ABSTRACT
Hepatitis E virus (HEV) is the leading cause of acute viral hepatitis. Numerous studies have investigated the dynamics of HEV infection markers, but the most suitable marker for diagnosing ongoing or recent HEV infection remains to be determined. Recent evidence suggests that serum antigen testing is superior to serum IgM and RNA quantification. Moreover, it has been found that infected individuals excrete HEV antigen in significant quantities through urine. To address this question, we conducted a longitudinal analysis involving 16 patients with acute or chronic HEV infection in an area where genotype 3 HEV is prevalent. Our findings indicate that the diagnostic and monitoring capabilities of antigen testing for HEV infection can be further enhanced by measuring it in urine. Additionally, we were able to demonstrate that this enhancement is likely due to the presence of HEV-reactive IgG in blood plasma, which hampers efficient detection of HEV antigen through sandwich ELISA. In conclusion, urine-based antigen testing appears to be superior to measuring anti-HEV antibodies or viral RNA for diagnosing suspected HEV infection and monitoring ongoing infections.
With great interest we read the article of Tang et al. entitled “Profile of clinical characteristics and serologic markers of sporadic hepatitis E in a community cohort study” who investigated the dynamics of serological tests including hepatitis E virus (HEV) antigen (Ag), anti-HEV IgM and HEV RNA for diagnosing ongoing and recent HEV infections in a patient cohort in China, where genotype 4 (GT4) HEV is most prevalent [Citation1]. The authors show a limited performance of current anti-HEV IgM assays. Accordingly, and due to our limited understanding of the dynamics of HEV markers HEV RNA detection remains the gold standard for the detection of HEV infection [Citation1–3], but other studies have indicated that HEV Ag might be a more sensitive marker for diagnosing HEV infections [Citation4,Citation5]. In their manuscript, the authors describe the superiority of HEV Ag testing over HEV RNA, IgM or IgG testing for diagnosing early HEV infection but also show a rapid decline in HEV Ag and HEV RNA after the second week after symptom onset [Citation1]. Ag testing may be faster and more affordable than nucleic acid tests and can potentially even be used as an on-site diagnostic to test for suspected HEV infection.
To further enhance our understanding of HEV infection and Ag dynamics and its utility for diagnostics, we evaluated the detection of HEV Ag in different body fluids of HEV GT3 infected patients. After it was demonstrated that HEV Ag can be detected in urine [Citation6,Citation7] and that the titres of Ag are notably higher in individuals with chronic infections [Citation5], we analysed longitudinal plasma, stool and urine samples from 16 patients from two centres in Germany. None of the patients had a history of travelling, indicating that all of these were infected with autochthonous transmitted HEV GT3. We assessed the practicability of HEV Ag using the Wantai HEV-Ag ELISA in plasma and urine samples, while also testing for HEV RNA in plasma and stool and anti-HEV IgG in plasma (, Supplementary Figure 1, and Supplementary Table 1). In all patients but one (patient F), in which RNA was measured in both, plasma and stool, a consistent overlap of RNA positivity was found between these two body fluids. Consistent with Tang et al., we observed a strong correlation between HEV RNA positivity in plasma and stool and Ag detection in plasma [Citation1]. Remarkably, and in line with previous findings that were reported for HEV GT4 [Citation7], we observed higher levels of Ag in the urine of both, individuals with acute and chronic HEV GT3 infection, compared to plasma (D). Even after achieving RNA negativity in the plasma, the Ag levels remained detectable in plasma and urine of all patients except one (patient L). Moreover, a delayed decline of Ag titres was evident in urine compared to plasma for all patients, except patient L. It is noteworthy that HEV Ag was still detectable in the urine of one patient 5 months after achieving RNA negativity (A, patient C). In addition, for one patient RBV treatment was stopped after achieving RNA negativity while Ag remained detectable in plasma and urine leading to a viral rebound ∼20 weeks after stopping RBV (A, patient J). These findings point to a superiority of HEV Ag over RNA for the diagnosis of HEV GT3 infections. Furthermore, testing for HEV Ag in urine might significantly enhance the sensitivity of the assay and extend the period of positive test results beyond the current gold standard of RNA testing.
Figure 1. Hepatitis E virus Ag detection in urine is more efficient than Ag detection in blood plasma due to the lack of ORF2-reactive IgG. A) Kinetics of HEV Ag in urine and blood plasma, anti-HEV IgG in plasma and HEV RNA in plasma and stool (positive + and negative −) in selected patients with acute or chronic hepatitis E virus infection. The duration of ribavirin treatment (off-label) is indicated. Red symbols indicate values that were defined as negative by the manufacturer’s instructions. The kinetics of each patient in the cohort are shown in supplementary Figure 1. B) To exclude non-specific effects of the body fluid on the detection of HEV Ag, we diluted HEV Ag positive urine or blood plasma in HEV negative urine and blood plasma and detected Ag levels by ELISA. Error bars indicate the SEM. The assay was performed in technical duplicates. C) To show that anti-HEV IgG is responsible for the reduced detection of HEV Ag in blood plasma when compared to urine we titrated the anti-HEV IgG reference serum in HEV Ag positive urine and subsequently analysed the samples by Ag ELISA. Error bars indicate the SEM. (n = 3) D) The HEV Ag signals measured in the simultaneously taken urine and plasma samples are plotted. Error bars indicate the SEM. Statistical significance was calculated using Student’s t-test. (n = 44) Schematics were created in BioRender (https://www.biorender.com/).
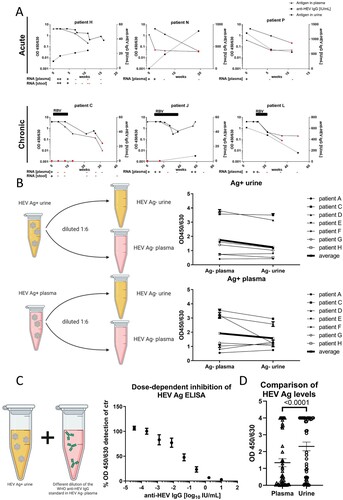
To exclude non-specific effects of urine or plasma on the performance of the ELISA, we diluted HEV Ag positive urine or plasma in Ag negative urine and plasma, respectively (B). Using this approach, we were able to rule out non-specific modulation of Ag detection in urine.
Therefore, we hypothesized that antibodies that are usually absent in urine might interfere with the efficient detection of HEV Ag in plasma. To test this hypothesis, we titrated the anti-HEV IgG reference serum in Ag positive urine and subsequently quantified HEV Ag by ELISA (C). We observed a robust and dose-dependent reduction of HEV Ag detection by the WHO reference serum, confirming the interference of anti-HEV IgG in plasma with reliable Ag measurement and indicating Ag-IgG complex formation that could also lead to an inhibition of IgG quantification.
Discussion
In congruence with the observation of Tang et al. HEV Ag detection seems to be superior in the detection of ongoing or recent infection than the detection of RNA also in a region where GT3 is most prevalent [Citation1]. The rapid decrease in HEV Ag levels observed by Tang et al. is likely due to a robust increase in anti-HEV IgG that is also evident in their data [Citation1]. This behaviour nicely reflects our observation that anti-HEV IgG interferes with HEV Ag detection in a dose-dependent manner. Such antibodies should be absent in the urine of the patients under normal conditions which may enhance the sensitivity of HEV Ag detection in this body fluid. However, our study is limited by the substantial fraction of immunosuppressed patients in which seroconversion might be limited, possibly increasing plasma antigen by the lack or reduced titres of anti-HEV IgG. Elevated hepatic antigen expression was previously shown in immunosuppressed rats and macaques and elevated HEV titres were observed in plasma and stool of immune-compromised mice with humanized livers [Citation8–10]. Recently, a higher sensitivity of Ag testing in the urine of mice and rabbits was described that was due to an enrichment of ORF2 in renal cells leading to accumulation of ORF2 in urine of the animals, possibly indicating a second explanation for the higher sensitivity of Ag testing in urine in addition to the lack of anti-ORF2 IgG [Citation6]. Interestingly, one of the patients in our cohort did not display comparable HEV Ag kinetics in urine and also suffered from haematuria that likely increased anti-HEV IgG in the urine and lead to reduced signal in the HEV Ag ELISA.
In summary, HEV Ag detection in the urine seems to be a fast and easy to perform assay for the diagnosis of ongoing and recent HEV infection that shows higher sensitivity than the detection of HEV RNA or Ag in plasma of acutely and chronically infected individuals. Due to the delayed reduction of Ag in urine, it can also be used to monitor HEV infections >2 weeks after symptom onset giving the clinicians the possibility to reliably detect a recent HEV infection even when patients are already HEV RNA negative. Since our study only includes four non-immunocompromised individuals, the performance of urine antigen testing needs to be evaluated in greater numbers and in particular immunocompetent patients. As HEV circulates as one serotype, our data is likely translatable for different genotypes.
Supplemental Material
Download MS Word (581.5 KB)Acknowledgements
The schematics of experimental procedures were created with BioRender (https://www.biorender.com/; the agreement numbers of the publication licenses are TM258TRAKT and RD25501UH3).
Disclosure statement
BM: funding: Altona Diagnostics, Roche, Fujirebio; consulting: Roche; lectures: Roche, Fujirebio; HW: clinical trials: Abbvie, Altimmune, Bristol-Myers-Squibb, Gilead, Janssen, MYR GmbH, Novartis, Vir Biotechnology; grants: Abbvie, Biotest AG, Gilead; consulting or advisory board: Abbvie, Aligos Therapeutics, Altimmune, Biotest AG, Bristol-Myers-Squibb, BTG pharmaceuticals, Dicerna Pharmaceuticals, Enanta Pharmaceuticals, Gilead, Janssen, Merck KGaA, MYR GmbH, Roche, Vir Biotechnology; lectures: Falk Foundation, Gilead, Merck KGaA. YS, IK, HH, BB, HHJS, TP, BS and PB have nothing to declare.
Additional information
Funding
References
- Tang ZM, Wen GP, Ying D, et al. Profile of clinical characteristics and serologic markers of sporadic hepatitis E in a community cohort study. Emerg Microbes Infect [Internet]. 2023 [cited 2023 Mar 7];12(1):2140613. Available from: https://pubmed.ncbi.nlm.nih.gov/36314245/.
- Lu J, Huang Y, Wang P, et al. Dynamics of Hepatitis E Virus (HEV) antibodies and development of a multifactorial model To improve the diagnosis of HEV infection in resource-limited settings. J Clin Microbiol [Internet]. 2021 [cited 2023 Mar 7];59(2):e02321-20. Available from: https://pubmed.ncbi.nlm.nih.gov/33239375/.
- Dalton HR, Kamar N, Baylis SA, et al. EASL clinical practice guidelines on hepatitis E virus infection q. J Hepatol. 2018;68:1256–1271.
- Wen GP, Tang ZM, Yang F, et al. A valuable antigen detection method for diagnosis of acute hepatitis E. J Clin Microbiol [Internet]. 2015 [cited 2023 Mar 7];53:782. Available from: /pmc/articles/PMC4390668/.
- Behrendt P, Bremer B, Todt D, et al. Hepatitis E virus (HEV) ORF2 antigen levels differentiate between acute and chronic HEV infection. J Infect Dis [Internet]. 2016 [cited 2023 Mar 13];214:361–368. Available from: https://academic.oup.com/jid/article/214/3/361/2577407.
- Ying D, He Q, Tian W, et al. Urine is a viral antigen reservoir in hepatitis E virus infection. Hepatology [Internet]. 2022 [cited 2023 Mar 7];77(5):1722–1734. Available from: https://onlinelibrary.wiley.com/doi/full/10.1002hep.32745.
- Geng Y, Zhao C, Huang W, et al. Detection and assessment of infectivity of hepatitis E virus in urine. J Hepatol. 2016;64:37–43.
- Sridhar S, Wu S, Situ J, et al. A small animal model of chronic hepatitis E infection using immunocompromised rats. JHEP Rep [Internet]. 2022 [cited 2023 Jul 19];4(10):100546. Available from: /pmc/articles/PMC9424580/.
- Gardinali NR, Guimarães JR, Melgaço JG, et al. Cynomolgus monkeys are successfully and persistently infected with hepatitis E virus genotype 3 (HEV-3) after long-term immunosuppressive therapy. Plos One [Internet]. 2017 [cited 2023 Jul 19];12:e0174070. Available from: https://journals.plos.org/plosone/article?id=10.1371journal.pone.0174070.
- Sayed IM, Verhoye L, Cocquerel L, et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut [Internet]. 2017 [cited 2023 Aug 1];66:920–929. Available from: https://gut.bmj.com/content/66/5/920.
