried out with stimulated culture supernatants or cells after 24 h. In order to detect the adaptive immune response, PBMCs were stimulated with the corresponding HK fungal particles for 7 days, and culture supernatants were gathered for further ELISA assays (IL-22, GM-CSF, and INF-γ). Residual living cells were re-stimulated with Cell Stimulation Cocktail (eBioscience, San Diego, CA, USA) for 5 h in an incubator to promote the intracellular accumulation of secreted cytokines. Human PBMCs were surface stained with FITC-conjugated anti-human CD4 antibody, fixed and permeabilized by Cytofix/Cytoperm solution (BD Biosciences), and stained with PE-conjugated anti-human IL-22 and Alexa®Fluor647-conjugated anti-human IL-17A antibodies. Data were collected on a BD FACS Calibur system and analysed with FlowJo7.6 software.
Results
Mycology
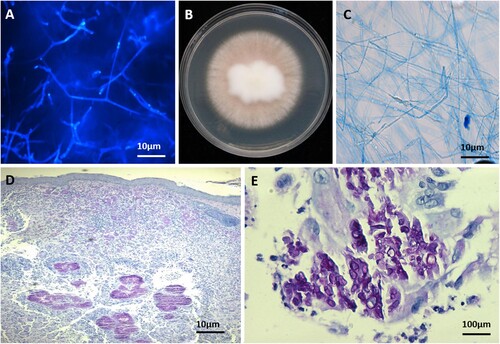
The fungal cultures from multi-sites samples on SGA showed radially 0.3 cm/day expanding, pale pigmented colony, with brownish orange centre, obverse and reverse becoming paler towards the margin. Furthermore, septate, branched hyphae were present with lactophenol cotton blue and calcofluor staining (). On Potato Dextrose Agar (PDA, BDTM) and on rice medium at 28°C, no conidia were seen. Poor conidiation was observed on SGA after 14 days, revealing irregularly shaped conidia morphologically. The rDNA ITS sequence generated with primers: ITS1/ITS4, ITS2/ITS5, and ITS3/ITS4, and the LSU sequence with primers LROR/LR5 identified the above isolates as Microsporum canis. The fungus showed optimal growth (measured after 7 days on SGA) at 28°C, reaching 2.5 cm diameter. At 35°C, the diameter was 1.5 cm, at 37°C 1.1 cm, and at 40°C 0.2 cm.
Figure 2. A. Direct microscopy of lesions revealed septate, branched hyphae (Calcofluor-white staining); B. macroscopy of the isolate with poor conidiation (PDA, 14 d, 28°C); C. microscopy of the cultured strain showing irregular hyphae morphologically (lactophenol cotton blue staining); D, E. skin biopsy specimens showing intense inflammatory infiltrates, aggregates of hyaline hyphae in the dermis with infectious granulomata (PAS staining; original magnification × 40 and × 400).
Before treatment, the patient's serum GM tests were negative, but the G-test showed a significantly elevated level of 895.80 pg/mL (>100). After undergoing six months of antifungal treatment, follow-up testing revealed persistently high levels of G (+) > 1000 pg/mL (60–80 pg/mL) and GM (+) 0.925 optical density index (ODI) (0.5 ODI). Microscopic examination of the affected area confirmed the presence of fungal hyphae, despite negative fungal cultures from the pus. Notably, brain MRI and lung CT scan did not reveal any signs of fungal infection.
Histopathology test
Histopathology of the skin tissue biopsy revealed infectious granulomata, with subcompact aggregates of hyaline hyphae in the dermis visible with periodic acid-Schiff (PAS) stain (). These hyphal aggregates were surrounded by granulomatous tissue. The deep infection of the skin and soft tissue exhibited various clinical manifestations, such as honeycomb folliculitis, pyogenic, subcutaneous and lymph node abscesses, and verrucous hyperplasia, leading to the diagnosis of subcutaneous fungal infection. Screening for HIV and other infectious parameters was negative.
Genetic studies
Based on previous reports linking CARD9 mutations to deep dermatophytosis [Citation12–14], as well as the chronic and recalcitrant characteristics of the infection, we suspected a genetic immunodeficiency. Therefore, genomic DNA was extracted from the peripheral blood of the patient and her son, and whole exome sequencing was carried out in the patient. Exome capture was carried out with a Bioanalyzer 2100 (Agilent) to examine the quality of the DNA sample in the captured library, and then sequenced using the NEXTSEQ500 System (Illumina) according to the concentration and depth requirement of the DNA sample in the captured library, following the manufacturer’s protocols. Upon analysis, no mutations were found in reported genes known to predispose individuals to subcutaneous fungal infections (Supplementary Table 1), such as CARD9 or STAT1. Nevertheless, the patient was found to have homozygous mutations in the caspase-9 monomer (CASP9, NM_001229) and in the interleukin-7 receptor (IL7R, NM_002185), which are surmised to increase the patient’s susceptibility to infection. Interestingly, the patient's son was also analysed and was found to be heterozygous for CASP9 (Supplementary Table 2).
Host immune responses
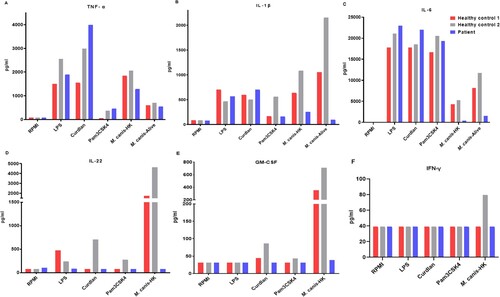
To further study the host immune responses against fungi, we isolated PBMCs from the patient and challenged with different stimuli for 24 h and 7 days. Compared with cells isolated from two healthy control participants, patient and controls showed comparable levels of TNF-α, IL-1β, and IL-6 in response to LPS, curdlan, and Pam3CSK4 stimulations. However, responses to HK or viable M. canis stimulation were partially lower than the healthy controls: IL-22, and GM-CSF production upon fungal stimulation was significantly decreased in the patient (). The IFN γ production in healthy control 2 was increased after fungal stimulation, whereas no obvious responses were noted in the patient and healthy control 1, suggesting that these stimuli were not strong inducers of IFN γ production. We also assessed the differentiation of T-helper (Th) cells in PBMCs from the patient and healthy control participants after stimulation for 7 days, and hardly isolated any CD4+ T cells in the patient (). These findings indicate a partially impaired innate immune response and a significantly impaired adaptive immune response in this patient.
Figure 3. Pro-inflammatory cytokines and adaptive immune responses in PBMCs derived from patients and healthy control individuals. A-C. Pro-inflammatory cytokines (A) TNF-α, (B) IL-1β, and (C) IL-6 in response to 24-h stimulations with LPS, curdlan, Pam3CSK4 and Microsporum canis (HK or viable resting) in supernatants detected by ELISA; D-F. cytokines (D) IL-22, (E), GM-CSF, (F), IFN-γ in response to curdlan and M. canis (HK or resting conidia) stimulation, were measured in cell culture supernatants after 6 d using commercial ELISA kits. LPS, lipopolysaccharide; PBMC, peripheral blood mononuclear cell.
Antifungal susceptibility testing
The in vitro antifungal susceptibility testing according to CLSI protocol [Citation11] yielded the following MIC values: amphotericin B 0.5 µg/mL, caspofungin 0.5 µg/mL, itraconazole 0.25 µg/mL, voriconazole 0.03 µg/mL, terbinafine 0.008 µg/mL. In combination, for itraconazole + terbinafine FICI = 2, and itraconazole + amphotericin B FICI = 0.625.
Discussion
Deep infections by dermatophytes including Microsporum species are exceptional. Majocchi’s granuloma, redefined by Durdu et al. [Citation5] as perifollicular inflammatory granuloma involving the dermis, is promoted by the use of steroid-containing creams [Citation15]. Subcutaneous infections, reported in the literature as pseudomycetomata are extremely rare [Citation6,Citation7,Citation16–18]. In contrast to eumycetoma, grain-formation and draining sinuses are mostly lacking. Pseudomycetomas represent granulomatous infiltrations with hyphal aggregates that form basophilic structures (grains) similar to eumycotic granules, and may arise through extension from hair follicles.
Microsporum canis is a zoophilic dermatophyte found particularly on cats, mostly residing asymptomatically in the fur [Citation19]. Local outbreaks of human infections, probably because of pet ownership, are known which are transmitted rapidly [Citation20,Citation21], but tend to die out within a few generations [Citation22]. Particularly tinea capitis, which via hair ectothrix leads to a clinical picture known as grey patch, is relatively common [Citation23]. In the genus Microsporum, human infections are classically caused by the anthropophilic species M. audouinii, which is morphologically characterized by reduced sporulation with deformed conidia, and is occasionally observed to cause pseudomycetoma [Citation24]. Our patient’s strain showed this type of morphology, but was genetically identified as M. canis. This suggests that loss of sporulation is one of the features accompanying the host jump to the unfavoured host, Homo sapiens.
The MIC results indicate that our patient's strain of M. canis is susceptible to most of the tested antifungal drugs, including amphotericin B, caspofungin, itraconazole, voriconazole, and terbinafine. The combination of itraconazole and terbinafine showed a synergistic effect, while the combination of itraconazole and amphotericin B showed a partial synergistic effect. However, in this case, the treatment did not yield favourable outcomes, and the infection remained uncontrolled. It's crucial to note that susceptibility testing outcomes must be interpreted along with clinical response, and the MIC values may not accurately reflect the in vivo effectiveness of the treatment. Therefore, a holistic approach is necessary when evaluating the efficacy of antifungal therapy for patients, considering both laboratory results and clinical manifestations.
In literature before 2010, cases regarding deep dermatophytosis were listed as occurring in otherwise healthy, immunocompetent patients. However, with the advances in genetics and primary immunodeficiencies, the underlying genetic background predisposing to chronic, deep and mutilating dermatophyte infections has been recognized [Citation25]. Lanternier et al. [Citation10] reviewed chronic mutilating cases and found solid organ transplant as the prime risk factor, and CARD9 defects as the second. Rouzaud et al. [Citation3] surmised that most of dermatophytic pseudomycetomata were in patients with a defective signalling pathway with impaired IL-6 and IL-17 production [Citation26,Citation27], resulting from CARD9-related immune defects. Cytokine release from innate immune cells is crucial for Th-cell differentiation. Underlying conditions were mostly SOT associated with immunodepression, whereas autosomal recessive CARD9 deficiency was second. In the present case, whole-exome sequencing did not suggest known pathogenic mutations, including CARD9 and genes leading to CD4 lymphocytopenia. However, we still cannot exclude the involvement of other genetic defects in the pathogenesis of the disease, which needs further study.
Although all currently known genetic parameters were proven to be negative, this patient showed a significant reduction in CD4+ T cells, both by peripheral blood lymphocyte subset analysis and by our functional studies. This led to the lethally reduced adaptive immune responses, as shown by significantly reduced IL-22 production upon fungal stimulation. Moreover, the innate immune response of the patient was also partially impaired compared to healthy controls. All of these led to a deficiency in antifungal immunity, which could explain the recalcitrant dermatophytic pseudomycetoma. Several authors mentioned the occurrence idiopathic CD4 lymphocytopenia (ICL) [Citation28–31] which leads to decreased production of TNF-α, IL-6, and IL-22 in seemingly immunocompetent patients. As main infectious agents, Cryptococcus, Mycobacterium, Pneumocystis, and Candida were prevalent [Citation32], but no dermatophytes were listed. The patient had homozygous CASP9 and IL7R mutations, which may have increased the patient’s susceptibility to infection, but similar mutations are known to occur in the healthy population. Our patient did not have HIV infection, and thus the reason for her CD4 lymphocytopenia remains a mystery. Considering she lived as farmer in a rural area of China, we cannot exclude exposure to toxic or harmful active substances, that might have contributed to her CD4 lymphocytopenia [Citation33,Citation34]. Further investigation and analysis are needed to fully understand the underlying causes and contributing factors to the patient's condition.
Nevertheless, we report a case of fatal dermatophyte pseudomycetoma caused by M. canis, without HIV-related mutations, but with CD4 lymphocytopenia. The present case indicates that ICL might also be a risk factor for chronic severe dermatophyte infection. Published non-AIDS cases might have experienced a similar, though still unexplained immune defect. The search for hidden immune disorders predisposing to severe dermatophyte infections is not over yet.
Ethics
The patient in the current study, her son and two ethnically matched healthy volunteers provided written consent for participation in the study, which was approved by the Clinical Research Ethics Committee of the Peking University First Hospital.
Acknowledgements
The authors would like to thank the patient, her son and the healthy donors for their participation in this study. This work was supported by the National High Level Hospital Clinical Research Funding (“Star of Outlook” Scientific Research Project of Peking University First Hospital).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data were shown in Results and supplementary tables.
Additional information
Funding
References
- Urban K, Chu S, Scheufele C, et al. The global, regional, and national burden of fungal skin diseases in 195 countries and territories: a cross-sectional analysis from the global burden of disease study 2017. JAAD Int. 2021;2:22–27.
- Gupta AK, Cooper EA. Update in antifungal therapy of dermatophytosis. Mycopathologia. 2008;166(5-6):353–367.
- Rouzaud C, Hay R, Chosidow O, et al. Severe dermatophytosis and acquired or innate immunodeficiency: a review. J Fungi (Basel). 2016;2(1):4.
- Ilkit M, Durdu M, Karakaş M. Majocchi's granuloma: a symptom complex caused by fungal pathogens. Med Mycol. 2012;50(5):449–457.
- Durdu M, Kandemir H, Ilkit M, et al. Changing concepts and current definition of Majocchi's granuloma. Mycopathologia. 2020;185(1):187–192.
- Wang R, Yao X, Li R. Mycetoma in China: a case report and review of the literature. Mycopathologia. 2019;184(2):327–334.
- Ruiz LRB, Zaitz C, Lellis RF, et al. Pseudomycetoma of the scalp caused by Microsporum canis. An Bras Dermatol. 2020;95(3):372–375.
- Vaezi A, Fakhim H, Abtahian Z, et al. Frequency and geographic distribution of CARD9 mutations in patients with severe fungal infections. Front Microbiol. 2018;9:2434.
- Song Y, Du M, Menezes da Silva N, et al. Comparative analysis of the black yeast Exophiala spinifera from a CARD9 deficiency patient combining short- and long-read genome sequencing technology. Front Microbiol. 2020;11:1880.
- Lanternier F, Barbati E, Meinzer U, et al. Inherited CARD9 deficiency in 2 unrelated patients with invasive Exophiala infection. J Infect Dis. 2015;211(8):1241–1250.
- CLSI. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. CLSI Standard M38, 3rd.
- Lanternier F, Pathan S, Vincent QB, et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med. 2013;369(18):1704–1714.
- Huang C, Peng Y, Zhang Y, et al. Deep dermatophytosis caused by Trichophyton rubrum. Lancet Infect Dis. 2019;19(12):1380.
- Zhang Y, Mijiti J, Huang C, et al. Deep dermatophytosis caused by Microsporum ferrugineum in a patient with CARD9 mutations. Br J Dermatol. 2019;181(5):1093–1095.
- Boral H, Durdu M, Ilkit M. Majocchi's granuloma: current perspectives. Infect Drug Resist. 2018;11:751–760.
- Berg JC, Hamacher KL, Roberts GD. Pseudomycetoma caused by Microsporum canis in an immunosuppressed patient: a case report and review of the literature. J Cutan Pathol. 2007;34(5):431–434.
- Chiapello LS, Dib MD, Nuncira CT, et al. Mycetoma of the scalp due to Microsporum canis: histopathologic, mycologic, and immunogenetic features in a 6-year-old girl. Diagn Microbiol Infect Dis. 2011;70(1):145–149.
- Tirado-González M, Ball E, Ruiz A, et al. Disseminated dermatophytic pseudomycetoma caused by Microsporum species. Case Rep Int J Dermatol. 2012;51(12):1478–1482.
- Mignon BR, Losson BJ. Prevalence and characterization of Microsporum canis carriage in cats. J Med Vet Mycol. 1997;35(4):249–256.
- Zhan P, Geng C, Li Z, et al. Evolution of tinea capitis in the Nanchang area, Southern China: a 50-year survey (1965–2014). Mycoses. 2015;58(5):261–266.
- Chiller K, Resneck J, Chiller TM, et al. An outbreak of Microsporum canis in the community demonstrating rapid transmission. Exogen Dermatol. 2002;1:18–21.
- Brosh-Nissimov T, Ben-Ami R, Astman N, et al. An outbreak of Microsporum canis infection at a military base associated with stray cat exposure and person-to-person transmission. Mycoses. 2018;61(7):472–476.
- Pratiwi FD, Setyaningrum T. Gray patch tinea capitis caused by Microsporum canis. Cermin Dunia Kedokteran. 2020:47(10).
- Diongue K, Boye A, Bréchard L, et al. Dermatophytic mycetoma of the scalp due to an atypical strain of Microsporum audouinii identified by MALDI-TOF MS and ITS sequencing. J Mycol Méd. 2019;29(2):185–188.
- Grumach AS, de Queiroz-Telles F, Migaud M, et al. A homozygous CARD9 mutation in a Brazilian patient with deep dermatophytosis. J Clin Immunol. 2015;35(5):486–490.
- Drewniak A, Gazendam RP, Tool AT, et al. Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood. 2013;121(13):2385–2392.
- Song Y, Menezes da Silva N, Vicente VA, et al. Comparative genomics of opportunistic Phialophora species involved in divergent disease types. Mycoses. 2021;64(5):555–568.
- Zonios D, Sheikh V, Sereti I. Idiopathic CD4 lymphocytopenia: a case of missing, wandering or ineffective T cells. Arthritis Res Ther. 2012;14(4):222.
- Ollé-Goig JE, Ramírez J, Cervera C, et al. Profound reduction of CD4+ lymphocytes without HIV infection: two cases from the horn of Africa. Afr Health Sci. 2012;12(3):331–333.
- Asher I, Mahlab-Guri K, Elbirt D, et al. Idiopathic CD4 lymphopenia: severe CD4 lymphopenia in the absence of human immunodeficiency virus infection. IMAJ. 2016;18(10):627–629.
- Khamanarong N, Insiripong S. CD4 lymphocytopenia without HIV infection. Southeast Asian J Trop Med Public Health. 2016;47(3):503–505.
- Ahmad DS, Esmadi M, Steinmann WC. Idiopathic CD4 lymphocytopenia: spectrum of opportunistic infections, malignancies, and autoimmune diseases. Avicenna J Med. 2013;3(2):37–47.
- Aroonvilairat S, Kespichayawattana W, Sornprachum T, et al. Effect of pesticide exposure on immunological, hematological and biochemical parameters in Thai orchid farmers – a cross-sectional study. Int J Environ Res Public Health. 2015;12(6):5846–5861.
- Wojtacha P, Trybowski W, Podlasz P, et al. Effects of a low dose of T-2 toxin on the percentage of T and B lymphocytes and cytokine secretion in the porcine ileal wall. Toxins (Basel). 2021;13(4):277.