ABSTRACT
Naturally occurring oscillations in glucocorticoids induce a cyclic activation of the glucocorticoid receptor (GR), a well-characterized ligand-activated transcription factor. These cycles of GR activation/deactivation result in rapid GR exchange at genomic response elements and GR recycling through the chaperone machinery, ultimately generating pulses of GR-mediated transcriptional activity of target genes. In a recent article we have discussed the implications of circadian and high-frequency (ultradian) glucocorticoid oscillations for the dynamic control of gene expression in hippocampal neural stem/progenitor cells (NSPCs) (Fitzsimons et al., Front. Neuroendocrinol., 2016). Interestingly, this oscillatory transcriptional activity is common to other transcription factors, many of which regulate key biological functions in NSPCs, such as NF-kB, p53, Wnt and Notch. Here, we discuss the oscillatory behavior of these transcription factors, their role in a biologically accurate target regulation and the potential importance for a dynamic control of transcription activity and gene expression in NSPCs.
Introduction
The generation of oscillatory transcription factor activity can provide an additional way to encode and transmit information, contained in the amplitude and period of the oscillations, to accurately regulate gene expression. Indeed, oscillations in transcription factor concentration, intracellular localization and activity are considered key components of several signaling pathways.Citation1-6
Oscillations in transcription factors: A dynamic way to control gene expression
Many studies aiming to understand transcription factor action assess target gene responses by a steady level of stimulation. However, steady inputs are infrequently observed under natural conditions and, at the cellular level, many signaling pathways have been optimized to result in a dynamic fluctuation in transcription factor activity. This suggests that a biologically accurate regulation of gene expression requires, or at least benefits from, an oscillatory mode of action in many cases.Citation7-10 Oscillating signaling pathways and downstream transcription factors may present “circadian” lower-frequency oscillations over the day/night period and/or ‘ultradian’ higher-frequency oscillations in the order of hours. Furthermore, they frequently present a common architecture consisting of negative feedback loops that introduce time delays responsible for their oscillatory activity.Citation11 Conceptually, this necessary time delay could be obtained by several mechanisms, such as: 1) the inclusion of a biological process that takes a minimum amount of time (e.g. transcription, translation, synthesis); 2) the inclusion of many such intermediate steps, with each step adding to the overall time delay; 3) the inclusion of a threshold concentration that must be reached before a molecule becomes biologically active, resulting in an on/off-like response; 4) the inclusion of a degradation/sequestration step, where the activity of a molecule is delayed by the formation of a saturated complexCitation8 (). Often, these mechanisms are combined within signaling pathways that induce oscillatory transcriptional activity.
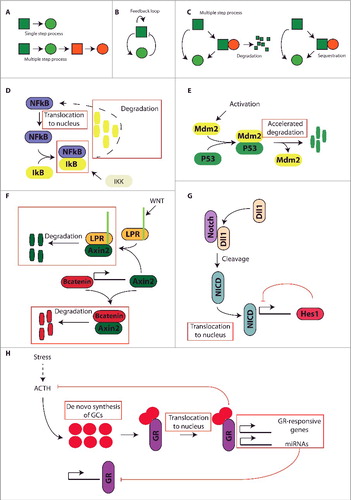
Figure 1. Different molecular mechanisms that can induce time-delay necessary for the generation of oscillations in transcription factor activity. A) Inclusion of multiple intermediate steps that take a minimum amount of time, such as transcription, translation, transportation; B) Inclusion of a threshold concentration, in the form of a feedback loop that generates an on/off-like response, necessary for a molecule to become biologically active; C) Inclusion of degradation/sequestration steps that prevent functionality. Examples are: inclusion of a saturated degradation step or inclusion of a complex sequestration step; D) The NFkB pathway is regulated by the sequestration of NFkB by IkB, rendering NFkB inaccessible. Only upon phosphorylation of IkB by IKK, IkB is degraded and NFkB becomes functionally active; E) Oscillatory behavior is generated by the P53-induced transcription of Mdm2, which in turn binds to P53 and results in accelerated degradation of P53; F) Axin2 governs at least a part of the oscillatory behavior of the Wnt pathway. Axin2 transcription is under control of B-catenin, and upon translation Axin2 can either bind B-catenin, inducing degradation, or bind the LRP receptor complex inducing degradation of Axin2 itself; G) A negative feedback loop controls oscillations in the Notch signaling pathway. Upon stimulation of the Notch receptors they are cleaved and their intracellular domain, NICD, is translocated to the nucleus where it promotes the expression of Hes1. Hes1 in its turn inhibits its own promotor, thereby creating a negative feedback loop; H) Glucocorticoid signaling is a prime example of several time-delay steps inducing oscillations. First, glucocorticoids are synthesized de novo upon stimulation by ACTH. Upon release, glucocorticoids bind to and activate the GR, the activated glucocorticoid receptor is translocated to the nucleus where it induces the transcription of several GR-responsive protein coding genes, as well as several microRNAs. Some of these microRNAs form negative feedback loops with the GR, locally inhibiting GR expression. Furthermore, the activated GR is engaged in a classic negative feedback loop to the brain, where it inhibits the production of ACTH and other upstream mediators of the HPA axis.
A delayed negative feedback loop is defined as a negative feedback loop with a time delay due to the inclusion of intermediate processes between the product and the repressor.Citation12 Delayed negative feedback loops are often required to induce oscillations in transcription factor activity. For example, in the circadian clock, longer delayed negative feedback loops are central for the reaction to regular external inputs such as light or feeding. The reaction to faster, more irregular signals, such as cellular stress, frequently requires faster (lower period) ultradian oscillations. These oscillations in activity are observed in well-characterized signaling pathways and downstream transcription factors such as NF-kB, p53, Wnt and Notch signaling and the glucocorticoid receptor (GR).Citation4,8,13 In the following sections we will discuss how the oscillatory behaviors of these transcriptions factors influence gene expression in in neural stem/progenitor cells (NSPCs).
The NF-kB pathway
The NF-kB pathway is composed of a group of transcription factors that bind to form homo- or hetero-dimers. Once formed, these protein complexes control several cellular functions such as the response to stress and the regulation of growth, cell cycle, survival, apoptosis and differentiation in NSPCs.Citation14-16 Oscillations in NF-kB were first observed in embryonic fibroblasts, this observation suggested that temporal control of NF-kB activation is coordinated by the sequential degradation and synthesis of inhibitor kappa B (IkB) proteins.Citation3
More recently, oscillations in the relative nuclear/cytosolic concentration of NF-kB transcription factors have been observed in single cells in vivo, indicating this may be an additional regulatory mechanism to control NF-kB-dependent transcriptional activity. Importantly, the frequency and amplitude of these oscillations changed in a cell-type dependent fashion and differentially affected the dynamics of gene expression,Citation5 indicating that NF-kB transcription factors may use changes in the frequency and amplitude of their oscillatory dynamics to regulate the transcription of target genes.Citation1,17 Thus, the NF-kB pathway provides a well-characterized example of how oscillatory transcription factor activity may encode additional, biologically relevant, information for an accurate control of gene expression.
The p53 pathway
p53 and some of its family members, such as p73 and p63, regulate various functions in NSPCs. In particular, p63 and p73 cooperate to regulate p53 in adult hippocampal NSPCsCitation18 and p53 negatively regulates proliferation and survival of adult NSPCs, controlling self-renewal and long-term maintenance of adult NSPCs.Citation19
Similar to NF-kB, the p53 pathway presents intrinsic oscillatory dynamics. The mechanism behind these oscillations may involve p53 and its negative regulator mouse double minute 2 homolog (MDM2) also known as E3 ubiquitin-protein ligase. Due to its ubiquitin-protein ligase activity MDM2 induces poly-ubiquitination and degradation of p53, bringing p53 half-life from the order of hours in the absence of MDM2 to the order of seconds in its presence, and thereby generating an oscillatory, stress-specific response.Citation20,21 Thus, this simple negative feedback loop provides a solid example of oscillatory transcription factor behavior induced by the inclusion of a saturated degradation step.Citation8
The Wnt signaling pathway
The canonical Wnt signaling, or Wnt/β-catenin, pathway is a complex signal transduction system composed of several proteins. It is activated by the binding of Wnt ligands to receptors of the Frizzled family members and leads to cytosolic accumulation and further translocation of β-catenin to the nucleus, where it acts as a coactivator of downstream transcriptions factors of the TCF/LEF family, which in turn regulate the expression of responsive genes, such as Axin 2. While still in the cytosol, β-catenin is targeted by a multiprotein complex designed to specifically promote β-catenin degradation. This complex includes members of the Axin family (i.e. Axin 2) and the glycogen synthase kinase 3 β (GSK-3 β), which constitutively phosphorylates β-catenin in the absence of Wnt ligands, thereby targeting it for degradation. Due to its complexity, the extensive description of the canonical Wnt signaling pathway is beyond the scope of this review and we refer to previous reviews.Citation22-24
The oscillatory activity of the Wnt signaling pathway was first identified in vertebrate somite formation during embryonic development. There, the Wnt signaling interacts with other oscillatory pathways, such as Notch, in the presomitic mesoderm to coordinate somite formation.Citation25,26 The presence of Wnt-dependent oscillatory gene expression in several other developmental processes such as limb outgrowth and NSPCs maintenance, suggests that it plays crucial roles at different stages during embryonic development.Citation27 Although Wnt oscillations have not been studied in great detail in (adult) NSPCs, Wnt signaling is known to regulate adult hippocampal neurogenesis, control Prospero-related homeobox 1 gene (Prox1) expression and mediate activation of NeuroD1 and retro-elements in adult NSPCs.Citation28-30
The central negative feedback loop resulting in oscillations in Wnt activity seems to involve the Wnt ligand-activated low density lipoprotein receptor-related protein (LRP) receptor complex, Axin-2 and β-catenin.Citation25 Axin-2 is crucial in this regulatory loop because it can bind both to β-catenin and to the Wnt ligand-activated LRP receptor complex. Binding to β-catenin provides a negative feedback step, as it promotes β-catenin degradation, but this binding is weak. Binding to the Wnt ligand-activated LRP receptor complex is much stronger and results in saturated degradation of Axin2. Thus, the Wnt/β-catenin pathway provides an interesting example of negative feedback loops and saturated degradation steps that can be incorporated as independent components into oscillatory signaling pathways involving transcription factors.Citation8
The Notch signaling pathway
The Notch signaling pathway is perhaps the best characterized oscillatory pathway in NSPCs, it promotes cell proliferation and maintenance, favoring a non-differentiated cellular state in the developing and adult brain.Citation31-33 Proteins of the Notch family are normally activated by cell-to-cell contact and act as transmembrane receptors for specific ligands expressed in neighboring cells, such as Delta-like 1 (Dll1). Upon activation, Notch proteins are proteolytically cleaved and release their Notch intracellular domain (NICD).Citation34 NICD then translocates to the nucleus to form the CSL (or RBP-J in mice) transcriptional complex, which activates the transcription of responsive genes of the Hes/Hey family, such as Hes1. The extensive description of the canonical Notch signaling pathway is beyond the scope of this review and we refer to previous reviews.Citation32,35
Levels of Hes1 oscillate in mouse embryonic NSPCs and progenitors, and this oscillatory expression promotes proliferation.Citation7,36 On the contrary, sustained (non-oscillatory) Hes1 expression is associated with inhibition of NSPCs proliferation and neurogenesis in the developing central nervous system.Citation37 Therefore, it has been suggested that oscillatory vs. sustained expression of Notch target genes may distinguish active and quiescent NSC pools.Citation32
In particular, sustained Hes1 expression inhibits the expression of proneural genes, Notch ligands and cell cycle regulators, suggesting that Hes1 oscillations are of key importance for their concerted function in NSPCs, in which Hes1 oscillations coordinate self-renewal and differentiation.Citation7,38 The mechanisms regulating oscillations in Notch signaling are not fully understood but evidence indicates that Hes1 oscillations are regulated by negative feedback loops with delayed timing.Citation39,40 Hes1 represses its own expression by direct binding to its own promoter, leading to a rapid downregulation of Hes1 mRNA and protein levels and upregulation of Dll1, thereby generating oscillations in Hes1 expression in NSPCs, which regulate their maintenance.Citation7,41 Further studies have demonstrated that Hes1 is engaged in double negative feedback loops involving specific microRNAs as well.Citation42,43 Interestingly, double negative feedback loops are common between transcription factors and microRNAs and have been frequently described in NSPCs.Citation44,45 Specifically, in the case of Notch signaling in NSPCs, miR-9 inputs into the Hes1 ultradian oscillator system to introduce a second negative feedback loops that controls the emergence and timing of alternative cell states in NSPCs.Citation42 Thus, the Notch pathway provides a relevant example demonstrating that different delayed negative feedback loops can be engaged in the generation of oscillatory behavior in transcription factor activity.
Glucocorticoid receptor signaling
Glucocorticoid receptor signaling has well-characterized effects on NSPCs. The most commonly reported observation is a marked inhibition of proliferation induced by activation of the GR by its natural or synthetic agonists.Citation46-49 Further research has established that glucocorticoids regulate other cellular functions in NSPCs, such as survival, senescence, cell fate and differentiation.Citation50-53 All in all, the available experimental evidence indicates that glucocorticoids regulate multiple cellular functions in NSPCs through the activation of the GR. For a more extensive discussion of this evidence we refer the reader to a recent review.Citation54 As a central component of the hypothalamus-pituitary-adrenal hormonal axis, signaling mediated by the ligand-activated transcription factor GR is regulated at multiple levels, including: 1) the hormone (ligand) synthesis level in the adrenals; 2) the hormone access, binding and activation of the GR; 3) the receptor translocation from the cytosol to the nucleus; 4) the transcription efficiency of target genes resulting from the interaction with transcriptional coregulators and other transcription factors and 5) the negative feedback regulation of releasing factors at the central hypothalamus and pituitary. A thorough description of GR signaling and its complex regulation is beyond the scope of this review and we refer to previous reviews.Citation55
Glucocorticoid signaling involves circadian and ultradian oscillations that regulate multiple organismal and cellular functions to coordinate energy availability and stress responsivity.Citation56 Ultradian and circadian CORT rhythms are intrinsically linked, as ultradian pulses are a necessary component of circadian oscillations. Ultradian oscillations in glucocorticoids have the highest amplitude around awakening, which then declines, effectively contributing to a phase response curve, characteristic of most circadian rhythms.Citation57-59 Circadian and ultradian glucocorticoid oscillations have likely evolved to help adaptation to predictable changes in environmental factors (i.e., light cycles), while the stress response may have evolved to rapidly adapt to acute unpredictable changes in environmental factors (i.e., stressors).Citation54
Several mechanisms may underlie the generation of oscillations in GR-mediated transcriptional activity. At the organism level, the glucocorticoid lipophilicity prevents it from being stored in membrane vesicles. Thereby, glucocorticoids need to be synthesized de novo in the adrenals, in response to ACTH stimulation. This crucial step introduces a built-in time-delay in the hypothalamus-pituitary-adrenal negative feedback loop, which then presents intrinsic oscillatory activity.Citation54,59 Due to the presence of ultradian oscillation, all the tissues tested, including the hippocampus where adult NSPCs reside, are exposed to a pulsatile GR activation, which has considerable consequences for the activation of responsive genes.Citation60-62
At the cellular level, other mechanisms take place that contribute to GR-dependent oscillatory activation of target genes: 1) NSPCs express specialized microtubule-associated proteins that tightly control GR cytosol-to-nucleus translocation.Citation63 This translocation step possibly introduces another time-delay within GR-mediated transcription of target genes, that may favor oscillatory behavior; 2) Ultradian glucocorticoid oscillations induce cyclic GR-mediated transcriptional regulation, both in cultured cells and in animal models. This “responsive gene pulsing” is driven by rapid GR exchange at DNA response elements and by intranuclear GR recycling through the chaperone machinery, which promotes GR activation/reactivation in response to the ultradian hormone release.Citation13 This GR recycling introduces another built-in time-delay, which may favor oscillatory behavior, and serve pulsatile and constant hormone stimulations to induce unique, treatment-specific patterns of gene and regulatory element activationCitation64; 3) The GR is targeted by microRNAs such as miR-124 and miR-433, with the latter dampening GR signaling and impacting on circadian rhythms.Citation65,66 miR-124 expression is in turn regulated by glucocorticoids through GR-binding elements in its promoter region, thereby generating a miR-124/GR negative feedback loop.Citation45,67 Thus, the GR signaling pathway presents a well-characterized example of oscillatory transcriptional regulation in which several processes interact with each other, i.e., the inclusion of several negative feedback loops, many intermediate steps, a threshold concentration that must be reached before the GR biologically active (ligand affinity), and saturated sequestration/degradation steps. Together, this provides built-in time delays that characterize model oscillatory systems.
Conclusion and future perspectives
We have discussed first some molecular mechanisms involved in the oscillatory behavior of specific signaling pathways that control key cellular functions in NSPCs, and second the importance that such oscillations may have in terms of transcriptional activity and the dynamic control of gene expression. We have further extended our discussion by providing 5 examples of well-characterized transcription factors that display oscillatory activity. In most cases the importance of this oscillatory activity has been characterized and clear differences between sustained and oscillatory activity have been observed. These data suggest that oscillations in transcription factor activity in NSPCs, and their functional consequences, may be much more widespread than hitherto demonstrated. Indicatively, a recent study has demonstrated that 43% of all coding genes show transcriptional oscillations in mice.Citation68 We propose that one of the reasons why a further characterization of transcription factor oscillations in NSPCs has not been fully achieved yet, may lie in the technical limitations associated with measuring and mimicking oscillatory activity in an appropriate manner, which require real-time imaging of individual cells and/or complex incubations with the adequate ligand, in the case of ligand-induced transcription factors such as the GR.Citation13,69
Studies of transcription factor action frequently measure gene responses after long-term stimulation or exogenous (over)expression. However, the mechanisms and examples we discuss here suggest that such treatments may not provide a complete and accurate view of their physiological activity. This concern may have relevance not only for studies assessing physiological transcription factor activity but also for the treatment of common human conditions such as chronic inflammatory diseases and neoplasias. In these cases, for example, patients are treated with high doses of synthetic GR ligands, ignoring the importance of dynamic oscillatory activity.Citation70 Furthermore, chronic sustained treatment with the GR agonist prednisolone represses the circadian oscillation of clock gene expression in mouseCitation71 and prenatal exposure to excess glucocorticoids induces depression-like behavior and impaired adult hippocampal neurogenesis in old mice, which correlates with the absence of circadian oscillations in hippocampal clock gene expression.Citation72 As we discussed here, glucocorticoids regulate multiple functions in hippocampal NSPCs and adult neurogenesis. However, the effects of circadian and ultradian glucocorticoid oscillations on hippocampal NSPCs remains poorly characterized. Ongoing experiments in our lab are aimed to address this question experimentally.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize to all colleagues whose work could not be cited due to space limitations.
Funding
This work has been funded by grants from the Netherlands Organization for Scientific Research (NWO), Innovational Research Incentives Scheme VIDI; Alzheimer Nederland and the International Foundation for Alzheimer Research (ISAO) to CPF and PJL.
References
- Nelson DE, See V, Nelson G, White MR. Oscillations in transcription factor dynamics: a new way to control gene expression. Biochem Soc Trans 2004; 32:1090-1092; PMID:15506974; https://doi.org/10.1042/BST0321090
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 1997; 386:855-858; PMID:9126747; https://doi.org/10.1038/386855a0
- Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 2002; 298:1241-1245; PMID:12424381; https://doi.org/10.1126/science.1071914
- Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet 2004; 36:147-150; PMID:14730303; https://doi.org/10.1038/ng1293
- Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science 2004; 306:704-708; PMID:15499023; https://doi.org/10.1126/science.1099962
- Wang HY, Huang YX, Zheng LH, Bao YL, Sun LG, Wu Y, Yu CL, Song ZB, Sun Y, Wang GN, et al. Modelling coupled oscillations in the Notch, Wnt, and FGF signaling pathways during somitogenesis: a comprehensive mathematical model. Comput Intell Neurosci 2015; 2015:387409; PMID:25866502
- Shimojo H, Ohtsuka, T, Kageyama R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 2008; 58:52-64; PMID:18400163; https://doi.org/10.1016/j.neuron.2008.02.014
- Mengel B, Hunziker A, Pedersen L, Trusina A, Jensen MH, Krishna S. Modeling oscillatory control in NF-kappaB, p53 and Wnt signaling. Curr Opin Genet Dev 2010; 20:656-664; PMID:20934871; https://doi.org/10.1016/j.gde.2010.08.008
- Wee KB, Yio WK, Surana U, Chiam KH. Transcription factor oscillations induce differential gene expressions. Biophys J 2012; 102:2413-2423; PMID:22713556; https://doi.org/10.1016/j.bpj.2012.04.023
- McMaster A, Jangani M, Sommer P, Han N, Brass A, Beesley S, Lu W, Berry A, Loudon A, Donn R, et al. Ultradian cortisol pulsatility encodes a distinct, biologically important signal. PLoS One 2011; 6:e15766; PMID:21267416; https://doi.org/10.1371/journal.pone.0015766
- Tiana G, Krishna S, Pigolotti S, Jensen MH, Sneppen K. Oscillations and temporal signalling in cells. Phys Biol 2007; 4:R1-17; PMID:17664651; https://doi.org/10.1088/1478-3975/4/2/R01
- Lei J. Time-delayed negative feedback. In: Dubitzky W, Wolkenhauer O, Cho K-H, Yokota H, editors. Encyclopedia of Systems Biology. New York: Springer; 2013. p. 2171.
- Stavreva DA, Wiench M, John S, Conway-Campbell BL, McKenna MA, Pooley JR, Johnson TA, Voss TC, Lightman SL, Hager GL, et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 2009; 11:1093-1102; PMID:19684579; https://doi.org/10.1038/ncb1922
- Zhang Y, Liu J, Yao S, Li F, Xin L, Lai M, Bracchi-Ricard V, Xu H, Yen W, Meng W, et al. Nuclear factor kappa B signaling initiates early differentiation of neural stem cells. Stem Cells 2012; 30:510-524; PMID:22134901; https://doi.org/10.1002/stem.1006
- Grilli M, Memo M. Possible role of NF-kappaB and p53 in the glutamate-induced pro-apoptotic neuronal pathway. Cell Death Differ 1999; 6:22-27; PMID:10200544; https://doi.org/10.1038/sj.cdd.4400463
- Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol 2012; 14:999-1012; PMID:22940906; https://doi.org/10.1038/ncb2562
- Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, Ryan S, Spiller DG, Unitt JF, Broomhead DS, et al. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science 2009; 324:242-246; PMID:19359585; https://doi.org/10.1126/science.1164860
- Fatt MP, Cancino GI, Miller FD, Kaplan DR. p63 and p73 coordinate p53 function to determine the balance between survival, cell death, and senescence in adult neural precursor cells. Cell Death Differ 2014; 21:1546-1559; PMID:24809925; https://doi.org/10.1038/cdd.2014.61
- Meletis K, Wirta V, Hede SM, Nistér M, Lundeberg J, Frisén J. p53 suppresses the self-renewal of adult neural stem cells. Development 2006; 133:363-369; PMID:16368933; https://doi.org/10.1242/dev.02208
- Hunziker A, Jensen MH, Krishna S. Stress-specific response of the p53-Mdm2 feedback loop. BMC Syst Biol 2010; 4:94; PMID:20624280; https://doi.org/10.1186/1752-0509-4-94
- Batchelor E, Loewer A, Lahav G. The ups and downs of p53: understanding protein dynamics in single cells. Nat Rev Cancer 2009; 9:371-377; PMID:19360021; https://doi.org/10.1038/nrc2604
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell 2009; 17:9-26; PMID:19619488; https://doi.org/10.1016/j.devcel.2009.06.016
- Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res 2010; 106:1798-1806; PMID:20576942; https://doi.org/10.1161/CIRCRESAHA.110.219840
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev 1997; 11:3286-3305 (); PMID:9407023; https://doi.org/10.1101/gad.11.24.3286
- Goldbeter A, Pourquie O. Modeling the segmentation clock as a network of coupled oscillations in the Notch, Wnt and FGF signaling pathways. J Theor Biol 2008; 252:574-585; PMID:18308339; https://doi.org/10.1016/j.jtbi.2008.01.006
- Dunty WC, Jr, Biris KK, Chalamalasetty RB, Taketo MM, Lewandoski M, Yamaguchi TP. Wnt3a/beta-catenin signaling controls posterior body development by coordinating mesoderm formation and segmentation. Development 2008; 135:85-94; PMID:18045842; https://doi.org/10.1242/dev.009266
- Aulehla A, Pourquie O. Oscillating signaling pathways during embryonic development. Curr Opin Cell Biol 2008; 20:632-637; PMID:18845254; https://doi.org/10.1016/j.ceb.2008.09.002
- Karalay O, Doberauer K, Vadodaria KC, Knobloch M, Berti L, Miquelajauregui A, Schwark M, Jagasia R, Taketo MM, Tarabykin V, et al. Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci U S A 2011; 108:5807-5812; PMID:21436036; https://doi.org/10.1073/pnas.1013456108
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci 2009; 12:1097-1105; PMID:19701198; https://doi.org/10.1038/nn.2360
- Lie DC, Colamarino SA, Song HJ, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005; 437:1370-1375; PMID:16251967; https://doi.org/10.1038/nature04108
- Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, Radtke F, Hsieh J, Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci 2010; 30:10484-10492; PMID:20685991; https://doi.org/10.1523/JNEUROSCI.4721-09.2010
- Giachino C, Taylor V. Notching up neural stem cell homogeneity in homeostasis and disease. Front Neurosci 2014; 8:32; PMID:24611040; https://doi.org/10.3389/fnins.2014.00032
- Basak O, Giachino C, Fiorini E, Macdonald HR, Taylor V. Neurogenic subventricular zone stem/progenitor cells are Notch1-dependent in their active but not quiescent state. J Neurosci 2012; 32:5654-5666; PMID:22514327; https://doi.org/10.1523/JNEUROSCI.0455-12.2012
- Lieber T, Kidd S, Alcamo E, Corbin V, Young MW. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev 1993; 7:1949-1965 (); PMID:8406001; https://doi.org/10.1101/gad.7.10.1949
- Mumm JS, Kopan R. Notch signaling: from the outside in. Dev Biol 2000; 228:151-165; PMID:11112321; https://doi.org/10.1006/dbio.2000.9960
- Imayoshi I, Isomura A, Harima Y, Kawaguchi K, Kori H, Miyachi H, Fujiwara T, Ishidate F, Kageyama R. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science 2013; 342:1203-1208; PMID:24179156; https://doi.org/10.1126/science.1242366
- Baek JH, Hatakeyama J, Sakamoto S, Ohtsuka T, Kageyama R. Persistent and high levels of Hes1 expression regulate boundary formation in the developing central nervous system. Development 2006; 133:2467-2476; PMID:16728479; https://doi.org/10.1242/dev.02403
- Pfeuty B. A computational model for the coordination of neural progenitor self-renewal and differentiation through Hes1 dynamics. Development 2015; 142:477-485; PMID:25605780; https://doi.org/10.1242/dev.112649
- Harima Y, Imayoshi I, Shimojo H, Kobayashi T, Kageyama R. The roles and mechanism of ultradian oscillatory expression of the mouse Hes genes. Semin Cell Dev Biol 2014; 34:85-90; PMID:24865153; https://doi.org/10.1016/j.semcdb.2014.04.038
- Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R. Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science 2002; 298:840-843; PMID:12399594; https://doi.org/10.1126/science.1074560
- Takebayashi K, Sasai Y, Sakai Y, Watanabe T, Nakanishi S, Kageyama R. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-1. Negative autoregulation through the multiple N box elements. J Biol Chem 1994; 269:5150-5156 (); PMID:7906273
- Goodfellow M, Phillips NE, Manning C, Galla T, Papalopulu N. microRNA input into a neural ultradian oscillator controls emergence and timing of alternative cell states. Nat Commun 2014; 5:3399; PMID:24595054; https://doi.org/10.1038/ncomms4399
- Jing B, Yuan J, Yin Z, Lv C, Lu S, Xiong H, Tang H, Ye G, Shi F. Dynamic properties of the segmentation clock mediated by microRNA. Int J Clin Exp Pathol 2015; 8:196-206 (); PMID:25755706
- Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol 2009; 16:365-371; PMID:19330006; https://doi.org/10.1038/nsmb.1576
- Eendebak RJ, Lucassen PJ, Fitzsimons CP. Nuclear receptors and microRNAs: Who regulates the regulators in neural stem cells? FEBS Lett 2011; 585:717-722; PMID:21295033; https://doi.org/10.1016/j.febslet.2011.01.039
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci 1992; 12:3642-3650 (); PMID:1527603
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience 1994; 61:203-209 (); PMID:7969902; https://doi.org/10.1016/0306-4522(94)90224-0
- Sundberg M, Savola S, Hienola A, Korhonen L, Lindholm D. Glucocorticoid hormones decrease proliferation of embryonic neural stem cells through ubiquitin-mediated degradation of cyclin D1. J Neurosci 2006; 26:5402-5410; PMID:16707792; https://doi.org/10.1523/JNEUROSCI.4906-05.2006
- Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci 2007; 26:3395-3401; PMID:18052970; https://doi.org/10.1111/j.1460-9568.2007.05972.x
- Bose R, Moors M, Tofighi R, Cascante A, Hermanson O, Ceccatelli S. Glucocorticoids induce long-lasting effects in neural stem cells resulting in senescence-related alterations. Cell Death Dis 2010; 1:e92; PMID:21368868; https://doi.org/10.1038/cddis.2010.60
- Wong EY, Herbert J. The corticoid environment: a determining factor for neural progenitors' survival in the adult hippocampus. Eur J Neurosci 2004; 20:2491-2498; PMID:15548194; https://doi.org/10.1111/j.1460-9568.2004.03717.x
- Fitzsimons CP, van Hooijdonk LW, Schouten M, Zalachoras I, Brinks V, Zheng T, Schouten TG, Saaltink DJ, Dijkmans T, Steindler DA, et al. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol Psychiatry 2013; 18:993-1005; PMID:22925833; https://doi.org/10.1038/mp.2012.123
- Chetty S, Friedman AR, Taravosh-Lahn K, Kirby ED, Mirescu C, Guo F, Krupik D, Nicholas A, Geraghty AC, Krishnamurthy A, et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol Psychiatry 2014; 19:1275-1283; PMID:24514565; https://doi.org/10.1038/mp.2013.190
- Fitzsimons CP, Herbert J, Schouten M, Meijer OC, Lucassen PJ, Lightman S. Circadian and ultradian glucocorticoid rhythmicity: Implications for the effects of glucocorticoids on neural stem cells and adult hippocampal neurogenesis. Front Neuroendocrinol 2016; 41:44-58; PMID:27234350; https://doi.org/10.1016/j.yfrne.2016.05.001
- de Kloet ER, Fitzsimons CP, Datson NA, Meijer OC, Vreugdenhil E. Glucocorticoid signaling and stress-related limbic susceptibility pathway: about receptors, transcription machinery and microRNA. Brain Res 2009; 1293:129-141; PMID:19332027; https://doi.org/10.1016/j.brainres.2009.03.039
- Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat Rev Endocrinol 2014; 10:303-310; PMID:24663223; https://doi.org/10.1038/nrendo.2014.22
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab 1971; 33:14-22; PMID:4326799; https://doi.org/10.1210/jcem-33-1-14
- Dallman MF, Engeland WC, Rose JC, Wilkinson CW, Shinsako J, Siedenburg F. Nycthemeral rhythm in adrenal responsiveness to ACTH. Am J Physiol 1978; 235:R210-218 (); PMID:215040
- Walker JJ, Terry JR, Lightman SL. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc Biol Sci 2010; 277:1627-1633; PMID:20129987; https://doi.org/10.1098/rspb.2009.2148
- Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, De Kloet ER, Lightman SL. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. J Neuroendocrinol 2010; 22:1093-1100; PMID:20649850; https://doi.org/10.1111/j.1365-2826.2010.02051.x
- Sarabdjitsingh RA, Isenia S, Polman A, Mijalkovic J, Lachize S, Datson N, de Kloet ER, Meijer OC. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology 2010; 151:1177-1186; PMID:20080870; https://doi.org/10.1210/en.2009-1119
- Qian X, Droste SK, Lightman SL, Reul JM, Linthorst AC. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology 2012; 153:4346-4353; PMID:22822164; https://doi.org/10.1210/en.2012-1484
- Fitzsimons CP, Ahmed S, Wittevrongel CF, Schouten TG, Dijkmans TF, Scheenen WJ, Schaaf MJ, de Kloet ER, Vreugdenhil E. The microtubule-associated protein doublecortin-like regulates the transport of the glucocorticoid receptor in neuronal progenitor cells. Mol Endocrinol 2008; 22:248-262; PMID:17975023; https://doi.org/10.1210/me.2007-0233
- Stavreva DA, Coulon A, Baek S, Sung MH, John S, Tesikova M, Hakim O, Miranda T, Hawkins M, et al. Dynamics of chromatin accessibility and long-range interactions in response to glucocorticoid pulsing. Genome Res 2015; 25:845-857; PMID:25677181; https://doi.org/10.1101/gr.184168.114
- Vreugdenhil E, Verissimo CS, Mariman R, Kamphorst JT, Barbosa JS, Zweers T, Champagne DL, Schouten T, Meijer OC, de Kloet ER, et al. MicroRNA 18 and 124a down-regulate the glucocorticoid receptor: implications for glucocorticoid responsiveness in the brain. Endocrinology 2009; 150:2220-2228; PMID:19131573; https://doi.org/10.1210/en.2008-1335
- Smith SS, Dole NS, Franceschetti T, Hrdlicka HC, Delany AM. microRNA-433 Dampens Glucocorticoid Receptor Signaling, Impacting Circadian Rhythm and Osteoblastic Gene Expression. J Biol Chem 2016; 291(41):21717-21728
- Dwivedi Y, Roy B, Lugli G, Rizavi H, Zhang H, Smalheiser NR. Chronic corticosterone-mediated dysregulation of microRNA network in prefrontal cortex of rats: relevance to depression pathophysiology. Transl Psychiatry 2015; 5:e682; PMID:26575223; https://doi.org/10.1038/tp.2015.175
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 2014; 111:16219-16224; PMID:25349387; https://doi.org/10.1073/pnas.1408886111
- Masamizu Y, Ohtsuka T, Takashima Y, Nagahara H, Takenaka Y, Yoshikawa K, Okamura H, Kageyama R. Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cells. Proc Natl Acad Sci U S A 2006; 103:1313-1318; PMID:16432209; https://doi.org/10.1073/pnas.0508658103
- Lightman S, Terry JR. The importance of dynamic signalling for endocrine regulation and drug development: relevance for glucocorticoid hormones. Lancet Diabetes Endocrinol 2014; 2:593-599; PMID:24731665; https://doi.org/10.1016/S2213-8587(13)70182-7
- Koyanagi S, Okazawa S, Kuramoto Y, Ushijima K, Shimeno H, Soeda S, Okamura H, Ohdo S. Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Mol Endocrinol 2006; 20:573-583; PMID:16269518; https://doi.org/10.1210/me.2005-0165
- Spulber S, Conti M, DuPont C, Raciti M, Bose R, Onishchenko N, Ceccatelli S. Alterations in circadian entrainment precede the onset of depression-like behavior that does not respond to fluoxetine. Transl Psychiatry 2015; 5:e603; PMID:26171984; https://doi.org/10.1038/tp.2015.94

