ABSTRACT
During vertebrate embryonic development, neural crest cells arise from the border region between neural and non-neural ectoderm, migrate to various locations of the embryonic body and differentiate into diverse tissues. In the cochlea of the inner ear, the neural crest cell-derived melanocyte is an important component to form the stria vascularis, a non-sensory structure crucial for auditory function. Our recent conditional knockout study in the mouse indicates that in the absence of HGF-c-MET signaling, neural crest cells properly migrate and find the right region of the prospective stria vascularis in the cochlear epithelium, but fail to start incorporating into the epithelium. Our study has shed light on a homing issue of migrating cells that is evident during development, or in cancer cell metastasis.
Suppose you were a wanderer and finally found your eternal homeland. But, how would you feel if you were refused entry? The neural crest cells may face this problem if they are not able to receive a key signal, Hepatocyte Growth Factor (HGF) signaling.
Neural crest cells arise from the lateral border of the neural plate during vertebrate embryonic development and migrate to specific locations. They differentiate into a wide variety of cell types, including neurons and glia of the peripheral nervous system, smooth muscle, cartilage, craniofacial mesenchyme, and melanocytes.Citation6 A subpopulation of the melanocytes migrate toward the developing otocyst and incorporate into the stria vascularis of the cochlear duct, forming an intermediate cell layer. The stria vascularis is a non-sensory structure of the mammalian cochlear duct which is crucial to cochlear homeostasis by maintaining the ionic composition of the endolymph of the Scala Media, the middle division of the cochlear duct.Citation10 Defects in the stria vascularis are one of the major causes of sensorineural hearing loss.Citation4
Correlations between melanocyte deficiency and deafness were recognized as far back as the 19th-century by Charles Darwin. In On the Origin of Species (1859), he wrote, “cats with blue eyes are invariably deaf; color and constitutional peculiarities go together…” although later studies showed not all blue-eyed cats are deaf.Citation1-3,5 Nearly 100 years after Darwin's observation, a Dutch ophthalmologist, Petrus Johannes Waardenburg, described a syndrome with pigmentation defects and congenital deafness, now called Waardenburg syndrome.Citation16 At present, several gene mutations from several subtypes of Waardenburg syndrome have been identified, including PAX3, MITF, SNAI2, EDNRB, EDN3 and SOX10, which are important for differentiation and migration of neural crest cell-derived melanocytes.Citation12
Our present study has shed light on a novel relationship between HGF-c-MET signaling in melanocytes and deafness.Citation14 Recently, Schultz and colleagues showed that an autosomal-recessive, nonsyndromic hearing loss, DFNB39, is caused by noncoding mutations of HGF.Citation13 Mujtaba and colleges have identified missense mutations in the c-MET locus from the DFNB97 family.Citation7
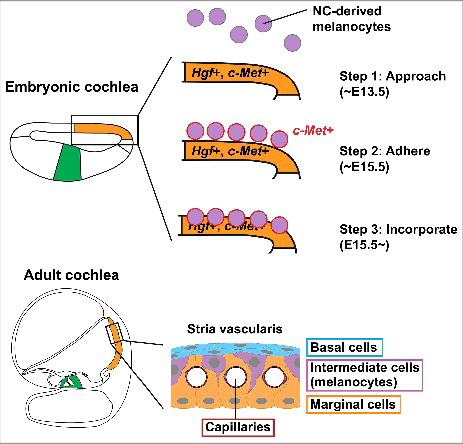
We analyzed 3 conditional mutant mice in which (1) Hgf is inactivated in the cochlear epithelium, (2) c-Met is inactivated in the cochlear epithelium and (3) c-Met is inactivated in neural crest cells. We found HGF-c-MET signaling in both epithelial and neural crest cells is required for the migrating melanocytes to incorporate into the epithelium of the prospective stria vascularis ().Citation14 HGF is also called “Scatter factor," promoting motility and invasiveness of the epithelial cells.Citation8,15 Peinado and colleagues reported that metastatic melanomas secret exosomes including active MET that “educate” bone marrow progenitor cells by increasing mobility and tumor niche by altering the extracellular matrix.Citation11 It is possible that developmentally normal mechanisms of cell migration and invasion are used for cancer metastasis in an uncontrollable manner.
Figure 1. Schematic drawing of melanocyte incorporation into the stria vascularis of developing mouse cochlea. During mouse development, the prospective stria vascularis is specified in the embryonic cochlear epithelium (orange), distinct from the prospective organ of Corti (green). The stria vascularis consists of capillaries (crimson), basal cell layer (blue), intermediate cell (melanocyte) layer (purple) and marginal cell layer (orange). Neural crest (NC)-derived melanocytes approach the embryonic cochlear epithelium around embryonic day (E) 13.5 (Step 1). Around E15.5, the melanocytes adhere to the cochlear epithelium in the region of prospective stria vascularis (Step 2). These melanocytes start expressing c-Met (red). c-Met is weakly expressed in the cochlear epithelium and Hgf is expressed in the prospective stria vascularis (orange). The melanocytes then incorporate into the cochlear epithelium from basal turn of the cochlea (Step 3). The present study suggests that HGF-c-MET signaling in both melanocytes and cochlear epithelium is required for the initiation of the incorporation movement.
We also observed that capillaries in the stria vascularis form in the HGF-c-MET conditional mutant mice at birth, but then disappear in the adult (unpublished). Depletion of the melanocytes in the mature stria vascularis caused leakiness of capillaries.Citation9,17 Thus, it is possible that the melanocytes per se or HGF-c-MET signaling in the melanocytes are important for maintenance of the capillary network in postnatal stria vascularis.
Our recent study indicates the importance of HGF-c-MET signaling as a trigger of melanocyte incorporation into the prospective stria vascularis during mouse inner ear development. It is still largely unknown which population of neural crest cells migrates into the otocyst, which molecules attract the neural crest cells toward the prospective stria vascularis, what molecules are used to adhere the neural crest cell-derived melanocytes on the epithelium of the prospective stria vascularis and the mechanisms of melanocyte incorporation movement. Further study is needed to resolve these questions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
I am very grateful to Drs. Andrew Groves (Baylor College of Medicine) and Andrej Kral (Hannover Medical School) for helpful comments on an earlier version of the manuscript. I am indebted to the late Dr. John K. Niparko for his outstanding support as the Chairman of the USC Tina and Rick Caruso Department of Otolaryngology – Head and Neck Surgery.
References
- Geigy CA, Heid S, Steffen F, Danielson K, Jaggy A, Gaillard C. Does a pleiotropic gene explain deafness and blue irises in white cats? Vet J 2007; 173:548-53; PMID:16956778; https://doi.org/10.1016/j.tvjl.2006.07.021
- Heid S, Hartmann R, Klinke R. A model for prelingual deafness, the congenitally deaf white cat–population statistics and degenerative changes. Hear Res 1998; 115:101-12; PMID:9472739; https://doi.org/10.1016/S0378-5955(97)00182-2
- Kral A, Lomber SG. Deaf white cats. Curr Biol 2015; 25:R351-3; PMID:25942543; https://doi.org/10.1016/j.cub.2015.02.040
- Locher H, de Groot JCMJ, van Iperen L, Huisman MA, Frijns JHM, Chuva de Sousa Lopes SM. Development of the stria vascularis and potassium regulation in the human fetal cochlea: Insights into hereditary sensorineural hearing loss. Dev Neurobiol 2015; 75:1219-40; PMID:25663387; https://doi.org/10.1002/dneu.22279
- Mair IW, Elverland HH. Hereditary deafness in the cat. An electron microscopic study of the stria vascularis and Reissner's membrane. Arch Otorhinolaryngol 1977; 217:199-217; PMID:303094; https://doi.org/10.1007/BF00665540
- Mayor R, Theveneau E. The neural crest. Development 2013; 140:2247-51; PMID:23674598; https://doi.org/10.1242/dev.091751
- Mujtaba G, Schultz JM, Imtiaz A, Morell RJ, Friedman TB, Naz S. A mutation of MET, encoding hepatocyte growth factor receptor, is associated with human DFNB97 hearing loss. J Med Genet 2015; 52:548-52; PMID:25941349; https://doi.org/10.1136/jmedgenet-2015-103023
- Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, Narsimhan RP, Hartmann G, Zarnegar R, Michalopoulos GK. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J 1991; 10:2867-78; PMID:1655405
- Neng L, Zhang F, Kachelmeier A, Shi X. Endothelial cell, pericyte, and perivascular resident macrophage-type melanocyte interactions regulate cochlear intrastrial fluid-blood barrier permeability. J Assoc Res Otolaryngol 2013; 14:175-185; PMID:23247886; https://doi.org/10.1007/s10162-012-0365-9
- Patuzzi R. Ion flow in stria vascularis and the production and regulation of cochlear endolymph and the endolymphatic potential. Hear Res 2011; 277:4-19; PMID:21329750; https://doi.org/10.1016/j.heares.2011.01.010
- Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18:883-91; PMID:22635005; https://doi.org/10.1038/nm.2753
- Pingault V, Ente D, Dastot-Le Moal F, Goossens M, Marlin S, Bondurand N. Review and update of mutations causing Waardenburg syndrome. Hum Mutat 2010; 31:391-406; PMID:20127975; https://doi.org/10.1002/humu.21211
- Schultz JM, Khan SN, Ahmed ZM, Riazuddin S, Waryah AM, Chhatre D, Starost MF, Ploplis B, Buckley S, Velásquez D, et al. Noncoding mutations of HGF are associated with nonsyndromic hearing loss, DFNB39. Am J Hum Genet 2009; 85:25-39; PMID:19576567; https://doi.org/10.1016/j.ajhg.2009.06.003
- Shibata S, Miwa T, Wu H-H, Levitt P, Ohyama T. Hepatocyte growth factor-c-MET signaling mediates the development of nonsensory structures of the mammalian cochlea and hearing. J Neurosci 2016; 36:8200-09; PMID:27488639; https://doi.org/10.1523/JNEUROSCI.4410-15.2016
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010; 11:834-48; PMID:21102609; https://doi.org/10.1038/nrm3012
- Waardenburg PJ. A new syndrome combining developmental anomalies of the eyelids, eyebrows and nose root with pigmentary defects of the iris and head hair and with congenital deafness. Am J Hum Genet 1951; 3:195-253; PMID:14902764
- Zhang W, Dai M, Fridberger A, Hassan A, Degagne J, Neng L, Zhang F, He W, Ren T, Trune D, Auer M, Shi X. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci U S A 2012; 109:10388-93; PMID:22689949; https://doi.org/10.1073/pnas.1205210109

