ABSTRACT
The chaetotaxy distribution pattern of surface nerve sensilla on the surface of both Schistosoma mansoni and S. haematobium was studied after exposure to silver nitrate treatment followed by light. This distribution pattern could not be achieved earlier due to namelessness, crowded nerve sensilla and twist of most parasites. In S. mansoni, silver impregnation technique displays nerves as ventral nerve cord, ventral sucker nerve that branches to three rings which innervate the ventral sucker. This technique shows the oral sucker ring, ring commissures and bipolar or multipolar nerve cells detected dorsally. In S. haematbium, nerves stained in S. mansoni were also stained in addition to the oral nerve and oral sucker ring. Furthermore, a number of crucial nerves were identified and their innervations to relevant organs were thoroughly examined. A confocal anti-FMRF amide technique was applied to whole-mount worms of both species. In addition to the general tegumental muscles stated previously and stated in this research specific nerves were also described and referred to a specific organ that assumed to innervate. In conclusion, the findings of the current study differentiate between S. mansoni and S. mansoni using chaetotaxy map as a step for spotlighting on FMRF amidergic components as antihelmintic target in future.
Introduction
The silver impregnated technique method for Lynch [Citation1] was used to identify the surface cell junctions on the monogeneas and digeneas. Shinn et al. [Citation2–4] have used such method as a chaetotaxy map to discriminant different species of monogeneas.
Chaetotaxy was used previously for the differentiation between species of the genus Brachylaima (trematoda) such as B. cribbi [Citation5], B. mascomain [Citation6] and B. aspersae [Citation7].
Histochemical and histological methods were employed by Bhatnager et al. [Citation8] to investigate the neuroendocrine and structural elements of Ceylonocotyle scoliocoelium. In addition, neurosecretory cells type A and type B of both Fasciola hepatica & F. gigantica were studied [Citation9,Citation10].
Over the past 15 years, numerous immunocytochemical studies have used confocal imaging with phalloidin-fluorescence technique for F-actin to demonstrate aminergie & peptidergic components of the nervous system of monogeneas and digeneas [Citation11–13].
Concerning F. hepatica, the localization and distribution of nervous system neuropeptides by indirect immuno- florescence technique were studied [Citation14,Citation15]. For the Frog-lung fluke, Haplometra cylindracea and Haematoloeches medioplexus; the nervous system cholinergic, serotoninergic and peptidergic components were studied [Citation16,Citation17].
FMRFamidergic neurons of trematoda Dicrocoelium lanceatum (lancet fluke) were identified in paired brain ganglia, in the brain commissure, longitudinal nerve cords and connective nerve commissures. The innervation of the oral and ventral suckers by peptidergic nerve structures was revealed [Citation18].
The immunocytochemical investigations and confocal scanning laser microscopy show the presence of serotonin and FMRFamide-like immunoreactivity in the nervous system elements in various parts of the digestive, reproductive and excretory systems of trematodes [Citation19].
For Schistosoma spps., the major muscle systems of both sex adult S. mansoni were studied using the filamentous actin marker, FITC- conjugated phalloidin [Citation15,Citation20]. The presence and distribution of neuropeptides belong to the both sex adult S. mansoni were investigated by indirect immunofluorescence technique [Citation21]. As a first step toward highlighting FMRF amidergic components as anthelmintic targets, it is imperative to pay close attention to the muscular systems of both Schistosoma spp., S. haematobium & S. mansoni, and to compare them using chaetotaxy maps.
Materials and methods
Worms of Schistosoma mansoni& S.heamatobium obtained from Biological Production Unite (BPU) of Theodore Bilharz Research Institute (TBRI, Giza, Egypt). Schistosoma mansoni was perfused from the hepatic portal vessels and mesenteric veins of infected hamster, while Schistosoma haematobium was perfused from veins of urinary bladder using perfusion pumb containing phosphate -buffered saline (PBS) and rapidly placed in culture media (RPMI-1640).
The body apertures and surface sensilla were localized using Lynch’s [Citation1] silver nitrate staining technique.-
Twenty five worms of S.haematobium and thirty worms of S. mansoni were used. Living, unflattened specimens expelled from a pipette into a dish containing hot (75° C-80° C) 1% silver nitrate solution. They placed in sunlight for 3-7 min until the color of the parasites changes to brown. The silver nitrate solution withdrawn and the parasites washed with distilled water. Finally, they dehydrated in ascending series of ethanol, cleared in terpinol, mounted in DPX and examined using the light microscope.
For neuromuscularation
For each species, 25 worms were used. Worms flattened between microscope slides and coverslips and fixed in 4% (w/v) paraformaldehyde (PFA) in 0.1 M phosphate-buffered saline (PBS) for 1 h. They were transferred to fresh fixative for further 3hrs.Worms were placed in 0.1 M antibody dilutent (AbD) and immediately air-mailed to Belfast.Worms were incubated with the following primary (antisera, anti-FMRFamide raised in New Zealand white rabbits) diluted 1:600 in AbD for 96 h at 4°C.Worms washed in AbD for 24 h.
Secondary antiserum (goat anti-rabbit IgG, conjugated to FITC) diluted 1:100 in AbD, applied for 48 h.Washed again in AbD for 24 h. Worms were incubated in phalloidin-TRITC diluted 1:100 in AbD for 72 h. Then, washed for 24 h in AbD and mounted in PBS/glycerol (1:9(v/v)).The samples were examined in a LSM 710 confocal laser scanning microscope operating at 488-543 nm (CLSM and Cell Culture Unit, Nanotechnology and advanced materials central lab. (NAMCL), ARC).
Results
Chaetotaxy of schistosoma mansoni and S. haematobium
The parasites have microscopic dark brown rings on their surface that are thought to be sensilla after being exposed to light and silver nitrate treatment.
Nerve sensilla of S. mansoni
The distribution pattern of surface nerve sensilla could be done neither dorsal nor ventral surface. This due to numberless crowded of nerve sensilla and twist of most parasites. In addition to the surface sensilla, some other surface features that different from the presumed sensilla also revealed by Lynch’s technique ().
Table 1. Showing the main differences between male schistosoma mansoni and S. haematobium as revealed by silver impregnation.
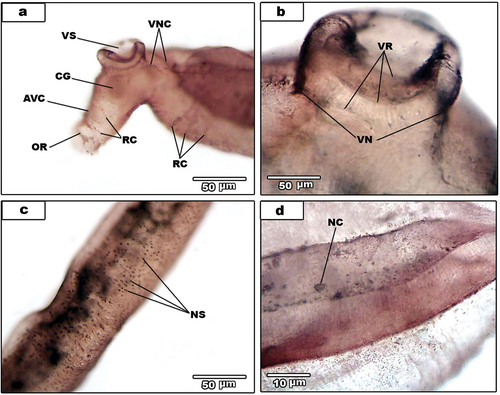
In addition to the surface sensilla, some little surface features are also detected by silver impregnation technique. Ventrally, the ventral nerve cord (VNC) () stained and give rise to ventral sucker nerve (VN) that branches to three rings (VR) (). Moreover, the cerebral ganglia stained and give rise to anteroventral connectives (AVC) (). The oral sucker ring (OR) is deeply staining and some ring commissures stained well (). The nerve cells are adjacent to dorsal nerve cord; each nerve is characterized by a prominent nucleus and two or more extensions (). Additionally, some bipolar and multipolar nerve cells (NC) are seen, situated posteriorly to the ventral sucker and close to the worm’s dorsal surface ().
Figure 1. Light micrographs for AgNO3-treated male S. mansoni. (a). Nerve sensilla (NS) of dorsal surface of the middle region. (b). Venteral sucker showing ventral nerve of the sucker (VN) and venteral sucker rings (VR). (c). Anterior region of the male. Showing anteroventral connectives (AVC), cereberal ganglia (CG), oral sucker ring (OR), ring commissures (RC), venteral nerve cord (VNC) and venteral sucker (VS). (d). Dorsal surface showing nerve cell (NC).
Nerve sensilla of S. haematobium
The edges of gynaecophoral canal (EGC) deeply stained and there is a thin nerve connects between them (EN) (). Ventrally, the ventral nerve cord deeply stains and gives rise to ventral sucker nerve (VN) (). The ventral sucker nerve is branched to three rings (VR) which innervate the ventral sucker () while, the oral sucker slightly stained (). Moreover, the anteroventral connectives (AVC) on each side () that give rise to oral nerve (ON) which from oral sucker ring (OR) (). Ventral sucker region showing dorsal nerve cord (DNC), lateral nerve cord (LNC) and ventral nerve cord (VNC) (). Some ventrolateral connectives were slightly stained ().
Figure 2. Light micrographs for AgNO3-treated male S. haematobium.
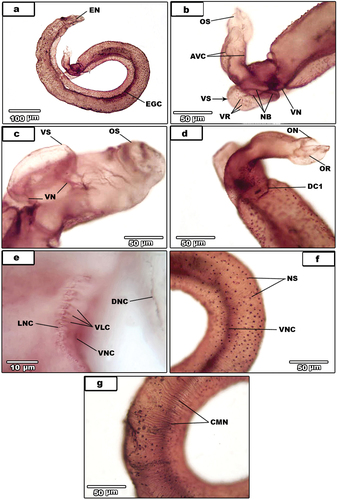
Peripheral cholinergic innervations were found in the dorsal connectives; DC1 is anteriorly located (), whereas other innervations are posteriorly located (). The distribution pattern of surface nerve sensilla () could not do neither dorsal nor ventral surface; owing to numberless, crowded of nerve sensilla and twist of most parasites. The peripheral cholinergic innervations also detected in the subtegumental circular muscles (). All tubercles throughout the whole body are deeply staining () as well as inner dorsal nerve cord (IDNC) (). The excretory pore is deeply staining () and innervating by two excretory pore nerves (EPN) (as in previous paper of nervous system). Additionally, the wall of the anterior region of the excretory vesicle is innervated by fine vesicular nerves (VEN) two in each side arising from dorsal connective11(DC11).
Figure 3. (A-e).Light micrographs for AgNO3-treated male S. haematobium. (a). magnification of nerve sensilla (NS). Note circular muscles nerves (black arrows). (b). dorsal surface showing tubercles (T) and ventral nerve cord (VNC). (C). male with dorsal connectives (DC) and the gynaecophoral canal (GC). (d). magnification of the posterior regionshowing dorsal connectives (DC), dorsal nerve cord (DNC) and inner dorsal nerve cord (IDNC). (e). excretory pore (EP). (f-h) light micrographs for AgNO3-treated female S. haematobium. (f). Whole female. (g). Magnification of posterior region showing ring commissures (RC) and ventral nerve cord (VNC). (3 H). magnification of anterior region (A), egg (E), ootype (OӦ) and uterus wall (UW) and the beginning of posterior region (B) showing ganglion knots (GK) and lateral nerve cord (LNC).
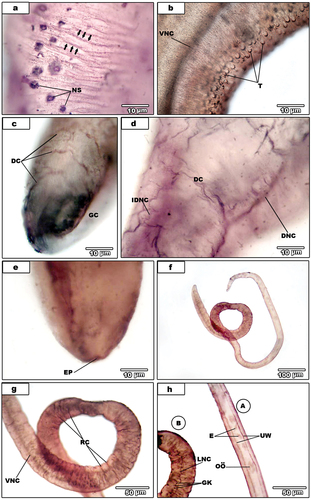
Regarding the female (), central nervous system; two ventral nerve cords (VNC), two lateral nerve cords (LNC) with many ganglion knots and numerous ring commissures (RC) are deeply staining (). The peripheral nervous system detected in the wall of uterus, eggs and oӧtype ().
Confocal activities of muscle and nerves in Schistosoma mansoni and S. haematobium using confocal laser scanning microscopy (CLSM)
In this study, confocal anti-FMRF amide techniques was applied to whole-mount Schistosoma spp. specimens to show the distribution and activity of different types of muscle and their innervation to study the normal neuro-muscular work in this particular important human parasite. The general muscles namely, circular muscles (C), longitudinal muscles (L), dorso-ventral muscles (DV), tegumental muscles (TM) and transverse muscles (TR) which are different from muscle fibers forming the general body organ. The nervous system innervating these muscles namely oral sucker nerve (OSN), ventral sucker nerve (VS), tegumental nerve (TN) and excretory pore nerve (EP).
Confocal activities of muscle in S. mansoni and S. haematobium
Confocal activity
Confocal activity detected in all types of muscles namely circular, longitudinal, transverse or diagonal muscle fibers that show the regular arrangement of Platyhelminthes. These muscles are located beneath the tegument and consist general body muscles. Circular () and longitudinal muscle fibers () are closely arranged, traveling perpendicular in a parallel way to the main body plan, respectively. Circular fibers are slender (1up to 1.5 µm in diameter) and be more superficial to longitudinal ones. Longitudinal muscle fibers, which lie immediately beneathcircular ones, appear thinner than circular fibers (0.5 up to 1 µm in diameter) and show a distinct spindle-shaped.
Figure 4. Confocal laser scanning micrographs of muscle activities in S. mansoni and S. haematobium.
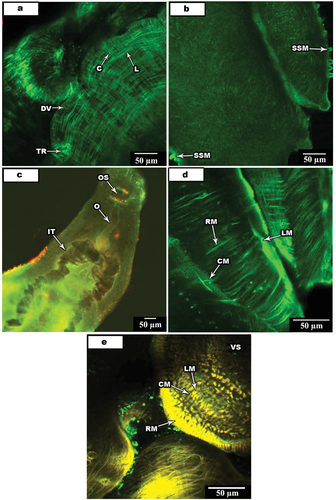
In addition to the above mention muscles, transverse tegumental muscle fibers that constitute the shape of the worm also observed ().
Both oral and ventral suckers have a similar musculature with three muscle fibers types (). They include circular, radial and longitudinal muscle fibers; circular muscle fibers found beneath the outer surface of the cavity of the sucker while radial fibers travel between the inner cavity of the sucker toward the outer surface (). The oral sucker with their dorsal and dorsolateral surfaces is continuous with the wall musculature of the parasite body in a similar structural pattern including circular, longitudinal and diagonal muscle fibers.
Schistosomes lack a pharynx, and the esophagus immediately succeeds the mouth opening, which is subapical and opens through the oral sucker on one hand side () and to the intestine bifurcates into the two intestinal ceca on the other hand side.
Confocal activities of nerves in Schistosoma mansoni and S. haematobium
Phalloidin-TRITC and anti-FMRF amid techniques carried on ten specimens of S. haematobium and S. mansoni and displayed a positive confocal activity. The central nervous system (CNS) of both male Schistosoma consists of two cerebral ganglia (CG) connected by a single ventral connection (VCO) at the posterior level of to the oral sucker (). Two antero-ventral connectives (AVC) arise from anterior and posterior neuronal pathways and inter-linked by cross-connectives and commissures.
Figure 5. Confocal laser scanning micrographs of nerve activities in S. mansoni and S. haematobium.
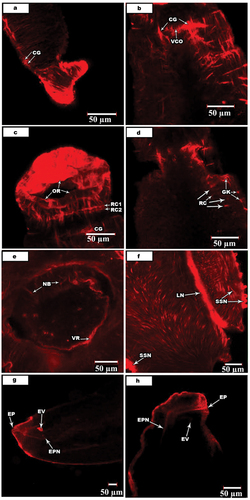
At the area between the oral sucker and cerebral ganglia there are two ring commissures (RC1, RC2). Each commissure like the dorsal nerve cords by the lateral nerve cords at four small ganglion knots (GK) is created (). Several ring sutures are extending from the cerebral ganglia ().
The peripheral nervous system (PNS) includes innervation of the alimentary tract, reproductive organs, oral and ventral suckers. The ventral sucker is innervated by two VN. It is known that ventral nerves arise from the ventral nerve cord and ventral sucker nerves meet in three nerve branches (NB) (). They, from each side, give rise to a ventral sucker ring (VR) that intern branch forming many fine nerves and unit as plexuses. The pharynx is innervated by several nerves, especially the pharyngeal lapel nerve (LN). In addition, there are several surface sensilla surrounding the pharynx that innervate by surface sensilla nerve (SSN) (). The excretory opening innervating by two excretory pore nerves (EPN) originates from dorsal nerve cords ().
Discussion
Lynch technique [Citation1] for the silver impregnation has been used to detect the locations of argentophilic rings, a sign for the existence of nerve sensilla on the surface of both Schistosoma mansoni and S. haematobium. The distribution pattern of surface nerve sensilla could not be done neither dorsal nor ventral surface. This is due to the namelessness crowded of nerve sensilla and the twist of most parasites.
The staining process with silver includes two steps; in the first step of the process (impregnation), silver salt is transformed to metallic silver not yet large enough to be seen. In the second step (development), excess untransformed silver is concentrated around the nuclei until they become observable [Citation22,Citation23]. So far the mechanism of staining is poorly understood. However, Switzer [Citation24] gives a complete historical example of the advance of neurohistological silver methods.
In addition to the surface sensilla, the technique showed the positions of anteroventral connectives, cerebral ganglia, oral sucker ring, ring commissures, ventral nerve cord, ventral sucker, ventral sucker nerve as well as the ring of the nervous system and nerve cells in S. mansoni. In S. haematobium male, the locations of anteroventral connectives, circular muscles nerves, dorsal connectives, dorsal nerve cord, edge nerve, edge of gynaecophoral canal, excretory pore, inner dorsal as well lateral nerve cords and, nerve branches were stained. Moreover, oral sucker, oral sucker nerve as well ring, tubercles, ventral nerve cord, ventrolateral connectives, ventral sucker, ventral sucker and ring were stained. While in S. haematobium female, ganglion knots, lateral and ventral nerve cords, and ring commissures have been detected. Also, the egg, ootype and uterus wall of the reproductive system were stained.
Chaetotaxy is a crucial taxonomic tool for differentiation between species of the genus Brachylaima (trematode) and other groups of digeneans [Citation7] and Trichobilharzia species [Citation25]. However, El-Bakery used chaetotaxy to differentiate between three monogeneans (Schilbetrematiodes aegypticus, Schilbetrema quadricornis schilbe and Schilbetrema acornis) [Citation26].
Similar to what was observed by acetylthiocholine iodide of [Citation27], some components of the nervous systems of both Schistosoma sp. were observed by silver nitrate impregnation [Citation1]. These components included anteroventral connectives, cerebral ganglia, dorsal, ventral, and lateral nerve cords, oral sucker ring, ring commissures, and ventral sucker nerve. But, most interesting remarkable structures detected by the silver nitrate impregnation technique and belonging to the nervous system were dorsal connectives and inner dorsal nerve cord. The present results are in agreement with El-Abbassy [Citation28], as the nerve cords and nerves of both Macrogyrodactylus clarii and M. congolensis were stained with silver nitrate impregnation. Also, excretory pores of the protonephridial system, openings of the mouth, and anterior adhesive sacs on the head lobes of five gyrodactylid monogeneans were detected by using silver nitrate impregnation [Citation28]. Bullock and Horridge [Citation29] have used Gomori [Citation30] technique which contains silver impregnation and has a wide and successful sign in the staining of the nervous systems of platyhelminthes.
It is reported that, acetylthiocholine iodide has been more successful than silver nitrate impregnation since it gave more details about the components of the nervous system. But silver nitrate impregnation is specific for nerve sensilla staining. In addition to chaetotaxy, pars prostatica of male reproductive system of Brachylaima aspersae were stained with silver nitrate impregnation [Citation7].
TRITC-phalloidin staining combined with Anti-FMRF amide under confocal scanning laser microscopy has detected unknown details of the regulation of the muscular and nervous systems of adult Schistosoma mansoni and S. haematobium. The regulation of the body wall musculature involved; an outer layer of circular muscle fibers, an intermediate layer of densely arranged spindle-shaped, longitudinal fibers, and a distinct inner layer of diagonal fibers running in two directions. Muscle layers of S. mansoni body wall agrees with the findings of the previous, morphological studies on the body wall musculature of both free-living [Citation31] and muscle acting failed to reveal innervations of longitudinal fibers. FMRF amide-like peptides has been used in identifying the nerves involved in innervations within the gland and sub-esophageal ganglia complex [Citation32].
The flatworm’s nervous system plays a central role in the realization of organism vital functions: such as locomotion, feeding, migration, host seeking and reproduction. Till now, neuromodulators and their functional value remain poorly studied. Using immunocytochemical in the localization of this substance is associated with the central and peripheral parts of the nervous system of helminthes in agreement with Kreshchenko et al.., [Citation33]. Also, the functions of serotonin in platyhelminthes remain poorly studied. However, the regulation of motor activity of parasitic worms is a major identified serotonin function. Several serotonin effects have been documented: the stimulation of the larval motility of free-swimming larvae (cercariae) of some trematodes [Citation34], sporocysts of S. mansoni [Citation35]. Moreover, serotonin was found to be essential for musculature functioning in S. mansoni [Citation36,Citation37].
According to Mair et al [Citation15]. and Halton et al. [Citation38]; there is a complete different of the muscle fiber of the body wall in Schistosoma from that in Fasciola hepatica or Diclidophora merlangi. In F. hepatica and D. merlangi; these muscle fibers have similar dimensions while, in body wall of Schistosoma, the longitudinal muscle fibers are broader than the other types of muscle fibers. These fibers appear short and have a different morphology while, circular as well as diagonal fibers are slender. These muscles in addition to the muscle that runs from the dorsal to ventral side keep the distinct shape of the worm. The transverse or diagonal fibers with the help of the previously mentioned muscle fibers give the worm the ability to explore for food and suitable position of an attachment.
The present results may indicate that the serotonin receptor can mediate the serotonin action and is a component of the parasite motor system already existing in the adults. Innervation of the oral sucker by serotonergic neurons in other trematodes was shown previously [Citation39]. These data predict the involvement of serotonin in the function of the sensitive organs of parasites. The present data are in agreement with the results obtained for S. mansoni, that serotonin may modulate the sensory circuits at the worm’s surface, representing an important mechanism of host-parasite interaction [Citation37].
The musculature of both suckers is extremely developed. The oral sucker dorsal surface is constant with the normal pattern of musculature and has circular, longitudinal as well as diagonal muscle fibers. The ventral sucker musculature developed more than the oral sucker, since the duty of attachment and survival of the worm is largely restricted to it.
Serotoninergic and peptidergic neurons of the suckers and their innervations were recorded. Suckers fibers that gave serotonin-positive reactions are frequent and anastomose between the various fiber groups. Peptidergic innervations have less intense in the oral sucker. From the anterior longitudinal nerve, a single pair of fibers is ascending from the sucker to the cerebral ganglia. Such records support the presence of neuronal regulation in the sucker musculature. This view goes in parallel with Terenina et al., [Citation19].
Data on the innervation of the attachment organ by serotonergic (neurons) was also suggesting an important role of serotonin in the regulation of the ventral sucker muscle activity. Our findings are in accordance with the results obtained on S. mansoni [Citation37].
In the digestive canal, circular and longitudinal fibers were seen in the esophagus wall. Moreover, wide circular fibers are forming the intestine walls.
Immuno-positive staining in nerve cells, located near the pharynx and in the neurons along the esophagus indicates that this region is also innervated by serotonergic neurons [Citation39]. The results suggest that serotonin can control the musculature of the digestive system of adult worms. Strong expression was also shown in the developing cecum of schistosomes larvae, implying a potential serotonin role in the regulation of the gut activity of the parasite [Citation37].
Phalloidine-TRITC stains intensively in the PNS of S. mansoni and expressed in an extensive sub-muscular nerve net. It is running in all parts of the worm. This is a specific character in both suckers’ muscle lining arranging pattern. The occurrence of serotoninergic nerve is varicose, indicating many sites on the axons responsible for release of the transmitter. In whole Schistosomes and muscle bands, Serotonin has found to be [Citation40–42]. On the other hand, in some studies of muscle fibers isolated from the worms; serotonin was variable and not directly in high level [Citation43]. This makes the serotonin action in the muscle indistinct in neuromuscular control.
Double-labeling experiments for TRITC-phalloidin staining combined with Anti-FMRF amide succeed in expressing specific nerve cells and fibers, the musculature of Schistosome [Citation44]. Both TRITC-phalloidin and Anti-FMRF amide immunoreactivities along the male Schistosomes body are restrictive to core nerve cords as well as the extensive plexus. These nerves are extended very close to the muscle fibers groups that run dorso-ventrally.
Finally, Serotonin receptor was abundantly expressed along the muscle fibers of the body musculature, following the musculature pattern identified by phalloidin staining. Punctate staining was observed along the longitudinal, circular and diagonal muscle fibers of the body wall, as well as along the muscle filaments around the intestine, along the muscle fibers extending from the body wall to the ventral sucker and in the musculature comprising the oral and ventral suckers. This finding can indicate the important role of the serotonin signaling system in the regulation of parasite muscle activity, confirmed by [Citation39].
In conclusion, the general organization of muscles in S. mansoni is extremely complex. Whole-mount investigation using CLSM aids in the detection of minute observations of all muscle details. Dual-labeling observations approved the possible functions for FaRPs, serotonin, and NPF in both male and female worms’ muscles. Moreover, many proofs are essential to detect the physiological processes. Finally [Citation45,Citation46], gave a chance of comparing the muscle association and related innervations of S. mansoni and S. haematobium with F. hepatica. All results support that muscle morphology and their innervations forms are detected in such trematodes. The results obtained on the morphological organization of nervous system support availability of the FMRFamidergic components as antihelmintic target.
List of abbreviations
| (CG) | = | Cereberal ganglia |
| (VS) | = | Ventral sucker |
| (VN) | = | Ventral sucker nerve |
| (VNC) | = | Ventral nerve cord |
| (OR) | = | Oral sucker ring |
| (RC) | = | Ring commissures |
| (AVC) | = | Anteroventral connectives |
| (VR) | = | Ventral sucker rings |
| (NS) | = | Nerve sensilla |
| (EGC) | = | Gynaecophoral canal edge |
| (NB) | = | Nerve branches |
| (OS) | = | Oral sucker |
| (ON) | = | oral sucker nerve |
| (DC1) | = | dorsal connective |
| (EN) | = | Edge nerve |
| (DC) | = | Dorsal connective |
| (LNC) | = | Lateral nerve cord |
| (VNC) | = | Ventral nerve cord |
| (VLC) | = | Ventrolateral connectives |
| (CMN) | = | Circular muscles nerves |
| (T) | = | Tubercles |
| (DC) | = | Dorsal connectives |
| (GC) | = | gynaecophoral canal |
| (IDNC) | = | Inner dorsal nerve cord |
| (EP) | = | Excretory pore |
| (E) | = | Eggs |
| (UW) | = | Uterus wall |
| (NC) | = | Nerve cell |
| (OӦ) | = | Oӧtype |
| (GK) | = | Ganglion knots |
| (DV) | = | Dorsoventral muscle fibers |
| (C) | = | Circular muscle fibers |
| (L) | = | Longitudinal muscle fibers |
| (TR) | = | Transverse muscle fibers |
| (SSM) | = | Surface sensillae muscle |
| (O) | = | Oesophagus muscle |
| (IT) | = | Intestinal tract |
| (RM) | = | Radial muscles |
| (CM) | = | Circular muscles |
| (LM) | = | Longitudinal muscles |
| (VCO) | = | Ventral connective |
| (RC1, RC2) | = | Ring commissures |
| (LN) | = | Pharyngeal lapel nerve |
| (SSN) | = | Surface sensilla nerve |
| (EV) | = | Execratory vesicle |
Author contributions
Prof. ASE & ESR participated in the design of the study and reviewed the final editing of the manuscript. Associate Prof. EAE conceived of the study; participated in its design, getting the worms, preparation process and helped to draft & final revision of the manuscript. SME performed the preparation process of worms and participated in the photographing. All authors read and approved the final manuscript.
Ethical approval
All deals with animals in this study were carried out according to international valid guidelines of experimental animal studies and research protocol was approved by the local ethical committee of the faculty of Science, Mansoura University with code number Sci-Z-P-2022-118.
Acknowledgments
Many thanks to Dr. Ramadan El-Naggar, Ass. Prof. in Literature- Dept. of English- Faculty of Science and Humanities- Shawra University, KSA for revision of our paper (language and grammer).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lynch JE. The miracidium of heronimus chelydrae MacCallum. Quart J Microsc Sci. 1933;76(301):13–33. doi: 10.1242/jcs.s2-76.301.13
- Shinn AP, Gibson DI, Sommerville C. Argentophilic structures as a diagnostic criterion for the discrimination of species of the genus gyrodactylus von Nordmann (Monogenea). Syst Parasitol. 1997;37(1):47–57. doi: 10.1023/A:1005771914482
- Shinn AP, Gibson DI, Sommerville C. Chaetotaxy of members of the gyrodactylidae (Monogenea), with comments upon their systematic relationships with the Monopisthocotylea and Polyopisthocotylea. Syst Parasitol. 1998a;39(2):81–94. doi: 10.1023/A:1005848407086
- Shinn AP, Gibson DI, Sommerville C. The application of chaetotaxy in the discrimination of gyrodactylus salaris Malmberg, 1957 (gyrodactylidae: monogenea) from species of the genus parasitising British salmonids. Int J Parasitol. 1998b;28(5):805–814. doi: 10.1016/S0020-7519(98)00028-9
- Butcher AR, Grove DI. Description of the life-cycle stages of brachylaima cribbi n. sp. (Digenea: brachylaimidae) derived from eggs recovered from human faeces in Australia. Syst Parasitol. 2001;49(3):211–221. doi: 10.1023/A:1010616920412
- Gracenea M, Moreno GO. Life cycle of brachylaima mascomai n. sp. (Trematoda: brachylaimidae), a parasite of rats in the llobregat delta (Spain). J Parasitol. 2002;88(1):124–133. doi: 10.1645/0022-3395(2002)088[0124:LCOBMN]2.0.CO;2
- Segade P, Crespo C, García N, et al. Brachylaima aspersae n. sp. (Digenea: brachylaimidae) infecting farmed snails in NW Spain: morphology, life cycle, pathology, and implications for heliciculture. Vet Parasitol. 2011;175(3–4):273–286. doi: 10.1016/j.vetpar.2010.10.026
- Bhatnagar AK, Gupta AN, Srivastava RC. Histology and cytochemistry of the neuroendocrine components of ceylonocotyle scoliocoelium (Digenea: trematoda). Z Parasitenkd. 1980;64(1):77–84. doi: 10.1007/BF00927058
- Shyamasundari K, Hanumantha RK. The structure and cytochemistry of the neurosecretory cells of fasciola gigantica cobbold and fasciola hepatica L. Z Parasitenk. 1975;47(2):103–109. doi: 10.1007/BF00382633
- Webb RA, Mizukawa K. Serotoninlike immunoreactivity in the cestode Hymenolepis diminuta. J Comp Neurol. 2004;234(4):431–440. doi: 10.1002/cne.902340403
- El-Naggar MM, Arafa SZ, El-Abbassy SA, et al. Neuromusculature of Macrogyrodactylus clarii, a monogenean gill parasite of the nile catfish Clarias gariepinus in Egypt. Parasitol Res. 2004;94(3):163–175. doi: 10.1007/s00436-004-1198-1
- El-Naggar MM, Arafa SZ, El-Abbassy SA, et al. Neuromusculature of Macrogyrodactylus congolensis, a monogenean skin parasite of the nile catfish clarias gariepinus. Parasitol Res. 2007;100(2):265–279. doi: 10.1007/s00436-006-0235-7
- Arafa SZ, El-Naggar MM, El-Abbassy SA, et al. Neuromusculature of Gyrodactylus rysavyi, a monogenean gill and skin parasite of the nile catfish clarias gariepinus. Parasitol Int. 2007;56(4):297–307. doi: 10.1016/j.parint.2007.06.005
- Magee RM, Fairweather I, Johnston CF, et al. Immunocytochemical demonstration of neuropeptides in the nervous system of the liver fluke, fasciola hepatica (trematoda, Digenea). Parasitology. 1989;98(2):227–238. doi: 10.1017/S0031182000062132
- Mair GR, Maule AG, Day TA, et al. A confocal microscopical study of the musculature of adult schistosoma mansoni. Parasitology. 2000;121(2):163–170. doi: 10.1017/S0031182099006174
- McKay DM, Halton DW, Johneston CF, et al. Occurrence and distribution of putative neurotransmitters in the frog-lung parasite haplometra cylindracea (Trematoda: Digenea). Parasitol Res. 1990;76(6):509–517. doi: 10.1007/BF00931056
- Humphries JE, Halton DW, Johnston RN, et al. Cholinergic, serotoninergic and peptidergic components of the nervous system of haematoloechus medioplexus (trematoda, digenea), characterised by cytochemistry. Int J Parasitol. 1997;27(5):517–525. doi: 10.1016/S0020-7519(97)00030-1
- Kreshchenko N, Terenina N, Mochalova N, et al. Neuromuscular system of the causative agent of dicrocoeliosis, dicrocoelium lanceatum. II. Neuropeptide FMRFamide immunoreactivity in nervous system. Zoology. 2022;155:1–12. doi: 10.1016/j.zool.2022.126054
- Terenina N, Kreshchenko N, Movsesyan S. Musculature and neurotransmitters of internal organs of trematodes (the digestive, reproductive and excretory systems). Zoology. 2022;150:1–12. doi: 10.1016/j.zool.2021.125986
- Neves RH, Costa-Silva M, Martinez EM, et al. Reproductive system abnormalities in Schistosoma mansoni adult worms isolated from nectomys squamipes (muridae: sigmodontinae): Brightfield and confocal laser scanning microscopy analysis. Mem Inst Oswaldo Cruz. 2003;98(3):361–365. doi: 10.1590/S0074-02762003000300011
- Skuce PJ, Johnston CF, Fairweather I, et al. Immunoreactivity to the pancreatic polypeptide family in the nervous system of the adult human blood fluke, schistosoma mansoni. Cell Tissue Res. 1990;261(3):573–581. doi: 10.1007/BF00313537
- Peters A. Experiments on the mechanism of silver staining. Ι. Impregnation. J Cell Sci. 1955a;96:84–102. doi: 10.1242/jcs.s3-96.33.84
- Peters A. Experiments on the mechanism of silver staining. Π. Development. J Cell Sci. 1955b;96:103–115. doi: 10.1242/jcs.s3-96.33.103
- Switzer RC. Application of silver degeneration stains for neurotoxicity testing. Toxicol Pathol. 2000;28(1):70–83. doi: 10.1177/019262330002800109
- Kock S, Bockeler W. Observations on cercarial chaetotaxy as a means for the identification of European species of Trichobilharzia Skrjabin & Zakharow, 1920 (Digenea: schistosomatidae). Syst Parasitol. 1998;43:159–166. doi: 10.1023/A:1006118619762
- El-Bakkery AT (2009). Biological studies on monogenean parasites of the freshwater fish Schilbe mystus inhabiting Nile Delta waters in Dakahlia Province. M. Sc. Thesis, Zoology Department, Faculty of Science, Mansoura University, Egypt.
- Rahemo ZIF, Gorgess NS. Studies on the nervous system of polystoma integerrimum as revealed by acetylthiocholine activity. Parasitol Res. 1987;73:234–239. doi: 10.1007/BF00578510
- El-Abbassy SA Biological and histochemical studies on some monogenean parasites of the catfish Clarias gariepinus inhabiting Nile Delta waters. M. Sc. Thesis, Zoology Department, Faculty of Science, Mansoura University, Egypt 2001.
- Bullock TH, Horridge GA. Structure and function in the nervous system of invertebrates. Vol.1. San Francisco and London: W.H. Freeman & Co; 1965. p. 798.
- Gomori G. Microscopic histochemistry. University of Chicago; 1952.
- Rieger RM, Salvenmoser W, Legniti A, et al. Phalloidin-rhodamine preparations of macrostomum hystricinum marinum (platyhelminthes): morphology and postembryonic development of the musculature. Zoomorphology. 1994;114:133–147. doi: 10.1007/BF00403261
- Moraes GD, Achaval M, DalPiva MM, et al. Ultrastructural analysis of the dorsal body gland of the terrestrial snail megalobulimus abbreviates (becquaert, 1948). Braz J Biol. 2010;70(2):341–350. doi: 10.1590/S1519-69842010005000017
- Kreshchenko N, Terenina N, Nefedova D, et al. The neuroactive substances and associated muscle system in rhipidocotyle campanula (Digenea, bucephalidae) from the intestine of the pike esox lucius. J Morphol. 2020;281(9):1047–1058. doi: 10.1002/jmor.21230
- Tolstenkov OO, Prokofiev VV, Pleskacheva MV, et al. Age and serotonin effects on locomotion in marine trematode cercariae. J Evol Biochem Phys. 2017;53(2):135–142. doi: 10.1134/S1234567817020069
- Boyle JP, Zaide JV, Yoshino TP. Schistosoma mansoni: effects of serotonin and serotonin receptor antagonists on motility and length of primary sporocysts in vitro. Exp Parasitol. 2000;94:217–226. doi: 10.1006/expr.2000.4500
- Ribeiro P, Gupta V, El-Sakkary N. Biogenic amines and the control of neuromuscular signaling in schistosomes. Invert Neurosci. 2012;12(1):13–28. doi: 10.1007/s10158-012-0132-y
- Patocka N, Sharma N, Rashid M, et al. Serotonin signaling in Schistosoma mansoni: a serotonin-activated G protein-coupled receptor controls parasite movement. PLOS Pathog. 2014;10:e1003878. doi: 10.1371/journal.ppat.1003878
- Halton DW, Maule AG, Mair GR, et al. Monogenean neuromusculature: some structural and functional correlates. Int J Parasitol. 1998;28(10):1609–1623. doi: 10.1016/S0020-7519(98)00063-0
- Terenina NB, Kreshchenko ND, Mochalova NV, et al. The new data on the Serotonin and FMRFamide localization in the nervous system of Opisthorchis felineus metacercaria. Acta Parasit. 2020;65(2):361–374. doi: 10.2478/s11686-019-00165-2
- Mellin TN, Busch RD, Wang CC, et al. Neuropharmacology of the parasitic trematode, schistosoma mansoni. Am J Trop Med Hyg. 1983;32:83–93. doi: 10.4269/ajtmh.1983.32.83
- Pax RA, Siefker CA, Bennett JL. Schistosoma mansoni: differences in acetylcholine, dopamine, and serotonin control of circular and longitudinal parasite muscles. Exp Parasitol. 1984;58(3):314–324. doi: 10.1016/0014-4894(84)90048-1
- Willcockson WS, Hillman GR. Drug effects on the 5-HT response of schistosoma mansoni. Comp Biochem Physiol. 1984;77(1):199–203. doi: 10.1016/0742-8413(84)90151-8
- Day TA, Maule AG, Shaw C, et al. Platyhelminth FMRFamide-related peptides (FaRps) contract schistosoma mansoni (Trematoda: Digenea) muscle fibres in vitro. Parasitology. 1994;109(4):455–459. doi: 10.1017/S0031182000080707
- McVeigh P, Mair GR, Atkinson L, et al. Discovery of multiple neuropeptide families in the phylum platyhelminthes. Int J Parasitol. 2009;39(11):1243–1252. doi: 10.1016/j.ijpara.2009.03.005
- Marks NJ, Halton DW, Maule AG, et al. Comparative analyses of the neuropeptide F (NPF)-and FMRFamide-related peptide (FaRP)-immunoreactivities in Fasciola hepatica and schistosoma spp. Parasitology. 1995;110:371–381. doi: 10.1017/S0031182000064714
- Mair GR, Maule AG, Shaw C, et al. Gross anatomy of the muscle systems of fasciola hepatica as visualized by phalloidin-fluorescence and confocal microscopy. Parasitology. 1998;117(1):75–82. doi: 10.1017/S0031182098002807

