ABSTRACT
Influenza A viruses (IAV) cause annual epidemics and occasional pandemics in humans. The most recent pandemic outbreak occurred in 2009 with H1N1pdm09. This virus, which most likely reassorted in swine before its transmission to humans, was reintroduced into the swine population and continues circulating ever since. In order to assess its potential to cause reassortants on a cellular level, human origin H1N1pdm09 and a recent Eurasian avian-like H1N1 swine IAV were (co-)passaged in the newly generated swine lung cell line C22. Co-infection with both viruses gave rise to numerous reassortants that additionally carry different mutations which can partially be found in nature as well. Reassortment most frequently affected the PB1, PA and NA segments with the swine IAV as recipient. These reassortants reached higher titers in swine lung cells and were able to replicate in genuine human lung tissue explants ex vivo, suggesting a possible zoonotic potential. Interestingly, reassortment and mutations in the viral ribonucleoprotein complex influence the viral polymerase activity in a cell type and species-specific manner. In summary, we demonstrate reassortment promiscuity of these viruses in a novel swine lung cell model and indicate a possible zoonotic potential of the reassortants.
Introduction
Influenza A viruses (IAV) cause seasonal epidemics (“flu”) in humans, which are associated with 294,000–518,000 deaths every year [Citation1]. A vaccine is available, but has to be adapted annually, due to the fast evolution of human-adapted IAV. Two major principles have to be differentiated regarding the evolution of these viruses and its impact on epidemics or even pandemics – the antigenic drift and the antigenic shift. Antigenic drift describes the accumulation (and immunological selection) of coding point mutations in parts of the viral genome encoding immunogenic epitopes due to the lack of proofreading activity of the viral RNA-dependent RNA polymerase. If these mutations occur in antigenic epitopes and allow the virus to escape from host immunity, this mechanism is at the basis of epidemics [Citation2]. Antigenic shift depends on the segmentation of the viral genome and co-infection with different parental viruses. When at least two IAV infect the same cell, they can exchange any of their eight genome segments, which theoretically can give rise to 254 new virus strains. This process is called reassortment and is of particular interest in the context of zoonotic IAV. Since IAV can infect not only humans, but also swine, birds and many other mammalian species, reassortants can originate from virus strains of different species origin. These reassortants can lead to occasional pandemics as last seen in 2009, when a reassortant originated from swine infected humans worldwide [Citation3]. The 2009 pandemic virus (pdm09) carried segments from the Eurasian avian-like (EA) swine influenza virus (SIV) lineage and a previously circulating triple reassortant that held segments from the classical swine H1N1 viruses, the north American avian lineage and a human seasonal H3N2 virus [Citation4]. After 2009, pdm09 was reintroduced into the swine population worldwide as a reverse zoonosis and is circulating since then. Importantly, it has been shown to create new reassortants with viruses of the EA swine lineage and the triple reassortant with some variants exhibiting elevated levels of infectivity in humans [Citation5].
Besides animal models, there are several traditional cell-based models classical to study IAV infections, like immortalized cell lines such as Madin-Darby Canine Kidney (MDCK) cells, Vero cells or human lung cells, e.g. A549 and Calu-3 cells [Citation6]. A problematic aspect of these models is the species-specific innate immune response to infections, rendering these cell lines insufficient to recapitulate SIV infection, cell-intrinsic adaptation, immune evasion and reassortment in the host, but alternative, more authentic swine cell models are rare [Citation7,Citation8].
In this study, we used a newly generated swine lung cell line to analyse the evolution of IAV in vitro, especially with respect to co-infection, reassortment and adaptation of pdm2009 and a recently circulating SIV known to give rise to reassortants in nature. Indeed, we identified numerous reassortants that might have a zoonotic potential, indicated by efficient replication in human lung tissue explants ex vivo.
Materials and methods
Biological resources
Viruses
Influenza A/Hamburg/04/09 (pdm09; EPI_ISL_30186) was taken from the virus collection of the Institute of Virology Muenster and A/swine/Germany/R1738/2010 (R1738/10; EPI_ISL_222129) was taken from the virus strain repository of the Friedrich-Loeffler-Institute, Greifswald, Isle of Riems, Germany.
Plaque-purified passaged viruses of A/Hamburg/04/09 are termed P-#, of A/swine/Germany/R1738/2010 R-# and from the co-infection Rpdm-x-#, with x being the segments derived from A/Hamburg/04/09.
Cell cultivation
All cell lines were cultivated at 37°C and 5% CO2. Madin-Darby canine kidney (MDCK) cells were grown in Minimum Essential Medium (MEM) with 5% fetal bovine serum (FBS). The cell line C22 was generated by subcloning from wild boar lung epithelial-like cell line WSL-R-HP (CCLV-RIE 1346, Collection of Cell Lines in Veterinary Medicine, Friedrich-Loeffler-Institute, Greifswald, Isle of Riems, Germany). Human embryonic kidney 293T (HEK293T) cells and human lung epithelial cell line A549 were originally purchased from ATCC and taken from the cell repository of the Institute of Virology Muenster, Germany. C22, A549 and HEK293T cells were cultivated in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS. At regular intervals, cells are checked for their identity by SNP-profiling (Multiplexion).
IAV infection and virus passaging
The initial infection of C22 cells was performed with multiplicity of infection (MOI) 4 for pdm09, MOI 5 for R1738/10 and MOI 4 + 5, respectively, for the co-infection for 8 h. Thereafter, supernatants were recovered and used for further passaging. Each well was infected with 500 μL virus dilution in triplicates. After 30 min of incubation at 37°C and 5% CO2, the virus was aspirated, cell monolayers were washed with PBS followed by incubation with DMEM high glucose, 1% 100× penicillin/streptomycin, 0.2% BSA, 0.01% MgCl2, 0.01% CaCl2, 0.02% TPCK-Trypsin (1 mg/ml in 1 mM acetic acid). For passaging, cells were washed with PBS and infected with successive 1:5 dilutions of the supernatant from the previous passage in PBS-infection containing 1% 100× penicillin/streptomycin, 0.2% BSA, 0.01% MgCl2, 0.01% CaCl2 as described above. After infection, cells were inspected every 24 h and supernatants were collected at 72 h post infection (h p.i.).
IAV infection of human lung explants
Tumor-free human lung tissue was obtained from patients undergoing lung surgery. Lung tissue was recovered in Roswell Park Memorial Institute 1640 (RPMI) medium and stored at 4°C until processing into blocks of ̴100 mg and incubated overnight at 37°C. For all experiments, untreated (mock-infected) tissue of the same donor served as a negative control. A virus dilution containing 1 × 106 PFU/ml was prepared in RPMI+++ (2 mM L-glutamine, 1% penicillin/streptomycin, 0.1% BSA) and lung pieces were infected in 2 ml. After 1 h of incubation at 37°C with 5% CO2, the tissue was washed twice, placed in fresh RPMI+++ and incubated at 37°C with 5% CO2 for 48 h. Supernatants were collected at 1, 24 and 48 h p.i..
Plaque assay
Virus-containing supernatants were serially diluted 1:10 in PBS-infection. MDCKII cells were washed with PBS and 500 μL of sample dilution were added to each well and cells were incubated at 37°C and 5% CO2. After 30 min, virus dilutions were aspirated, and cells were overlaid with freshly prepared pre-warmed plaque assay medium (9.9% 10X MEM, 0.2% BSA, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 0.3% DEAE-Dextran and 1.5% NaHCO3, 0.9% Agar, 0.01% MgCl2, 0.01% CaCl2, 0.02% TPCK-Trypsin (1 mg/ml in 1 mM acidic acid)). After agar solidification, plates were incubated for 2–3 days until plaques were visible.
Plaque purification
Once plaques from standard plaque assays were visible, selected plaques were collected by pinching through the agar layer with a 1 ml sterile micropipette tip and aspirating the agar plug. Each agar plug was collected in 500 μL of PBS-infection and was kept overnight at 4°C. At least 15 plaques were collected from each virus population.
Plaque morphology
Standard plaque assays were performed as described before and photos were taken at the time of counting. Pictures were analysed with the measurement tool of ImageJ 1.53t. For each virus, at least 30 plaques were counted, and plaque size was normalized to parental virus R1738/10.
Cytokine assay
Supernatants from mock- or IAV-infected human lung explants from 48 h p.i. were analysed using the bead-based immuno-assay LEGENDplex™ Human Anti-Virus Response Panel. Cytokine capturing was performed with a modified version of the manufacturer’s protocol by reducing the reaction volumes by half.
Minireplicon assay
C22 or HEK293T cells were transfected with a plasmid mixture of bidirectional pHW2000 vectors [Citation9] encoding the IAV polymerase subunits, polymerase basic proteins 1 and 2 (PB1 and PB2), polymerase acidic protein (PA) (200 ng each), and nucleoprotein (NP) (400 ng) as well as a polymerase I-driven plasmid (100 ng) expressing a negative-sense RNA coding for a luciferase under control of a viral promoter. Transfection was performed with Lipofectamine 2000 in OptiMEM for 4 h at 37°C. Afterwards, cells were washed with PBS and the medium was changed to DMEM 10% FBS for the next 20 h.
Subsequently, cells were lysed, and the luciferase activity was measured with a luminometer after addition of 1 mM luciferin in 5 mM KH2PO4 solution. Measured relative light units (RLU) were normalized to protein amounts measured by Bradford assay and the luciferase activity measured for the R1738/10 RNP was arbitrarily set to 100%.
RT-qPCR genotyping
For viral RNA isolation from virions, 140 μL of virus stock or supernatant from infected cells were used with the QIAamp Viral RNA Mini Kit according to the manufacturer´s instructions (“RT–PCR method B”). RT-qPCR genotyping was performed as described earlier [Citation10] with the primers listed in Table S1.
Sample preparation for next generation sequencing
For RT–PCR, we used primers that were designed to amplify each viral segment from either strain (Table S1). The One-Step RT–PCR system was used to amplify viral segments.
Illumina sequencing
Illumina Nextera XT chemistry and the MiSeq instrument were used. Briefly, ̴1 ng of pooled PCR products (5 μL of each segment) was used as template and introduced into library preparation with the Nextera XT DNA Sample preparation Kit. Then, samples were paired-end sequenced with the 2x 250 bp MiSeq Reagent Kit v2 with an average insert size of 300 bp on a MiSeq instrument. Library preparation and sequencing were conducted as recommended by the manufacturer. After automatic demultiplexing on the MiSeq instrument, the resulting fastq files were analysed with a specific bioinformatic pipeline (see Data Availability). At least three Illumina runs contained NA from pdm09 in a phW2000 vector under a T7 promoter as an error control that was processed in parallel with the experimental samples.
Bioinformatic analysis
The reads from each sequencing library were subjected to stringent quality control. Using Trimmomatic (v0.36), adapters and all reads with an average Phred quality score <20 were removed [Citation11]. Poor quality bases were trimmed using the parameter SLIDINGWINDOW:4:15 and only reads longer than 35 bases were retained for further analysis. Then, we aligned the processed reads from each sample to both the swine and pandemic viral genomes (genomic sequences shared via GISAID [Citation12], GISAID EpiFlu database accession numbers: EPI_ISL_222129, EPI_ISL_30186) using the Bowtie2 aligner (v2.3.4) [Citation13] in very-sensitive-local mapping mode. Next, using a custom script, we compared the alignment scores for each individual read in the mapped sample against both reference genomes assuming that each individual read must have better alignment quality to the reference genome segment it belongs to. Only those reads were retained in the mapped SAM file, which had a greater alignment score for that particular reference genome. In case that the read had the same alignment score for both references, the mapped read was retained in both reference alignment SAM files.
For each individual sequenced sample, both mapped SAM files were converted to BAM format, sorted and indexed using Samtools (v0.1.19) [Citation14]. Next, we used pysamstats (v1.1.2) to calculate the per base nucleotide frequency and per base mapping depth calculation for both reference mappings.
LoFreq (v2.1.2) [Citation15] was used for calling small insertions/deletion and SNP variants. Only positions with the coverage of at least 30 reads were tested for calling variants. Additionally, variants were filtered out, if the alternate allele count was less than 10 reads using BCFtools (v1.9) [Citation14]. Next, variants were classified into major and minor variants based on the allele frequency cut off of 0.51. Finally, all detected major variants in both reference alignments were subsequently incorporated into their corresponding genomes to produce consensus genomes.
Homology modelling
The amino acid sequences of HA from both viruses were used as targets for homology modelling using the SWISS MODEL server [Citation16]. The HA structure from A/Korea/01/2009 (H1N1) strain (PDB: 4EDA) was selected as template (sequence identity to our targets ≈99%) for the pdm09 HA. The HA structure of A/BrevigMission/1/1918 (H1N1) strain (PDB: 4EEF) was selected as template for R1738/10 HA (sequence identity to our targets ≈81%). The best 3D homology models were validated using the SAVES server.
Results
IAVs R1738/10 and pdm09 exhibit adaptational mutations upon serial passaging in swine lung cells
To model the cell-intrinsic evolution of IAV in the swine lung, we used the newly generated cell line C22, that was obtained by subcloning of WSL-R-HP, which were generated by spontaneous immortalization of epithelial-like cells from a wild boar swine lung. These cells were infected with either pdm09, R1738/10 or both viruses together at a high MOI, proven to reach at least 50% infection rate after single infection with each virus (supplementary Fig. S1A and B). After one replication cycle (8 h), new C22 cells were infected with serial dilutions of the supernatants derived from the initial single or co-infection, to achieve low MOI infections (< 0.5 MOI) allowing for 72 h of viral replication with moderate cytopathic effects. After seven blind passages (∼64 virus generations), we performed random selection of plaques for purification from each infection setup (A). All viral pools reached titers of at least 105 PFU/ml in all passages, and MOIs were efficiently kept below 0.5 PFU/cell (supplementary Fig. S1B). Thus, our passaging approach resulted in the generation of efficiently replicating viruses after seven passages.
Figure 1. Passaging in C22 swine lung cells affects viral replication. (A) Schematic outline of the experimental strategy. (B, C) C22 cells were infected with (B) R1738/10 (parental strain) or C22-passaged variants (R-1, R-2, R-5, R-7, R-13) or with (C) pdm09 (parental strain) or C22-passaged variants (P-1, P-2, P-5, P-6, P-14) with MOI 0.001. Virus-containing supernatants were collected at 24 h p.i. and virus titers were determined by standard plaque assays. Virus titers are depicted as mean ± SD of three biological replicates. One representative out of two independent experiments is shown. Statistical significance was determined by one-way ANOVA and Dunnett’s multiple comparison test against R1738/10 with *p ≤ 0.033, ** p ≤ 0.002, *** p ≤ 0.001.
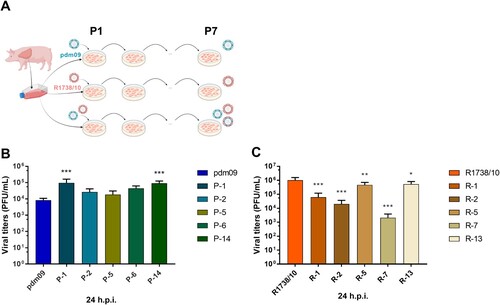
Next, we evaluated whether there were changes in viral fitness observable for plaque-purified viruses as a potential sign of further adaptation to the swine cells. Virus variants generated during separate passaging of pdm09 (termed as P#) exhibited similar or significantly higher titers compared to the parental virus (B). On the other hand, viruses developed during passaging of R1738/10 (termed as R#) all showed similar or significantly lower titers compared to the parental virus (C).
In order to identify potential mutations in the passaged viruses, virus genomes were analysed by NGS with a high sequencing depth (). Several high-frequency mutations (>51%) could be identified in all segments and the majority were coding. Of special interest was the S416N mutation in the NA segment of R-7, that seems to decrease viral replication in C22 cells by one order of magnitude compared to the already decreased replication of R-2. According to our models, position 416 is not close to the neuraminidase active site and the mutation from an uncharged polar amino acid to another amino acid of this kind, does not alter the electrostatic potential or hydrophobicity at this site (Supplementary Fig. S2B). However, position 416 is exposed on the upper surface of the protein and might therefore still slightly alter neuraminidase activity, which would explain the further decreased replication of R1738/10-R-7.
Table 1. Coding mutations in segments derived from R1738/10 respectively pdm09.
We can conclude that passaging of R1738/10 rather led to the introduction of neutral or detrimental mutations, while passaging of pdm09 resulted in the generation of mutations with neutral or beneficial effects on virus replication, potentially indicating further adaptation of pdm09 to C22 cells. In contrast, R1738/10 already seems to be highly adapted to swine cells and variation is therefore mostly disadvantageous.
IAVs R1738/10 and pdm09 efficiently reassorted during co-passaging in swine lung cells
As co-infection was performed to investigate reassortment in swine cells, we analysed the plaque-purified viruses by RT-qPCR genotyping [Citation10] and NGS regarding the parental origin of each viral segment. NGS data were analysed by a newly developed bioinformatic pipeline (A) allowing to clearly distinguish between pdm09 and R1738/10 segments with a high sequencing depth over the full length for all genomic segments (B).
Figure 2. The model gave rise to numerous reassortants. (A) Schematic depiction of the novel bioinformatic pipeline used to process sequencing data. (B) Sequencing depth (number of reads aligning to reference segments) versus nucleotide position are plotted. Reads from pdm09 were aligned against the reference genomes of pdm09 or R1738/10 strains and the best match was kept. Reads mapping against the pdm09 (blue) or R1738/10 (orange) reference are shown for each segment. (C) Segment origin of all strains was determined by RT-qPCR genotyping and confirmed by NGS. Segments derived from R1738/10 are depicted in orange and those derived from pdm09 in blue. (D) C22 or A549 cells were infected with parental strains R1738/10 or pdm09 or with co-infection isolates at low MOI (C22: 0.001; A549: 0.05). Virus-containing supernatants were collected at the indicated time points and viral titers were determined by standard plaque assays. Viral titers are depicted as mean ± SD of three independent experiments. Statistical significance was determined by two-way ANOVA and Dunnett’s multiple comparison test against pdm09 with *p ≤ 0.033, ** p ≤ 0.002, *** p ≤ 0.001. Significant differences were only found 48 h p.i. For C22: RCo-1, Rpdm-PB1/PA-1, Rpdm-PB1/PA/NA-1 and Rpdm-PB1/PA/NA-3 ***, RCo-2 **. For A549: RCo-1, RCo-2 and Rpdm-PB1/PA-1 ***, Rpdm-PB1/PA/NA-1 **.
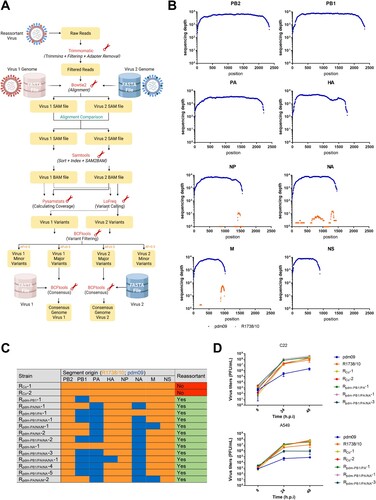
Surprisingly, 13 out of 15 randomly purified viruses were indeed reassortants (Rpdm-x-#, C). We identified in total eight different genotypes present in the viral population, indicating a high diversity of variants. All reassortments occurred in the R1738/10 background (recipient), with one to four segments deriving from pdm09 (donor). Reassortment was most frequent for segments PB1, PA and NA, while occurring only rarely for segments HA and M. In order to evaluate the effects of the observed reassortments/mutations on viral replication, C22 cells were infected with the parental strains or different virus variants (D top). The latter showed similar or even higher titers compared to the parental strain R1738/10, indicating an efficient adaptation of all reassortant viruses to C22 cells that exceeds that of R1738/10.
In order to test if these viruses can infect and replicate in human lung cells, we infected A549 cells (D) and all reassortants replicated more efficiently than pdm09 and, with exception of Rpdm-PB1/PA/NA-3, also reached higher titers than R1738/10. Thus, the reassortants have neutral or beneficial replication abilities not only in swine but also in human lung cells.
As an indicator for virus transmissibility, we evaluated the plaque morphology of these virus variants. Interestingly, we observed a significantly increased plaque size for the reassortants carrying pdmPB1/PA and especially pdmNA, however, plaque morphologies did not seem to correlate with the respective viral replication efficiencies of parental viruses and reassortants (supplementary Fig. S2A).
Interestingly, most frequent mutations in all experimental setups, and therefore potentially causative for the observed replication phenotypes, were found in HA proteins with amino acid 129 being mutated in all virus variants isolated from the single-infection and passaging of pdm09, and residue 186 that showed substitution in all viruses isolated after passaging of R1738/10 and two-thirds of the co-passaged viruses. We analysed the protein structure of these HAs using 3D homology models generated with a Swiss model server [Citation16]. Interestingly, both residues are exposed on the surface of the globular head domain of HA and a change from asparagine to aspartate or arginine to serine, respectively, modifies the electrostatic potential in the surrounding area (A and B). Positions 129 and 186 have been reported to be part of the receptor binding region [Citation17] and HA substitution N129D has already been shown to significantly increase the binding to α2,6-linked glycans, 6'-Sialyl-N-acetyllactosamine, 6’-Sulfo-6′SLN and 3′SLN glycans [Citation18]. Thus, these mutations most likely alter the receptor binding abilities of the respective viruses to C22 cells.
Figure 3. The originated mutations and reassortments could change essential protein functions. 3D homology models (partially shown) of (A) HA of pdm09 or of variant P-5, which carries the N129D mutation and (B) of HA1 of R1738/10 or of variant R-5, which carries the R186S mutation. Surface view of HA1 is shown, where the globular head domain (blue), all receptor binding sites (magenta) and (A) residue 129 or (B) residue 186 (red) are indicated. Gradient colourings indicating Coulombic electrostatic potential are shown. Position (A) 129 or (B) 186 and its surroundings are indicated by a black circle. (C) C22 or (D) HEK293T cells were transfected with the vRNP and a luciferase under a viral promotor. Luciferase activity was measured 24 h p.i. In reassorted RNPs, the proteins derived from pdm09 are indicated in the index. RLUs were normalized to R1738/10, and activities (coloured bars) are the mean of 3–8 independent experiments. Grey lines indicate the corresponding virus titer in (C) C22 or (D) A549 cells as determined in and , normalized to R1738/10 and shown logarithmic on the right y-axis as relative mean ± SD. Statistical significance was determined by one-way ANOVA and Dunnett’s multiple comparison test against R1738/10 with *p ≤ 0.033, ** p ≤ 0.002, *** p ≤ 0.001.
Further analysis of isolated sequences from swine H1N1 in Europe and Asia showed cooccurring motifs around position 186 with a clear change over the decades (supplementary Table S2). We see two different motifs that came up after 1999, the first is PTDS with an overall frequency of 45% and the other one is STSA with an overall frequency of 17%. Coming back to our identified mutation R186S this means, that we have to consider the surrounding amino acids to evaluate this position. 183PTD185 is in 96,1% of the analysed sequences followed by S186, which, together with the rise of this general motif over time, implicates a beneficial effect of this sequence in swine cells, respectively a disadvantageous effect of R186, considering that S186 is the annotated sequence for R1738/10 (EPI_ISL_222129) and changed predominantly to R186 after propagation in MDCK cells, while we still see about 30% of the virus carrying the serine.
We next investigated whether the mutations that emerged from our cell-based model would also occur in nature. First, we explored the frequency of the respective mutations in representative natural SIVs collected from different European countries between 2014 and 2017. Interestingly, some mutations in the R1738/10 segments were also quite frequent in natural isolates (), highlighting that a cell-intrinsic selection pressure may give rise to these specific mutations. In contrast, most of the mutations in the pdm09 segments are quite rare in natural swine isolates. As expected, silent mutations were in general more frequent than nonsynonymous mutations (supplementary Tabs. S3 and S4).
Different mutations and reassortments influence viral polymerase activity in a cell type specific manner
With the exception of the described HA mutations and one mutation in NS and NA, respectively, the other mutations were found in the segments coding for the viral polymerase or NP (). Due to the high variability of reassortment, the effects of these mutations cannot be evaluated solely by analysis of virus titers. Hence, we analysed the effects of mutations/reassortments on viral polymerase activity.
In C22 cells, the polymerase complex of R1738/10 showed a higher activity compared to the pdm09 polymerase complex, which is also reflected in the replication abilities of these viruses (C). To discriminate between effects caused by the mutations and effects of the segment reassortments, we first analysed the most common segment combination seen in isolated reassortants, mainly pdm09 PB1 and PA together with R1738/10 PB2 and NP (Ctrlpdm-PB1/PA). Interestingly, polymerase activity of this reassortant complex was similar to that measured for pdm09. Furthermore, a single reassortment with pdm09 PA (Ctrlpdm-PA) did not affect polymerase activity of the R1738/10 vRNP, whereas introduction of only the pdm09 PB1 segment (Ctrlpdm-PB1) induced a significant reduction. Furthermore, identified mutations in the proteins of the viral polymerase complex partially alter viral polymerase activity, but these effects cannot fully explain the changes observed in viral replication.
To analyse if these observations are also true in human cells, we investigated the activity of the same polymerase complexes in HEK293T cells (D). Here, the full pdm09 polymerase still showed a lower activity compared to R1738/10, but the reassortment with pdmPB1 and pdmPA did not significantly reduce viral polymerase activity, rather pointing towards cell/species specific activities. Rpdm-PB1/PA/NA-3 however showed a significantly reduced viral polymerase activity, caused by the unique mutations of this variant ().
The reassortants might hold a potential zoonotic risk for humans
To evaluate a potential zoonotic risk for humans posed by the identified reassortants and variants generated after co-infection, we infected genuine human lung tissue ex vivo and analysed viral replication (A).
Figure 4. Reassortants efficiently infect human lung tissue explants ex vivo. (A, B) Human lung tissue explants were infected with parental strains R1738/10 or pdm09 or with co-infection isolates. Virus-containing supernatants were collected at 1, 24 and 48 h p.i. and (A) virus titers were determined by standard plaque assays. Virus titers were normalized to 100 mg of lung tissue and are depicted as mean ± SEM of 5–6 independent experiments. Statistical significance was determined by two-way ANOVA and Dunnett’s multiple comparison test against pdm09 with *p ≤ 0.033, ** p ≤ 0.002, *** p ≤ 0.001. Only R1738/10 showed significant differences with * at 24 h p.i. and *** at 48 h p.i. (B) Cytokine/chemokine levels in the supernatants were determined and normalized to 100 mg of lung tissue. Results from 5–6 independent experiments are depicted with the mean indicated as a column. Statistical significance was determined by Kruskal Wallis non-parametric test against Mock with *p ≤ 0.033, ** p ≤ 0.002, *** p ≤ 0.001.
Here, passaged viruses did not show higher viral titers compared to R1738/10 as observed in A549 cells (D). Remarkably, only the reassortants were able to replicate in human lung tissue ex vivo, while RCo-1 and RCo-2 were not.
This difference might be the result of a stronger restriction of the non-replicating viruses by the innate immune response. Thus, we determined the levels of 13 different cytokines/chemokines in the supernatants of the infected human lung explants compared to mock infection (B).
However, for all induced chemokines/cytokines analysed, expression patterns correlated with the replication efficiencies of the respective viruses (A, B and supplementary Fig. S3), thus, decreases in viral replication cannot be explained by a stronger antiviral cytokine/chemokine response. The slow replicating virus Rpdm-PB1/PA−1 only induced detectable levels of IP-10, but none of the other chemokines/cytokines.
Taken together, reassortants derived from in vitro passaging in swine lung cells can efficiently infect and replicate not only in these cells, but also in human cell lines and lung tissue explants and, therefore, might carry a potential zoonotic risk for humans.
Discussion
IAV are known to induce seasonal epidemics in the human population. However, every 10–50 years, occurrence of a new IAV strain of zoonotic origin leads to a global pandemic in humans, as it happened last time in 2009 [Citation2,Citation19]. Since pandemic strains are antigenically distinct from previously circulating strains, they often spread rapidly and cause increased morbidity among the naïve human population [Citation3].
Swine are commonly regarded as a “mixing vessel” for the generation of pandemic influenza strains and, therefore, IAV infection of pigs is also crucial to human health [Citation20]. It is generally accepted that swine, but also other animal species, can serve as an intermediate host for avian influenza viruses and can transmit IAV to humans [Citation21]. Important for the relationship between swine and humans, humans can also infect swine in a reverse zoonotic manner [Citation22]. This has also been the case for pdm09 that is since then still circulating in the swine population [Citation23].
As these viruses are constantly developing in the swine population worldwide, it would be of great advantage to understand and model zoonotic spill over events.
In this study, we used a novel swine cell line as a model to analyse the evolution of two compatible H1N1 viruses and detected reassortants of an EA virus strain and pdm09. About 87% of our purified viruses from the co-infection and passaging were reassortants, reflecting the previously estimated reassortment rate of 88% between viruses without any segment mismatch [Citation24]. However, none of the isolated reassortant viruses showed the genetic composition of the reassortants isolated from swine in nature, which usually carry all internal genes or the full viral ribonucleoprotein (vRNP) of pdm09 [Citation5,Citation25,Citation26].
The PB2 segment from the EA SIV strain was retained in all isolated reassortants and carries the D701N mutation, while PB2 of pdm09 does not. This mutation is known to enhance polymerase activity and promote nuclear import of vRNPs into the nucleus in mammalian cells [Citation27]. It has also been shown to play an important role in the adaptation of avian viruses to mammalian hosts, especially to the pro-viral host proteins ANP32A, which is the most common protein family member in swine, dogs and horses. In human and mice, ANP32B is the preferred cofactor, where PB2 E627 K is the respective adaptational mutation [Citation28]. Accordingly, PB2 D701N has been shown to enhance viral replication and pathogenicity of EA like swine influenza viruses and of pdm09 viruses in mice and ferrets [Citation29,Citation30]. Furthermore, this mutation might also contribute to an increased pathogenicity in humans [Citation31]. The fitness advantage and the high selection pressure in our cell system could be possible explanations for the presence of EA PB2 in all isolated reassortants.
Among six different subtypes of influenza viruses, EA SIV was recently estimated to have the highest risk to cause a pandemic in humans [Citation32]. Along this line, we were able to show that reassortants of pdm09 and an EA SIV cannot only infect swine lung cells, but also human A549 cells and human lung explants ex vivo, without any previous adaptation or passaging in the human host. Replication efficiency in human lung explants exceeded that of human-derived pdm09, suggesting a possible zoonotic potential of these kind of reassortants as a prerequisite of a pandemic potential that was indicated before. Another interesting finding in the human lung tissue explants was the observation that R1738/10 was able to replicate to high viral titers. This fits with data from the literature, as EA SIV have in principle already been shown to be able to infect humans and replicate in human lung tissue and harbour full resistance to human MxA [Citation33, Citation29].
Taken together, we demonstrated that we can model the evolution of IAV in vitro, including reassortment events as well as mutations in the whole genome, showing correlation to what has been detected in nature. This indicates that the selection pressure, that provokes many of the changes seen in nature, already occurs on a cell-intrinsic level. Although the found mutations and reassortants do not fully reflect these processes, they give interesting insights into the interplay of the two viral strains that are likely to play a role in the generation of future pandemics. We furthermore substantiated a possible zoonotic potential of the generated reassortants, underlining the importance of further studies on these virus strains and their reassortants. These studies should be performed in appropriate models with respect to the situation in swine, as illustrated by the cell specific effects we observed in this study.
Ethics approval and consent to participate
All patients gave their written consent to donate lung tissue for scientific purpose. Ethical approval was given by the ethical council of the Deutsche Ärztekammer (AZ: 2016-265-f-S).
Availability of data and materials
The data underlying this article are available in the NCBI Sequence Read Archive (SRA), at https://www.ncbi.nlm.nih.gov/sra/PRJNA901904.
The bioinformatic pipeline used for processing of the next generation sequencing data is available at https://github.com/shreygandhi1990/ViReassort.
Molecular graphics and analyses were performed with UCSF ChimeraX, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from National Institutes of Health R01-GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases [Citation34].
Comparison of found mutations with the natural situation was performed using the Influenza research database [Citation35]. The frequency was estimated with available data in August 2022 in at least 1748 sequences younger than 2009 from R1738/10 isolated worldwide.
Supplemental Material
Download Zip (5 MB)Acknowledgements
We thank Michael Wojak, Michael Sulk, Saskia Hinse and Katharina Schlug for their support in experimental work. We gratefully acknowledge all data contributors, i.e. the authors and their originating laboratories responsible for obtaining the specimens, and their submitting laboratories for generating the genetic sequence and metadata and sharing via the GISAID Initiative, on which this research is based. (A) and (B) were created with BioRender.com. LB, MB, YB, TH and SL are members of the German FluResearchNet.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Paget J, Spreeuwenberg P, Charu V, et al. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR project. J Glob Health. 2019;9:20421.
- Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers. 2018;4:3.
- Peteranderl C, Herold S, Schmoldt C. Human influenza virus infections. Semin Respir Crit Care Med. 2016;37:487–500.
- Arias CF, Escalera-Zamudio M, MdLD S-DR, et al. Molecular anatomy of 2009 influenza virus A (H1N1). Arch Med Res. 2009;40:643–654.
- Sun H, Xiao Y, Liu J, et al. Prevalent Eurasian avian-like H1N1 swine influenza virus with 2009 pandemic viral genes facilitating human infection. Proc Natl Acad Sci U S A. 2020;117:17204–17210.
- Chua SCJH, Tan HQ, Engelberg D, et al. Alternative experimental models for studying influenza proteins, host-virus interactions and anti-influenza drugs. Pharmaceuticals (Basel). 2019: 12. doi:10.3390/ph12040147.
- Thomas M, Pierson M, Uprety T, et al. Comparison of porcine airway and intestinal epithelial cell lines for the susceptibility and expression of pattern recognition receptors upon influenza virus infection. Viruses. 2018: 10. doi:10.3390/v10060312.
- Meliopoulos V, Cherry S, Wohlgemuth N, et al. Primary swine respiratory epithelial cell lines for the efficient isolation and propagation of influenza A viruses. J Virol. 2020: 94. doi:10.1128/JVI.01091-20.
- Hoffmann E, Neumann G, Kawaoka Y, et al. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci U S A. 2000;97:6108–6113.
- Henritzi D, Zhao N, Starick E, et al. Rapid detection and subtyping of European swine influenza viruses in porcine clinical samples by haemagglutinin- and neuraminidase-specific tetra- and triplex real-time RT-PCRs. Influenza and Other Respiratory Viruses [Internet]. 2016;10:504–517. Available from: https://onlinelibrary.wiley.com/doi/10.1111irv.12407.
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120.
- Shu Y, McCauley J. GISAID: global initiative on sharing all influenza data - from vision to reality. Euro Surveill. 2017: 22. doi:10.2807/1560-7917.ES.2017.22.13.30494.
- Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359.
- Danecek P, Bonfield JK, Liddle J, et al. Twelve years of SAMtools and BCFtools. Gigascience. 2021: 10. doi:10.1093/gigascience/giab008.
- Wilm A, Aw PPK, Bertrand D, et al. Lofreq: a sequence-quality aware, ultra-sensitive variant caller for uncovering cell-population heterogeneity from high-throughput sequencing datasets. Nucleic Acids Res. 2012;40:11189–11201.
- Waterhouse A, Bertoni M, Bienert S, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303.
- Sriwilaijaroen N, Suzuki Y. Molecular basis of the structure and function of H1 hemagglutinin of influenza virus. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:226–249.
- Lee N, Khalenkov AM, Lugovtsev VY, et al. The use of plant lectins to regulate H1N1 influenza A virus receptor binding activity. PLoS One. 2018;13:e0195525.
- Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939.
- Chauhan RP, Gordon ML. A systematic review analyzing the prevalence and circulation of influenza viruses in swine population worldwide. Pathogens. 2020: 9. doi:10.3390/pathogens9050355.
- van Poucke SGM, Nicholls JM, Nauwynck HJ, et al. Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J. 2010;7:38.
- Nelson MI, Vincent AL. Reverse zoonosis of influenza to swine: new perspectives on the human-animal interface. Trends Microbiol. 2015;23:142–153.
- Sooksawasdi Na Ayudhya S, Kuiken T. Reverse zoonosis of COVID-19: lessons from the 2009 influenza pandemic. Vet Pathol. 2021;58:234–242.
- Marshall N, Priyamvada L, Ende Z, et al. Influenza virus reassortment occurs with high frequency in the absence of segment mismatch. PLoS Pathog. 2013;9:e1003421.
- Feng Z, Zhu W, Yang L, et al. Epidemiology and genotypic diversity of eurasian avian-like H1N1 swine influenza viruses in China. Virol Sin. 2021;36:43–51.
- Henritzi D, Petric PP, Lewis NS, et al. Surveillance of European domestic Pig populations identifies an emerging reservoir of potentially zoonotic swine influenza A viruses. Cell Host Microbe. 2020;28:614–627.
- Sediri H, Schwalm F, Gabriel G, et al. Adaptive mutation PB2 D701N promotes nuclear import of influenza vRNPs in mammalian cells. Eur J Cell Biol. 2015;94:368–374.
- Peacock TP, Sheppard CM, Lister MG, et al. Mammalian ANP32A and ANP32B proteins drive differential polymerase adaptations in avian influenza virus. J Virol. 2023: e0021323.
- Liu S, Zhu W, Feng Z, et al. Substitution of D701N in the PB2 protein could enhance the viral replication and pathogenicity of Eurasian avian-like H1N1 swine influenza viruses. Emerg Microbes Infect. 2018;7:75.
- Zhou B, Pearce MB, Li Y, et al. Asparagine substitution at PB2 residue 701 enhances the replication, pathogenicity, and transmission of the 2009 pandemic H1N1 influenza A virus. PLoS One. 2013;8:e67616.
- Nieto A, Pozo F, Vidal-García M, et al. Identification of rare PB2-D701N mutation from a patient with severe influenza: contribution of the PB2-D701N mutation to the pathogenicity of human influenza. Front Microbiol. 2017;8:575.
- Yang H, Chen Y, Qiao C, et al. Prevalence, genetics, and transmissibility in ferrets of eurasian avian-like H1N1 swine influenza viruses. Proc Natl Acad Sci U S A. 2016;113:392–397.
- Parys A, Vandoorn E, King J, et al. Human infection with eurasian avian-like swine influenza A(H1N1) virus, The Netherlands, September 2019. Emerg Infect Dis. 2021;27:939–943.
- Pettersen EF, Goddard TD, Huang CC, et al. UCSF chimerax: structure visualization for researchers, educators, and developers. Protein Science: A Publication of the Protein Society [Internet]. 2021;30:70–82. Available from: https://pubmed.ncbi.nlm.nih.gov/32881101/.
- Zhang Y, Aevermann BD, Anderson TK, et al. Influenza research database: An integrated bioinformatics resource for influenza virus research. Nucleic Acids Res [Internet]. 2017;45:D466–D474. Available from: https://pubmed.ncbi.nlm.nih.gov/27679478/.

