ABSTRACT
Background: This study aims to evaluate waning effectiveness against severe and fatal COVID-19 with two to three doses of CoronaVac/BNT162b2, where data are limited. Methods: A case–control study included individuals aged ≥18 years, unvaccinated or received two to three doses of CoronaVac/BNT162b2, using electronic healthcare databases in Hong Kong. Those with first COVID-19-related hospitalization, severe complications, or mortality between 1 January and 15 August 2022 were defined as cases and matched with up-to-10 controls by age, sex, index date, and Charlson Comorbidity Index. Vaccine effectiveness (VE) against COVID-19-related outcomes was estimated at different time intervals from second and third-dose vaccination (0–13 up-to 210–240 days) using conditional logistic regression adjusted for comorbidities and medications. Results: By 211–240 days after second dose, VE against COVID-19-related hospitalization reduced to 46.6% (40.7–51.8%) for BNT162b2 and 36.2% (28.0–43.4%) for CoronaVac, and VE against COVID-19-related mortality were 73.8% (55.9–84.4%) and 76.6% (60.8–86.0%). After third dose, VE against COVID-19-related hospitalization decreased from 91.2% (89.5–92.6%) for BNT162b2 and 76.7% (73.7–79.4%) for CoronaVac at 0–13 days, to 67.1% (60.4–72.6%) and 51.3% (44.2–57.5%) at 91–120 days. VE against COVID-19-related mortality for BNT162b2 remained high from 0–13 days [98.2% (95.0–99.3%)] to 91–120 days [94.6% (77.7–98.7%)], and for CoronaVac reduced from 0–13 days [96.7% (93.2–98.4%)] to 91–120 days [86.4% (73.3–93.1%)]. Conclusions: Significant risk reduction against COVID-19-related hospitalization and mortality after CoronaVac or BNT162b2 vaccination was observed for >240 and >120 days after second and third doses compared to unvaccinated, despite significant waning over time. Timely administration of booster doses could provide higher levels of protection.
Introduction
The Omicron (B.1.1.529) variant of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become the dominant strain of Coronavirus disease (COVID-19) that swept the globe. Many countries, such as China, Singapore and Australia, have achieved vaccination rates over 80% for the primary series of COVID-19 vaccine [Citation1], but the number of confirmed cases worldwide still surged during the Omicron outbreak. In Hong Kong, after more than 6 months of almost zero new cases of COVID-19 and related deaths in 2021, Hong Kong faced a major outbreak with more than 50,000 new cases and close to 300 deaths recorded daily at its peak in early March 2022 [Citation2], which have continued to place a significant burden on the healthcare system to date.
COVID-19 vaccines of two major vaccine platforms, namely BNT162b2 from Fosun-BioNTech (equivalent to Pfizer-BioNTech, mRNA vaccine) and CoronaVac from Sinovac Biotech (HK) Limited (inactivated vaccine) have been available in Hong Kong for individuals aged ≥16 years since 23 February 2021 for CoronaVac and aged ≥18 years since 6 March 2021 for BNT162b2. COVID-19 booster shots were made available to priority groups on 11 November 2021, and subsequently expanded to the general population from 1 January 2022. Individuals have a choice between BNT162b2 or CoronaVac for their first dose and are restricted to the same vaccine for their second dose. For the booster shot, either a homologous or heterologous booster was permitted. Since the launch of mass vaccination program in many countries in 2021, it has been more than a year after the completion of the primary series in those who started vaccination earliest. Nonetheless, the coverage of the booster dose lags far behind that of the primary series, resulting in a population susceptible to COVID-19 infection as protection offered by the primary series might have waned over time.
The phenomenon of waning immunity after natural infection or COVID-19 vaccination has been well described in previous studies. A Chinese study reported that the IgG antibody level in COVID-19 convalescent plasma declined with time to around 35.7% of individuals’ baseline by 9 months [Citation3]. In Qatar, the estimated effectiveness of BNT162b2 (Pfizer–BioNTech) vaccine against hospitalization and death dropped from 96.0% (93.9–97.4) to an insignificant value 6 months after the second dose during an outbreak when Delta was the dominant variant [Citation4]. Meanwhile, the United Kingdom (UK) recorded a vaccine effectiveness (VE) of 91.9% (88.5–94.3) against Delta variant-related death by 20 weeks after the second dose of BNT162b2 [Citation5]. The extent of the decline in terms of the protection offered by the third dose is less explored. In Israel, VE of BNT162b2 against infection decreased from 53.4% (47.7–58.6) to 16.5% (13–19.9) after three months since third dose vaccination during the Omicron wave [Citation6], but the decline in VE against hospitalizations or deaths was not found to be significant because of a small number of outcome events recorded by the end of 2021 [Citation6]. In South Africa, VE of the third dose of BNT162b2 against hospitalization declined to 50% (4.4–73.9) during the BA.1–BA.2 outbreak after 3–4 months [Citation7], representing a large discrepancy in VE across different countries. Estimates for waning VE other than BNT162b2, such as inactivated vaccines, remain limited. In view of the rise of newer variants or sub-lineages, whether three doses of vaccination provide adequate protection remains uncertain. This information may be useful in determining the optimal timing of a third or even fourth dose to boost protection against severe COVID-19.
Despite being the most widely used COVID-19 vaccine globally [Citation8], CoronaVac has been scarcely studied in terms of the effect of waning immunity. A serological study reported a substantial decrease in IgG seropositivity in Chileans who received two doses of CoronaVac [Citation9], but this does not necessarily equate to a decline in real-life effectiveness as immunity against SARS-CoV-2 is not solely contributed by neutralizing antibodies [Citation10]. It is in our interest to evaluate the extent of the decline in terms of VE against severe and fatal outcomes. Considering the high COVID-19 death rate observed locally [Citation11], this study aims to examine the phenomenon of waning effectiveness of BNT162b2 and CoronaVac against COVID-19-related hospitalization, severe complications and mortality during the Omicron-dominant outbreak in HK where the coverage of booster doses is suboptimal.
Methods
Study design and population
This is a case–control study conducted among individuals aged ≥18 years. Routine electronic health records were extracted from the clinical management system (CMS) under the Hospital Authority (HA) of Hong Kong. The CMS manages data on demographics, diagnoses, prescriptions, and laboratory tests, and provides real-time data support and monitoring for routine clinical management across all clinics and hospitals in HA. Individuals who had received either none or at least two doses of vaccinations between 1 January 2022 and 15 August 2022 were identified and included in the cohort. Vaccination records were extracted from the Department of Health (DH) of the Government of the Hong Kong Special Administrative Region (HKSAR). The DH manages and retains the database for all vaccination records in Hong Kong. The Centre for Health Protection (CHP) maintains a database of all confirmed COVID-19 cases, based on both mandatory and voluntary reporting of positive Rapid Antigen Test (RAT) and Polymerase Chain Reaction (PCR) test results. These databases are linked based on unique personal identifiers and have been used previously to conduct studies on the risk of adverse effects after COVID-19 vaccinations and other COVID-19 pharmacovigilance studies [Citation12–20].
To evaluate waning in VE after two or three doses of COVID-19 vaccines against outcomes after contracting predominantly Omicron variant [Citation21], the inclusion period for each outcome ranged from 1 January 2022 to 15 August 2022. Trends of new COVID-19 cases during the study period were presented in Supplementary Figure 1. Those who had a previous COVID-19 infection before the index date, or had received only one dose or the fourth dose of COVID-19 vaccine were excluded from the analysis. Due to the limited proportion of individuals receiving a heterologous booster since the commencement of the booster dose mass vaccination, these individuals were also excluded from the analysis.
Definitions of vaccine exposure
Time since vaccination was defined as index date minus the date of vaccination of the latest COVID-19 vaccine dose. To estimate VE against each outcome of the second dose of BNT162b2 or CoronaVac, nine time since-vaccination intervals (0–13, 14–30, 31–60, 61–90, 91–120, 121–150, 151–180, 181–210 and 210–240 days) were investigated; whilst VE of the third dose of vaccination was estimated for seven time since-vaccination intervals (0–13, 14–30, 31–60, 61–90, 91–120, 121–150, and 151–180 days). Previous studies suggested that the vaccines elicit full immune response in most patients by 14 days after receiving the second dose [Citation22,Citation23], thus we investigated vaccine effectiveness for the 0–13 days interval in addition to the subsequent monthly intervals. Those who did not receive any COVID-19 vaccine before the index date were considered unvaccinated.
Definitions of COVID-19-related outcomes
The outcomes investigated in this study were (i) COVID-19-related hospitalization, (ii) COVID-19-related mortality, and (iii) COVID-19-related severe complications. COVID-19-related hospitalization was defined as hospital admission within 28 days after a PCR-confirmed COVID-19 infection. COVID-19-related mortality was defined as all-cause mortality within 28 days after a PCR-confirmed COVID-19 infection. All-cause mortality data were based on the Hong Kong Deaths Registry, which officially records all registered deaths of Hong Kong residents. COVID-19-related severe complications were defined as the admission to the intensive care unit (ICU) or use of ventilatory support within 28 days after a PCR-confirmed COVID-19 infection. Use of ventilatory support, including intubation, mechanical ventilation, and oxygen supplementation, identified using the International Classification of Diseases, Ninth Revision, clinical modification (ICD-9-CM) procedure codes (39.65, 89.18, 93.90, 93.95, 93.96, 96.04, 96.7×). COVID-19 infection was defined as a positive polymerase chain reaction (PCR) test confirmed by the Centre of Health Protection of the HKSAR government. PCR test results were recognized as the gold standard diagnostic criteria for COVID-19 infection given its high specificity of >99% [Citation24]. The Hong Kong government has implemented extensive PCR testing for SARS-CoV-2 in public hospitals and clinics for close contacts of confirmed cases and those who presented with COVID-like symptoms. The government also set up territory-wide community testing centres to screen asymptomatic individuals and provide regular testing to various staff groups with a high risk of exposure to COVID-19, such as those working in nursing homes.
Statistical analysis
Case and control matching was conducted separately for each outcome. Patients with the outcome of interest during the inclusion period were included as cases; while all other patients with attendance to any HA health services (i.e. hospital admissions, emergency departments, and outpatient clinics) but without the outcome of interest were selected as controls. Up to ten controls were randomly matched with the cases according to sex, age (5-year band), date of attendance (within three calendar days), and Charlson Comorbidity Index (0, 1–2, 3–4, ≥5) [Citation25].
For each time since-vaccination interval, only eligible matched pairs, in which both the case and controls were either unvaccinated or fell within the specific time since-vaccination interval, were included to derive the corresponding estimates. Conditional logistic regression adjusted for chronic comorbidities (cancer, chronic kidney disease, respiratory disease, diabetes mellitus, cardiovascular disease, dementia), and the use of chronic medications (renin-angiotensin-system agents, beta-blockers, calcium channel blockers, diuretics, nitrates, lipid-lowering agents, insulins, antidiabetic drugs, oral anticoagulants, antiplatelets, immunosuppressants) was used to evaluate the association between vaccination and the risk of COVID-19-related outcome. Vaccine effectiveness (VE) was estimated by (1 - adjusted OR) × 100%. Subgroup analyses stratified by age (<65; ≥65 years), sex (male; female), and Charlson Comorbidity Index (<2; ≥2) were conducted. Simple linear regression on the VE point estimates was also used to test the linear trend in rate of change of VE after second or third-dose vaccination (Supplementary Figure 2).
Figure 1. Selection of cases and controls. (A) COVID-19-related hospitalization; (B) COVID-19-related mortality; COVID-19-related severe complications (ICU admission/ventilatory support).
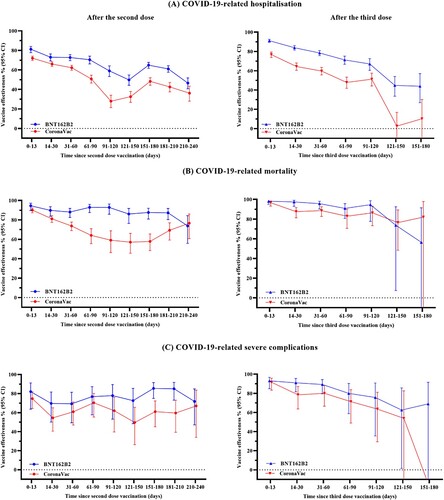
Figure 2. Vaccine effectiveness against COVID-19 outcomes over different time intervals after COVID-19 vaccination. This figure shows the vaccine effectiveness against COVID-19 outcomes over different time intervals after vaccination of BNT162b2 and CoronaVac. Results presented in this figure should not be interpreted as a direct comparison of effectiveness of the two vaccines.
All statistical tests were two-sided, and P values less than 0.05 were considered statistically significant. Statistical analysis was conducted using R version 4.0.3 (www.R-project.org). At least two investigators (VY, EW) conducted the statistical analyses independently for quality assurance. STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement checklists were followed to guide transparent reporting of the case–control study [Citation20].
Ethics approval
This study was approved by the Central Institutional Review Board of the Hospital Authority of Hong Kong (CIRB-2021-005-4) and the DH Ethics Committee (LM171/2021). Informed consent was waived by the ethics committee since this study only uses anonymized patient data.
Results
A total of 39,916 cases of COVID-19-related hospitalization, 7468 cases of COVID-19-related mortality, and 1950 COVID-19-related severe complications were matched with 406,687; 72,199 and 20,550 controls, respectively (). Baseline characteristics of cases and controls are summarized in . Time trends of new COVID-19 cases, hospitalization, mortality and severe complications during the study period are presented in Supplementary Figure 1.
Table 1. Baseline characteristics of cases and controls.
A significant risk reduction against COVID-19-related hospitalization with CoronaVac or BNT162b2 was observed for at least 240 days after the second dose and 120 days after the third dose when compared to the unvaccinated individuals, despite significant waning in VE (, and ). VE (95% CI) against COVID-19-related hospitalization decreased from 81.1% (77.7–84.1%) for BNT162b2 and 72.0% (69.7–74.1%) for CoronaVac at 0–13 days, to 46.6% (40.7–51.8%) for BNT162b2 and 36.2% (28.0–43.4%) for CoronaVac at 211–240 days after the second dose. For COVID-19-related mortality, VE for BNT162b2 remained consistently high from 0–13 days [94.6% (89.6–97.1%)] to 181–210 days [87.3% (80.2–91.8%)] after the second dose, and reduced to 73.8% (55.9–84.4%) by 211–240 days, whereas VE for CoronaVac waned gradually from 0–13 days [90.2% (87.3–92.3%)] to 211–240 days [76.6% (60.8–86.0%)] after the second dose. No significant waning of VE for COVID-19-related severe complications was observed, where VE was 82.1% (63.7–91.1%) for BNT162b2 and 74.7% (64.3–82.0%) for CoronaVac at 0–13 days, and 71.8% (47.2–84.9%) for BNT162b2 and 67.1% (34.0–83.6%) for CoronaVac at 211–240 days after the second dose.
Table 2. Vaccine effectiveness against COVID-19 outcomes over different time intervals after second-dose COVID-19 vaccination.
Table 3. Vaccine effectiveness against COVID-19 outcomes over different time intervals after third-dose COVID-19 vaccination.
Similar trends were observed for VE after the third vaccine dose (, ). VE against COVID-19-related hospitalization decreased from 91.2% (89.5–92.6%) for BNT162b2 and 76.7% (73.7–79.4%) for CoronaVac at 0–13 days, to 67.1% (60.4–72.6%) for BNT162b2 and 51.3% (44.2–57.5%) for CoronaVac at 91–120 days after the third dose. For COVID-19-related mortality, VE for BNT162b2 remained consistently high from 0–13 days [98.2% (95.0–99.3%)] to 91–120 days [94.6% (77.7–98.7%)] after the third dose, whereas VE for CoronaVac reduced gradually from 0–13 days [96.7% (93.2–98.4%)] to 91–120 days [86.4% (73.3–93.1%)] after the third dose. VE against COVID-19-related severe complications was 93.1% (84.9–96.8%) for BNT162b2 and 91.6% (83.3–95.8%) for CoronaVac at 0–13 days; and reduced to 75.7% (35.5–90.8%) for BNT162b2 and 63.6% (29.4–81.2%) for CoronaVac at 91–120 days after the third dose. We found no significant risk reduction against COVID-19-related mortality and severe complications for both CoronaVac and BNT162b2 by 151–180 days after the third dose compared to the unvaccinated, albeit this should be interpreted with caution due to the limited number of events.
In general, consistent trends of waning VE were also observed in all subgroups (Supplementary Table 2). Notably, VE against COVID-19-related hospitalization was generally higher with slower waning among individuals aged ≥65 years who received two doses of BNT162b2. Results from the main analyses were robust to sensitivity analyses (Supplementary Table 3). Estimated linear trend in waning VE was consistent with the main findings (Supplementary Figure 2), but should not be interpreted as a formal comparison between different vaccine platforms or second versus third dose.
Discussion
This study revealed that VE against severe and fatal COVID-19 with two to three doses of BNT162b2 and CoronaVac both decreased with time. The decline in VE was most notable within the first 90 days after the second dose, which is in line with the observation of waning immunity against Omicron infection after vaccination in other studies [Citation26,Citation27]. Although it was believed that protection against severe outcomes, in comparison to infection, generally persisted with time [Citation4,Citation5]. Our findings demonstrated a notable decline in VE after the third dose in terms of COVID-19-related hospitalization. Our findings highlight the importance of timely administration of additional doses in order to maintain protection against severe COVID-19.
In contrast to the previously reported effectiveness of ≥90% against COVID-19-related hospitalization and deaths after 20 weeks in the UK [Citation5] and up to 6 months after the second dose in the US [Citation28] against the Delta variant, our study recorded much lower values. During the current Omicron BA.2 epidemic in Hong Kong, the risk reduction with BNT162b2 observed at six months after the second dose was 61% against COVID-related hospitalization, 87% against COVID-19 mortality, and 85% against severe COVID-19 disease, respectively. This corresponds to the previous studies that described a lower VE against the Omicron variant when compared to earlier variants [Citation29,Citation30]. Our data are comparable with the observed effectiveness of BNT162b2 against hospitalization during the Omicron outbreak at 6–8 months in the US (42%, [34–50]) [Citation29] and at 5–6 months in South Africa (46% [39–51]) [Citation7]. VE of the third dose against hospitalization in our study was 67% (60–73) at 91–120 days and 45% (34–54) at 121–150 days, which is also similar to that in the US (4-<6 months: 66% [63–70]) and South Africa (3–4 months: 50% [4–74]) [Citation7]. Meanwhile, the waning effectiveness of CoronaVac has been less discussed. An increase in the cumulative incidence of COVID-19 infection among CoronaVac recipients over time has been documented [Citation31]. Another study conducted before the Omicron era compared COVID-19-related ICU admission and death rates between “early vaccinees” and “late vaccinees” and concluded that VE of CoronaVac against infection waned with time but effectiveness against mortality was persistent [Citation32]. At present, direct evidence is lacking. Our study bridged the research gap by demonstrating a decline in effectiveness of CoronaVac during the Omicron outbreak with time. At six months after the second dose, the effectiveness of CoronaVac was 43% against COVID-19-related hospitalization, 69% against COVID-19 mortality, and 60% against severe complications, respectively. Waning VE was also observed among three-dose recipients, especially in COVID-19-related hospitalization and severe COVID-19, while effectiveness against COVID-19-related mortality remained high within 120 days after the third dose, reaching 95% and 86% in BNT162b2 and CoronaVac recipients, respectively. Owing to the small number of outcome events recorded, the estimated VE beyond 120 days after the third dose should be interpreted with caution.
In general, the trend of waning protection against severe COVID-19 corresponds to the decline in the titre of neutralizing antibody against Omicron and spike-specific CD4+ and CD8+ T cells in the serum of healthy volunteers three months after the second dose of vaccine [Citation33]. It was observed that the titre of neutralizing antibody against Omicron decreased to the detection limit after three months among people who received CoronaVac [Citation33]. While other studies reported that spike-specific antibodies could last more than 6 months after natural infection despite a rigorous decline after 6 weeks [Citation34]. Nonetheless, some argued that the decline in humoral immunity does not necessarily predict a wane in vaccine protection [Citation35]. Yet the exact mechanism remains to be elucidated. On the other hand, it was postulated that SARS-CoV-2-specific humoral immunity provides more persistent protection against severe COVID-19 [Citation34,Citation36]. In the present study, we demonstrated that while vaccine protection against severe COVID-19 continued to wane over 8 months after the second dose, a substantial degree of protection remains. Nevertheless, vaccination with the third dose of vaccine would be warranted to provide a higher level of protection.
Our findings demonstrated that VE of both BNT162b2 and CoronaVac waned over time. In particular, a greater extent of decline was noted in people who received CoronaVac. Considering the effectiveness against COVID-19-related mortality, both BNT162b2 and CoronaVac offered at least 90% protection at the beginning. However, the effectiveness of CoronaVac decreased to 59% after 3 months since the second dose of vaccination while that of BNT162b2 was maintained at 93%. Although inactivated vaccines were also shown to elicit T cell response in addition to humoral response [Citation37], the disparity in the extent of T cell response has been reported in a study comparing blood samples from BNT162b2 recipients and CoronaVac recipients, which showed that more BNT162b2 recipients developed spike-specific CD4+ T cells and CD8+ T cells [Citation33]. While some studies hypothesized that there might be qualitative differences in addition to the quantitative differences amongst the T cell response triggered by mRNA vaccine and inactivated vaccine [Citation38], evidence that directly compares T cell response induced by BNT162b2 and CoronaVac remains limited, and further studies are warranted. In contrast, the effectiveness against mortality at 3 months after the third dose of BNT162b2 (94.6%) and CoronaVac (86.4%) was comparable, suggesting the importance of getting booster shots, especially in people who completed a primary series of CoronaVac.
By and large, waning immunity against Omicron after vaccination was observed. Nevertheless, booster shots were still largely effective. Based on a phase 4 randomized trial in Brazil, booster shots with either BNT162b2 or CoronaVac were able to raise IgG antibody levels substantially and increase neutralizing capacity against Omicron [Citation26]. In the present study, the effectiveness of BNT162b2 against hospitalization was 33.9% at 8 months after the second dose, but the effectiveness was as high as 83.9% one month after the third dose. The effectiveness study in the US also suggested a rise in the effectiveness of BNT162b2 against hospitalization from 45% at 8 months after the third dose, to 76% shortly after the fourth dose among adults above 65 years of age [Citation29]. This reinforces the importance of booster doses in combating different variants of concern during the COVID-19 pandemic.
This study is among the first to evaluate waning in VE of two to three doses of CoronaVac against Omicron in a Chinese population, where current evidence remains scarce in contrast to mRNA vaccines which are being studied more frequently. Owing to the suboptimal vaccine coverage in HK, this study enrolled a significant proportion of unvaccinated persons, hence allowing evaluation of the real-world protection of these vaccines. Our findings demonstrated that despite vaccine protection against severe or fatal COVID-19 waning significantly 8–9 months after the second dose, a substantial degree of protection remains. Nevertheless, timely vaccination with the third dose of vaccine, especially in those who received two doses of CoronaVac, would be warranted to provide a higher level of protection against severe COVID-19. Our results also shed some light on discussion of the optimal timing of the fourth vaccine dose.
This study has several limitations. First, VE beyond 180 days after the third dose of vaccine could not be estimated due to insufficient samples as it has been less than 10 months since the rollout of booster vaccination in the general population locally. Second, it was possible that some asymptomatic COVID-19 infections were not captured, since universal COVID-19 screening was not implemented in HK, as with most countries worldwide. Misclassification due to false negatives in PCR tests was also possible. However, PCR remains the gold standard for diagnosis owing to its high specificity >99% [Citation24], and the risk of false negatives was minimal in the analysis for severe or fatal COVID-19 disease. Third, there might be underdiagnosis as the need for ventilatory support was determined by the procedural codes in the electronic database. The rate of ICU admission was limited by its maximal capacity, and we could not eliminate the possibility that some severe cases who deteriorated rapidly died before being transferred to ICU. Nonetheless, these cases would have been captured by the COVID-19-related death outcome. Fourth, this study did not account for possible differences in health-seeking behaviour among vaccinated and unvaccinated individuals that may potentially put them at a higher risk of contracting COVID-19. Further, as with any observational studies, the possibility of confounding and selection bias could not be ruled out. Lastly, it should be noted that the findings of this study may not be generalizable to other COVID-19 vaccines.
Conclusion
Both CoronaVac and BNT162b2 were associated with a significant risk reduction against COVID-19-related hospitalization, death, and severe complications for at least 8 months after the second dose and 4 months after the third dose, when compared to the unvaccinated. However, a significant waning in VE over time was observed for both vaccines against COVID-19-related hospitalization and for CoronaVac against COVID-19-related mortality. Timely administration of booster doses could provide a higher level of protection against COVID-19-related hospitalization and mortality.
Data sharing
Data are not available as the data custodians (the Hospital Authority and the Department of Health of Hong Kong SAR) have not given permission for sharing due to patient confidentiality and privacy concerns. Local academic institutions, government departments, or non-governmental organizations may apply for the access to data through the Hospital Authority’s data sharing portal (https://www3.ha.org.hk/data).
Supplemental Material
Download MS Word (1.7 MB)Acknowledgements
We gratefully acknowledge the Centre for Health Protection, Department of Health and Hospital Authority for facilitating data access. ICKW is partially supported by the Laboratory of Data Discovery for Health (D24H) funded by AIR@InnoHK administered by the Innovation and Technology Commission. The corresponding authors had full access to all the data in the study and took final responsibility for the decision to submit for publication. Concept and design were prepared by VKCY, EYFW, ICKW, EWC. Acquisition, analysis, or interpretation of data was formulated by VKCY, EYFW, XY, FTTL, CSLC, XL, CKHW, PHL, TM, SQ, ICKW, EWC. Drafting of the manuscript was bone by VKCY, EYFW, AHYM. Critical revision of the manuscript for important intellectual content was done by all the authors. Statistical analysis: VKCY, EYFW, XY. Administrative, technical, or material support was given by ICKW, EWC. ICKW, EWC supervised the whole process.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Coronavirus Resource Center of the Johns Hopkins University School of Medicine. Understanding vaccination progress by country 2022 [29 Apr 2022]. Available from: https://coronavirus.jhu.edu/vaccines/international.
- HKSAR Government. Latest situation of reported cases of COVID-19 in Hong Kong 2022 [16 Apr 2022]. Available from: http://www.chp.gov.hk/files/misc/latest_situation_of_reported_cases_covid_19_eng.csv.
- Li C, Yu D, Wu X, et al. Twelve-month specific IgG response to SARS-CoV-2 receptor-binding domain among COVID-19 convalescent plasma donors in Wuhan. Nat Commun. 2021;12(1):1–9.
- Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24):e83.
- Andrews N, Tessier E, Stowe J, et al. Duration of protection against mild and severe disease by COVID-19 vaccines. N Engl J Med. 2022;386(4):340–350.
- Patalon T, Saciuk Y, Peretz A, et al. Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun. 2022;13(1):1–7.
- Collie S, Nayager J, Bamford L, et al. Effectiveness and durability of the BNT162b2 vaccine against omicron sublineages in South Africa. N Engl J Med. 2022;387(14):1332–1333.
- Mallapaty S. China’s COVID vaccines have been crucial – now immunity is waning. Nature. 2021 [cited 14 Oct 2021]. Available from: https://www.nature.com/articles/d41586-021-02796-w.
- Sauré D, O'Ryan M, Torres JP, et al. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis. 2022;22(1):56–63.
- Moderbacher CR, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19.
- Taylor L. COVID-19: Hong Kong reports world’s highest death rate as zero COVID strategy fails. Br Med J. 2022;376(o420):35177535.
- Wan EYF, Chui CSL, Lai FTT, et al. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. Lancet Infect Dis. 2021;21:00451–00455.
- Chua GT, Kwan MYW, Chui CSL, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination. Clin Infect Dis. 2022;75(4):673–681.
- Li X, Tong X, Yeung WWY, et al. Two-dose COVID-19 vaccination and possible arthritis flare among patients with rheumatoid arthritis in Hong Kong. Ann Rheum Dis. 2022;81(4):564–568.
- Lai FTT, Huang L, Chui CSL, et al. Multimorbidity and adverse events of special interest associated with COVID-19 vaccines in Hong Kong. Nat Commun. 2022;13(1):1–8.
- Lai FTT, Li X, Peng K, et al. Carditis After COVID-19 Vaccination With a Messenger RNA Vaccine and an Inactivated Virus Vaccine : A Case-Control Study. Ann Intern Med. 2022;175(3):362–370.
- Lai FTT, Huang L, Peng K, et al. Post-COVID-19-vaccination adverse events and healthcare utilization among individuals with or without previous SARS-CoV-2 infection. J Intern Med. 2022 Jun;291(6):864–869. doi:10.1111/joim.13453
- Li X, Tong X, Wong ICK, et al. Lack of inflammatory bowel disease flare-up following two-dose BNT162b2 vaccine: a population-based cohort study. Gut. 2022;71(12):2608–2611.
- Wan EYF, Chui CSL, Wang Y, et al. Herpes zoster related hospitalization after inactivated (CoronaVac) and mRNA (BNT162b2) SARS-CoV-2 vaccination: a self-controlled case series and nested case-control study. The Lancet Regional Health – Western Pacific. 2022;21:100393.
- Xiong X, Wong CKH, Au ICH, et al. Safety of inactivated and mRNA COVID-19 vaccination among patients treated for hypothyroidism: a population-based cohort study. Thyroid. 2022;32(5):505–514.
- Mesfin Y, Chen D, Bond H, et al. Epidemiology of infections with SARS-CoV-2 Omicron BA. 2 variant in Hong Kong, January–March 2022. medRxiv. 2022.
- Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers – eight U.S. locations, December 2020-march 2021. MMWR Morb Mortal Wkly Rep. 2021 Apr 2;70(13):495–500.
- Tanriover MD, Doğanay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021 Jul 17;398(10296):213–222.
- Miller TE, Garcia Beltran WF, Bard AZ, et al. Clinical sensitivity and interpretation of PCR and serological COVID-19 diagnostics for patients presenting to the hospital. FASEB J. 2020;34(10):13877–13884.
- Charlson ME, Groll D, To T, et al. Charlson comorbidity index. Nursing Research (New York). 2013;62(1):2.
- Costa Clemens SA, Weckx L, Clemens R, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–529.
- Andrews N, Stowe J, Kirsebom F, et al. COVID-19 vaccine effectiveness against the omicron (B. 1.1. 529) variant. N Engl J Med. 2022;386(16):1532–1546.
- Tartof SY, Slezak JM, Fischer H, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416.
- Ferdinands JM, Rao S, Dixon BE, et al. Waning of vaccine effectiveness against moderate and severe COVID-19 among adults in the US from the VISION network: test negative, case-control study. Br Med J. 2022;379:e072141.
- Gram MA, Emborg H-D, Schelde AB, et al. Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the alpha, delta, or omicron SARS-CoV-2 variant: a nationwide Danish Cohort Study. PLoS Med. 2022;19(9):e1003992.
- Can G, Acar HC, Aydin SN, et al. Waning effectiveness of CoronaVac in real life: a retrospective cohort study in health care workers. Vaccine. 2022;40(18):2574–2579.
- Suah JL, Husin M, Tok PSK, et al. Waning COVID-19 vaccine effectiveness for BNT162b2 and CoronaVac in Malaysia: an observational study. Int J Infect Dis. 2022;119:69–76.
- Peng Q, Zhou R, Wang Y, et al. Waning immune responses against SARS-CoV-2 variants of concern among vaccinees in Hong Kong. EBioMedicine. 2022;77:103904.
- Govender M, Hopkins FR, Göransson R, et al. T cell perturbations persist for at least 6 months following hospitalization for COVID-19. Front Immunol. 2022;13:931039.
- Krause PR, Fleming TR, Peto R, et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398(10308):1377–1380.
- Cevik M, Grubaugh ND, Iwasaki A, et al. COVID-19 vaccines: keeping pace with SARS-CoV-2 variants. Cell. 2021;184(20):5077–5081.
- Deng Y, Li Y, Yang R, et al. SARS-CoV-2-specific T cell immunity to structural proteins in inactivated COVID-19 vaccine recipients. Cell Mol Immunol. 2021;18(8):2040–2041.
- Vályi-Nagy I, Matula Z, Gönczi M, et al. Comparison of antibody and T cell responses elicited by BBIBP-CorV (Sinopharm) and BNT162b2 (Pfizer-BioNTech) vaccines against SARS-CoV-2 in healthy adult humans. GeroScience. 2021;43(5):2321–2331.

