ABSTRACT
Neoblasts are motile pluripotent stem cells unique to the flatworm phyla Platyhelminthes and Acoela. The role of neoblasts in tissue regeneration has received much attention in recent studies. Here we review data pertinent to the structure and embryonic origin of these stem cells, and their participation in normal cell turnover. Next, we present data proving that neoblasts also account for the addition of cells during postembryonic growth. Bromodeoxyuridine (BrdU) pulse chase experiments demonstrate that the incorporation of neoblast-derived cells into the different tissues of the juvenile worm follows a stereotyped pattern, whereby cells within the parenchymal layer (muscle, gland) incorporate new cells most rapidly, followed by the epidermal domain surrounding the mouth, dorsal epidermis, and, lastly, the nervous system.
Introduction
Platyhelminthes (flatworms), a phylum included within the superclade of lophotrochozoa, are characterized by a system of motile, pluripotent stem cells termed neoblasts. Whereas cells of all differentiated tissues of flatworms are postmitotic, neoblasts continue to divide throughout the life time of the animal. Part of their offspring renews the neoblast population; other daughter cells become associated with the differentiated tissues and replace cells damaged by aging or wear and tear [Citation1–5]. Neoblasts are also responsible for the high degree of regenerative capacity that has been observed for many flatworm species. Lastly, neoblasts play a central role in normal organ development, including neurogenesis, a function addressed in this paper. The only other animal phylum that possesses neoblasts are the acoels, a clade of simple worms showing many characteristics of platyhelminths, but residing outside the lophotrochozoa at the very base of the bilaterian tree [Citation6–8].
Based on the fact that they represent the only proliferating cell type, neoblasts of platyhelminths and acoels have been visualized light-microscopically using autoradiography [Citation9,Citation10] or Bromodeoxyuridine (BrdU) incorporation [Citation6,Citation11–15]. Electron microscopic studies established the ultrastructure of neoblasts of planarian flatworms (taxon Tricladida [Citation16–22];, macrostomids [Citation10,Citation23,Citation24] and acoels [Citation8]. In adult planarians and acoels, neoblasts are scattered throughout the mesenchymal layer ( = parenchyma). In macrostomids, a clade considered basal among the platyhelminths [Citation25,Citation26], neoblasts are more restricted, forming two bilateral bands of cells associated with the longitudinal nerve cords, in addition to neoblasts scattered along the wall of the gut (“gastrodermal neoblasts”; 11).
Ultrastructurally, three different types, or developmental stages, of neoblasts were distinguished [Citation23]. (1) Stage 1 neoblasts are round cells devoid of organelles except ribosomes and few mitochondria. The heterochromatin of these neoblasts is broken up into numerous small, regularly spaced domains forming a “checkerboard” pattern. Stage 1 cells were interpreted to represent the “ground state” within the life cycle of neoblasts; they are also present during regeneration adjacent to the regeneration blastema [Citation19]. A characteristic feature noted for stage 1 neoblasts in planarians and the microturbellarian Microstomum lineare, but not for Macrostomum sp. or acoels, is the chromatoid body, an electron-dense cytoplasmic inclusion close to the nuclear membrane, formed by RNA-protein complexes [Citation19,Citation22,Citation27]. (2) Stage 2 neoblasts possess heterochromatin that forms an irregular pattern of clumps, interconnected by strands. Stage 2 neoblasts constitute the bulk of undifferentiated cells of the blastema in regenerating tissues. (3) Finally, in stage 3 neoblasts, the cytoplasm has increased in volume and acquired Golgi complexes and abundant endoplasmic reticulum. Molecular studies (e.g., 28) demonstrated that the pathway from self-renewing proliferation towards commitment and differentiation of neoblasts is reflected in profound changes of the gene expression profile.
The role of neoblasts as pluripotent tissue replacement cells has been studied in great detail under experimental conditions, where parts of the animal were cut, and the appearance of differentiation markers in neoblast-derived cells were recorded (e.g., brain: [Citation29]; pharynx: [Citation30]; protonephridia: [Citation31]; gut: 32). For the gut, the incorporation of BrdU-positive cells was also followed in normal (non-regenerating) animals. In planarians, similar to other bilaterians more generally, the midgut epithelium contains absorptive enterocytes, aside from glandular cells and endocrine cells. The epithelium is separated by a basement membrane from the surrounding parenchyma, which houses neoblasts. After applying BrdU it took a 72hr chase for BrdU-positive cells to penetrate the basement membrane and become incorporated into the midgut epithelium [Citation32].
As a result of the fact that the journey of a replacement cell from neoblast to differentiated cell, in normal cell turn-over or regeneration, takes several days (see also findings reported in this paper below), live imaging of this process has not yet been accomplished. Instead, electron microscopy studies have frequently presented figures of cells which are highly suggestive of developmental pathways from neoblast to differentiated cell (e.g., neoblast to gland cell: 21; neoblast to epidermis: 23, 25; neoblast to muscle cell: 20). In all cases, the cells interpreted to represent transitional stages between neoblast and differentiated cells were characterized by combining signs of differentiation (e.g., for nascent muscle cells, formation of Golgi complexes, followed by arrays of microtubules and myofilaments) with primitive features [Citation20,Citation23].
The role of neoblasts in adult tissue maintenance and regeneration has been studied in great detail, and detailed gene expression data for neoblasts have been assembled [Citation33–38]. Less is known about the developmental origin of neoblasts and their participation in embryonic and postembryonic tissue growth. Numerous embryological studies of flatworms and acoels document a generalized mitotic activity throughout roughly the first half of embryonic development (stages 1–4 after 38–41; 42; ). Subsequently, differentiation sets in, and definitive organ primordia (brain and nerve cords, epidermis, pharynx, musculature, protonephridia) make their appearance (). At this point, cells exit the cell cycle and will remain postmitotic for the rest of their life. The only cells that remain proliferative in the later stages of embryogenesis, as well as during postembryonic growth, were interpreted as the nascent neoblast population [Citation42]. At a molecular level, the embryonic origin of neoblasts has been recently documented in detail for the planarian Schmidtea mediterranea [Citation28]. Here, embryonic neoblasts with the molecular footprint of the previously characterized adult neoblasts arise during the stage of organogenesis (stages 5, 6) from within a larger pool of Piwi-positive, cycling progenitor cells. As the majority of these early progenitors, which transcriptomically differ from definitive neoblasts, exit the cell cycle to differentiate into the different organs, a subset of cells remains mitotically active and switches to the “definitive neoblast mode” of gene expression. It can be surmised that the formation of neoblasts proceeds along similar mechanism in other flatworm taxa.
Figure 1. Structure and development of neoblasts. (A) (A-C) Schematic sections of platyhelminth embryo at early stage (A; cleavage, forming blastomeres), intermediate stage (B; embryonic primordium) and late stage (C; differentiated organs). “Neoblast progenitors” form a pool of scattered inner cells that remain mitotically active at a stage (B) when all other cells have exited the cell cycle and assemble into organ primordia; these progenitors become the definitive neoblasts of the late embryo that continue to add cells to growing tissues, and replace cells during adult life.
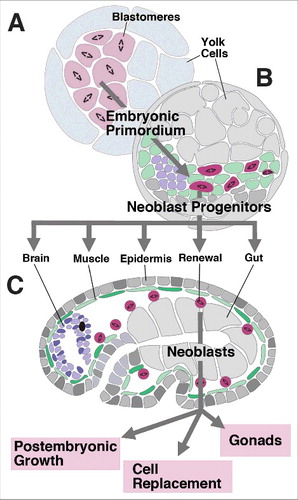
Given that, in terms of cell number, embryonic organ primordia (e.g., brain, epidermis or pharynx) are much smaller than the adult structures they give rise to it follows that neoblasts contribute substantially to the final size and shape of organs. In other words, one can subdivide the process of organogenesis into two phases. During an early phase, organ primordia assemble from spread-out populations of mitotic progenitors, similar to what happens during the development of bilaterian animals in general. This early phase results in small organ primordia that may already possess all the cell types and structural elements characteristic of the later, adult organs [Citation38–41,Citation43,Citation44]; ). During a secondary phase, more cells, derived from neoblasts, become incorporated into the primordia, causing them to grow until a prespecified adult size has been reached (). This process of “secondary organogenesis” in flatworms or acoels has not previously been investigated. We chose the microturbellarian Macrostomum lignano, which is much smaller and shows a faster and more ordered mode of development than the planarians Schmidtea or Dugesia to begin to address the role of neoblasts in organogenesis. Starting with the postembryonic growth phase, which is experimentally more accessible than embryogenesis, we used BrdU pulse chase experiments to analyze the spatio-temporal pattern describing the incorporation of neoblast-derived cells into different organs of the head of the juvenile. We show that (1) neoblast proliferation and differentiation accounts for the addition of cells within the epidermis, muscle-gland system, and brain that occurs during postembryonic growth; (2) the addition of cells follows a stereotyped pattern, whereby cells within the parenchymal layer (muscle, gland) incorporate new cells most rapidly, followed by the epidermal domain surrounding the mouth, dorsal epidermis, and nervous system.
Materials and methods
Animal culture and fixation
Macrostomum lignano lives in laboratory cultures of the diatom Nitzschia curvilineata. Petri dishes with the artificial growth medium “F2” (see 38) are inoculated with Nitzschia. By 10–14 days the algae form a dense lawn at the bottom of the dish. Groups of adult Macrostomum (approximately 1.5 mm in length) are transferred onto the algal dish. They produce eggs continuously, and the eggs, if left undisturbed, will develop into sexually mature adults in approximately 3 weeks. For preparation, animals at different stages were collected and fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS).
Immunohistochemistry and bromodeoxyuridine (BrdU) labeling
In preparation for immunohistochemistry, fixed animals were washed in PBT (PBS plus 0.3% Triton X-100; pH 7.2; for washing, PBT solution was changed three to five times over a 10-min period). Preparations were transferred to blocking solution [normal goat serum) and then incubated overnight in PBT containing primary antibody [anti-BrdU antibody (Becton Dickinson, Mountain View, CA; dilution of 1:40); anti-Tyrosinated tubulin (Sigma; dilution 1:1,000); anti-FMRF (Jackson Labs; 1:100 dilution)].
After another washing step in PBT the preparations were incubated for 4 h in PBT containing the secondary antibody (FITC-conjugated rabbit anti-mouse or anti rabbit immunoglobulin; Jackson Labs; dilution 1:800). The nuclear marker sytox (Molecular Probes; 1:10,000 dilution) were used to label nuclei.
To label proliferating neoblasts in pulse and chase experiments, worms at defined ages were kept in a solution of BrdU (1mg/ml) in artificial sea water for 1 hour. Following chase intervals of defined length, specimens were fixed, rinsed and treated with 2N HCI at 37oC for 1 hour to denature DNA. After 3 PBT rinses for 10 minutes each, specimens were incubated in blocking solution followed by incubation with anti-BrdU antibody.
Confocal microcopy
Labeled preparations were viewed as whole mounts in Vectashield mounting medium
H-1000 (Vector Laboratories) by confocal microscopy (Biorad Model MRC1024ES and Laser Sharp version 3.2 software. Complete series of optical sections were taken from preparations at 2mm intervals. Captured images were processed by ImageJ or FIJI (NIH; http://rsbweb.nih.gov/ij/ and http://fiji.sc/). Schematics were made in Adobe Illustrator and figures assembled in Adobe Photoshop.
Cell counts
To assess the ratio of BrdU-positive cells at different stages, z-projections of three consecutive 1micron horizontal sections were generated from specimens labeled with sytox and anti-BrdU. For each BrdU chase analyzed (0 days, 3 days, 7 days, 21 days after pulse; see ), four animals were taken. For each animal, all nuclei visible in three adjacent z-projections that included the central part of the body (brain neuropil, ventral nerve cords) were counted and the ratio of BrdU-positive nuclei to all nuclei was determined (see )
Results and discussion
Pulses of BrdU followed by immediate fixation resulted in labeling of S-phase neoblasts. As reported in previous works [Citation11], these cells were distributed in bilateral bands that extended throughout the trunk of the animal. Neoblasts lie in close proximity to the nerve cords and the layer of muscle and gland cells that form a “mesenchymal layer” in between the epidermis and intestine (; ). The distribution of neoblasts was similar in freshly hatched juveniles (1-3 days) and subadults (21-28 days), except for the increase in number that occurred at the later stages (). The increase in number of neoblasts reflected the overall increase in numbers of cells in all tissues. Cell counts performed on representative horizontal confocal sections of the head of animals over the period from 1–21days after hatching showed that cells in all tissue layers (epidermis, brain, parenchymal layer containing muscle and gland cells) increased by more than 50% ().
Figure 2. Postembryonic growth of Macrostomum lignano. (A, B) Z-projections of horizontal confocal sections of Macrostomum hatchling (A) and adult (B) pulsed for 30min with BrdU and fixed right thereafter. Preparations were labeled with antibody against BrdU (green) and tyrosinated tubulin (tyrTub; magenta). BrdU-labeled neoblasts form bilateral columnar populations of cells. (C-D’’) Z-projections of three consecutive horizontal confocal sections of specimen 3 days after hatching (D3; C-C’’) and 21 days after hatching (D21; D-D’’). Nuclei are labeled with Sytox (magenta in C, D; white in C’, C’’, D’, D’’); cilia and elongated processes of neurons and gland cells are labeled with anti-Tyrosinated tubulin (C, D; green). Z-projections represents central (“equatorial”) level of worm, defined by characteristic compartmentalization of brain neuropile into centro-medial domain (cm in D’), flanked by centro-lateral “wings” (cl in D’). Location, size and shape of nuclei allow one to distinguish several tissue layers, including epidermis (ep), parenchymal layer (muscle/glands; ms/gl), brain (br), nerve cords (nc), pharynx (ph) and gut. Individual nuclei of cells filling these layers in the z-projection presented in (C-D’’) are differentially colored (C’’, D’’). (E) Histogram showing average numbers of cells contained within horizontal section. For epidermis and muscle/gland layer, counts were started at the level of pharynx [arrow in (C’’, D’’)]. Abbreviations: br brain; cx cortex; ep epidermis; gl gland; ms muscle; nb neoblast; nc nerve cord; np neuropil; ph pharynx; Bar: 25μm.
![Figure 2. Postembryonic growth of Macrostomum lignano. (A, B) Z-projections of horizontal confocal sections of Macrostomum hatchling (A) and adult (B) pulsed for 30min with BrdU and fixed right thereafter. Preparations were labeled with antibody against BrdU (green) and tyrosinated tubulin (tyrTub; magenta). BrdU-labeled neoblasts form bilateral columnar populations of cells. (C-D’’) Z-projections of three consecutive horizontal confocal sections of specimen 3 days after hatching (D3; C-C’’) and 21 days after hatching (D21; D-D’’). Nuclei are labeled with Sytox (magenta in C, D; white in C’, C’’, D’, D’’); cilia and elongated processes of neurons and gland cells are labeled with anti-Tyrosinated tubulin (C, D; green). Z-projections represents central (“equatorial”) level of worm, defined by characteristic compartmentalization of brain neuropile into centro-medial domain (cm in D’), flanked by centro-lateral “wings” (cl in D’). Location, size and shape of nuclei allow one to distinguish several tissue layers, including epidermis (ep), parenchymal layer (muscle/glands; ms/gl), brain (br), nerve cords (nc), pharynx (ph) and gut. Individual nuclei of cells filling these layers in the z-projection presented in (C-D’’) are differentially colored (C’’, D’’). (E) Histogram showing average numbers of cells contained within horizontal section. For epidermis and muscle/gland layer, counts were started at the level of pharynx [arrow in (C’’, D’’)]. Abbreviations: br brain; cx cortex; ep epidermis; gl gland; ms muscle; nb neoblast; nc nerve cord; np neuropil; ph pharynx; Bar: 25μm.](/cms/asset/4c595390-f78b-460f-9605-6d02168cbe0d/kngs_a_1469944_f0002_oc.jpg)
Figure 3. Incorporation of BrdU-positive (neoblast-derived) cells into tissue layers, following chase intervals of different lengths. Top row panels (A, C, E, G) show Z-projections of horizontal confocal sections representing central (“equatorial”) level of juvenile worms treated with 30min pulse of BrdU 1 day after hatching, and chased for 0 days (A; D1Ch0), 3 days (C; D1 Ch3), 7 days (E; D1 Ch7) and 21 days (G; D1 Ch21). BrdU-positive cells in green; all nuclei (labeled by Sytox) are in magenta. Second row panels (B, D, F, H) Schematic depictions of horizontal sections with tissue layers (epidermis, brain, muscle/gland, pharynx/gut) differentiated by different shading. Colored circles indicate pattern of BrdU-positive cells at represented chase intervals. (I) Ratio of BrdU-positive cells (in % of total) in different tissue layers at different chase intervals. (J) Representative confocal section of specimen chased for 21 days. Nuclei are in blue (Sytox), cilia and processes of neurons/glands in red (anti-Tyrosinated tubulin). (K) Dorsal view of head of hatchling, showing location of cut (arrow anterior to eyes). (L, M) Neoblast derived cells following BrdU pulse at day 1 in control (L) and following cut (M). Arrows point at BrdU-positive neurons in brain cortex. (N-T) Representative confocal sections of specimens chased for 7 days (N-P) or 21 days (Q, R). (N, O) are horizontal sections showing ventral epidermis, surrounding pharynx (ph; N) and dorsal epidermis (O). (P-R) are digitally tilted cross sections at level of brain (P, Q) and posterior gut (R). For 7 day chase (N-P) note abundance of BrdU-positive epidermal cells ventrally, compared to dorsally. After 21 day chase, anterior epidermis is almost completely filled with BrdU-positive cells (Q); posterior epidermis (R) contains far less BrdU-positive cells. (S, T) Z-projections of horizontal confocal sections of specimen chased for 11 days (S) or 14 days (T). Subset of neurons is labeled by antibody against FMRFamide (red); general nuclei are in blue (Sytox), BrdU-positive cells in green. Select FMRF-positive cells of central cluster (CE) have incorporated BrdU (arrowheads). Abbreviations: br brain; CE central cluster of FMRF neurons; cx cortex; ep epidermis; fsc fascicle; gl gland; ms muscle; nb neoblast; nc nerve cord; np neuropil; ph pharynx; Bars: 25μm (A-D; J; K-M; N-Q).
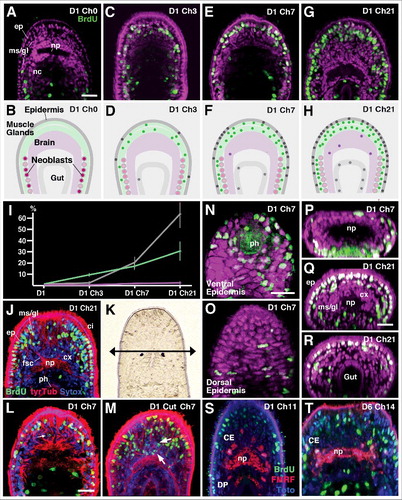
Chasing the BrdU pulse by time intervals of different lengths (3 days, 5 days, 7 days, 14 days, 21 days) resulted in labeling of differentiated cells, demonstrating that neoblast proliferation and differentiation represents a mechanism of postembryonic growth (). The distribution of BrdU-positive nuclei at successive time points indicated that the addition of cells to different tissues follows a distinct pattern. Following a 3 day chase, BrdU-positive nuclei were still restricted to the mesenchymal layer. However, labeled cells had spread anteriorly, surrounding the brain on all sides (). Aside from neoblasts (recognizeable by their relatively large diameter and interior position), nuclei of muscle cells and gland cells (smaller and more regular in shape; closely spaced and aligned in straight rows) showed BrdU label (). Their number was low, approximating less than 10% of the overall number of cells present in the parenchymal layer. Only very few labeled nuclei were found in the epidermis; none were present in the brain (). After a 7day chase (), the number of BrdU-positive cells of the parenchymal layer had increased to approximately 15%; at this stage, labeled nuclei were also found in the epidermis where they amounted to almost 20% of the overall number. The brain was still essentially devoid of BrdU label (). By 21 days after BrdU application, the fraction of labeled cells had continued to increase more or less linearly in the mesenchymal layer (28.7%); in the epidermis, the number had risen to over 60% (). At this stage, a small but significant number of BrdU-positive nuclei was also found in the brain cortex (). Following removal of the tip of the head [BrdU pulse 1 day after hatching, immediately followed by cut () and 7 day interval for regeneration] the number of BrdU-labeled cells in the brain and anteriorly adjacent gland/muscle/epidermis could be greatly enhanced ().
The above data demonstrated that the addition of neoblast-derived cells to the different tissues follows a distinct spatio-temporal pattern. This same principle also applies when analyzing the pattern of BrdU incorporation in individual tissues, such as the epidermis or brain. For example, in all animals fixed after a 7day chase, BrdU-positive cells were strongly concentrated at the anterior tip of the head and surrounding the mouth opening (); much fewer cells were found in the dorsal epidermis (). By 21 days after BrdU application, epidermal cells occupied mostly dorsal and lateral position, but were still more numerous anteriorly than posteriorly (). To obtain more detailed information regarding the differentiation of neurons from neoblast-derived cells we double-labeled preparations with an antibody against the neuropeptide FMRFamide, which is expressed in six discrete clusters of brain neurons in Macrostomum juveniles [Citation45]. BrdU application to freshly hatched animals, followed by 11–14 days chases, resulted in labeling of 1–2 neurons restricted to the central cluster (CE) of FMRFamide-positive neurons ().
Our measurements concerning the amount of cells added during postembryonic growth are comparable with previous findings; for example, Rieger and colleagues (46; for Macrostomum) concluded that the number of circular muscle fibers near the tail end of the body more than doubles (2.7:1) between the hatchling stage and adult; a similar figure (2.5:1) has been published for the planarian Dugesia tigrina [Citation47]. The ratio established in our study (1.6:1 for muscle/gland) is probably lower because we counted cell increases in the head, which grows substantially less postembryonically than the trunk or tail [Citation46]. Rieger and colleagues [Citation46] surmised that the source of added circular muscle fibers was the neoblast population, without providing experimental evidence. Our data clearly show that neoblasts, labeled by a short BrdU pulse at the time around hatching, become incorporated into the differentiated musculature after a lag phase of 2–3 days (). Interestingly, the addition of neoblast-derived cells to other tissues occurred at a slower pace, and followed a stereotyped pattern. In the epidermis, significant numbers of BrdU-positive nuclei were present only after a chase of 5–7 days; these newly added cells were always concentrated in the ventral epidermis around the mouth opening, suggesting that a “growth spurt” occurs in the perioral epidermis at an early stage, followed by a more delayed growth of epidermis at dorsal levels. In the nervous system, BrdU labeled cells appeared after more than two weeks, and remained at a fairly low number, even though overall brain growth is comparable to that of epidermis or muscle/gland (). It is possible that the low number of BrdU-positive brain cells after a 3 week chase could be due to higher mitotic activity (dilution of BrdU) in neoblast-derived progenitors entering the nervous system, compared to other tissues. The number mitoses that occur between the time when a neoblast commits to a particular cell fate and the time when it exits the cell cycle is unknown for Macrostomum (and other flatworms), and it is conceivable that neoblasts entering different lineages follow different proliferative schedules.
Most tissues and organs across different animal species are in a constant state of turnover. Loss of cells, caused by apoptosis or autophagy, is balanced by the production of new cells. In most cases, including the central nervous system, specialized stem cells residing within the organ itself account for the controlled production of new cells. For example, in the brain of song birds, defined regions undergo massive apoptotic cell death, while concomitantly new cells are generated by local populations of neural stem cells [Citation48,Citation49]. Neuron production can be halted, or increased, by experimentally manipulating the amount of neuronal death. In platyhelminths, including both planarians and macrostomids, the balanced destruction/production of neurons and other cells goes beyond that seen in other systems. In these animals, body/organ size fluctuates dramatically across the life time of the animal. Food scarcity brings about a decline in cell number by apoptosis and reduced neoblast proliferation; feeding immediately reverses this effect [Citation13,Citation50,Citation51]. Neoblasts “sense” cell numbers and ratios between different cell types, and adjust their proliferation/choice of fate accordingly. Neoblast proliferation restores sizes of organs and cell populations in a manner that is controlled by a genetically programmed set point, integrated with specific environmental conditions, such as food supply. During this process, ratios of specific cell types, including different neuron subpopulations, remain fixed, as shown for the planarian Dugesia japonica [Citation52]. Some of the pathways, among them Insulin signaling and Wnt signaling, that link cell numbers to neoblast proliferation begin to be elucidated for planarians [Citation51,Citation53]. We speculate that the same pathways will act during development, controlling the spatial and temporal pattern of neoblast proliferation and differentiation that bring about the proper shapes and sizes of organs. Macrostomum, with its relatively low overall cell number and fast reproduction, well studied genome [Citation54–56], and experimental tractability, carries much promise as a suitable model to obtain more insight into these mechanisms.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Rossi L, Salvetti A, Batistoni R, et al. Planarians, a tale of stem cells. Cell Mol Life Sci. 2008;65(1):16–23.
- Baguñà J. The planarian neoblast: the rambling history of its origin and some current black boxes. Int J Dev Biol. 2012;56(1-3):19–37.
- Rink JC. Stem cell systems and regeneration in planaria. Dev Genes Evol. 2013;223(1-2):67–84.
- Reddien PW. Specialized progenitors and regeneration. Development. 2013;140(5):951–7.
- Zhu SJ, Pearson BJ. (Neo)blast from the past: new insights into planarian stem cell lineages. Curr Opin Genet Dev. 2016;40:74–80.
- Gschwentner R, Ladurner P, Nimeth K, et al. Stem cells in a basal bilaterian. S-phase and mitotic cells in Convolutriloba longifissura (Acoela, Platyhelminthes). Cell Tissue Res. 2001;304(3):401–8.
- Egger B, Steinke D, Tarui H, et al. To be or not to be a flatworm: the acoel controversy. PLoS One. 2009;4(5):e5502.
- De Mulder K, Kuales G, Pfister D, et al. Characterization of the stem cell system of the acoel Isodiametra pulchra. BMC Dev Biol. 2009;9:69.
- Drobysheva IM. Physiological regeneration of the digestive parenchyma in Convoluta convoluta and Oxyposthia praedator (Turbellaria, Acoela). Hydrobiologia. 1986;132:189–93.
- Palmberg I. Stem cells in microturbellarians. Protoplasma. 1990;158(3):109–20.
- Ladurner P, Rieger R, Baguñà J. Spatial distribution and differentiation potential of stem cells in hatchlings and adults in the marine platyhelminth Macrostomum sp.: a bromodeoxyuridine analysis. Dev Biol. 2000;226(2):231–41.
- Newmark PA, Sànchez Alvarado A. Bromodeoxyuridine specifically labels the regenerative stem cells of Planarians. Dev Biol. 2000;220(2):142–53.
- Nimeth KT, Mahlknecht M, Mezzanato A, et al. Stem cell dynamics during growth, feeding, and starvation in the basal flatworm Macrostomum sp. (Platyhelminthes). Dev Dyn. 2004;230(1):91–9.
- Nimeth KT, Egger B, Rieger R, et al. Regeneration in Macrostomum lignano (Platyhelminthes): cellular dynamics in the neoblast stem cell system. Cell Tissue Res. 2007;327(3):637–46.
- Collins JJ, 3rd, Wang B, Lambrus BG, et al. Adult somatic stem cells in the human parasite Schistosoma mansoni. Nature. 2013;494(7438):476–9.
- Pedersen KJ. Cytological studies on the planarian neoblast. Zeitschr Zellforsch. 1959;50(6):799–817.
- Pedersen KJ. Studies on regeneration blastemas of the planarianDugesia tigrina with special reference to differentiation of the muscle-connective tissue filament system. Wilhelm Roux Arch Entwickl Mech Org. 1972;169(2):134–69.
- Lentz TL. Rhabdite formation in planaria: The role of microtubules. J Ultrastructure Res. 1967;17(1-2):114–26.
- Morita M, Best JB, Noel J. Electron microscopic studies of planarian regeneration. I. Fine structure of neoblasts in Dugesia dorotocephala. J Ultrastruct Res. 1969;27(1):7–23.
- Sauzin-Monnot MJ. Etude ultrastructurale des neoblastes de Dendrocoelum lacteum au cours de la regeneration. J Ultrastruct Res. 1973;45:206–22.
- Hay ED, Coward SJ. Fine structure studies on the planarian Dugesia I. Nature of the “neoblast” and other cell types in noninjured worms. J Ultrastruct Res. 1975;50(1):1–21.
- Hori I. An Ultrastructural Study of the Chromatoid Body in Planarian Regenerative Cells. J Electron Microsc. 1982;31(1):63–72.
- Rieger R, Legniti A, Ladurner P, et al. Ultrastructure of neoblasts in microturbellaria: significance for understanding stem cells in free living Platyhelminthes. Invert Reproduct Dev. 1999;35(2):127–40.
- Bode A, Salvenmoser W, Nimeth K, et al. Immunogold-labeled S-phase neoblasts, total neoblast number, their distribution, and evidence for arrested neoblasts in Macrostomum lignano (Platyhelminthes, Rhabditophora). Cell Tissue Res. 2006;325(3):577–87.
- Ehlers U. Das phylogenetische system der plathelminthes. Stuttgart, New York: Gustav Fischer Verlag; 1985.
- Riutort M, Álvarez-Presas M, Lázaro E, et al. Evolutionary history of the Tricladida and the Platyhelminthes: an up-to-date phylogenetic and systematic account. Int J Dev Biol. 2012;56(1-3):5–17.
- Auladell C, Garcia-Valero J, Baguna J. Ultrastructural localization of RNA in the chromatoid bodies of undifferentiated cells (neoblasts) in planarians by the Rnase-Gold complex technique. J Morph. 1993;216:319–26.
- Davies EL, Lei K, Seidel CW, et al. Embryonic origin of adult stem cells required for tissue homeostasis and regeneration. Elife. 2017;6:e21052.
- Nishimura K, Inoue T, Yoshimoto K, et al. Regeneration of dopaminergic neurons after 6-hydroxydopamine-induced lesion in planarian brain. J Neurochem. 2011;119(6):1217–31.
- Girstmair J, Schnegg R, Telford MJ, et al. Cellular dynamics during regeneration of the flatworm Monocelis sp. (Proseriata, Platyhelminthes). Evodevo. 2014;5:37.
- Scimone ML, Srivastava M, Bell GW, et al. A regulatory program for excretory system regeneration in planarians. Development. 2011;138(20):4387–98.
- Forsthoefel DJ, Park AE, Newmark PA. Stem cell-based growth, regeneration, and remodeling of the planarian intestine. Dev Biol. 2011;356(2):445–59.
- Reddien PW, Bermange AL, Murfitt KJ, et al. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev Cell. 2005;8(5):635–49.
- Eisenhoffer GT, Kang H, Sánchez Alvarado A. Molecular analysis of stem cells and their descendants during cell turnover and regeneration in the planarian Schmidtea mediterranea. Cell Stem Cell. 2008;3(3):327–39.
- Fernández-Taboada E, Rodríguez-Esteban G, Saló E, et al. A proteomics approach to decipher the molecular nature of planarian stem cells. BMC Genomics. 2011;12:133.
- Solana J, Kao D, Mihaylova Y, et al. Defining the molecular profile of planarian pluripotent stem cells using a combinatorial RNAseq, RNA interference and irradiation approach. Genome Biol. 2012;13(3):R19.
- Rodríguez-Esteban G, González-Sastre A, Rojo-Laguna JI, et al. Digital gene expression approach over multiple RNA-Seq data sets to detect neoblast transcriptional changes in Schmidtea mediterranea. BMC Genomics. 2015;16:361.
- Younossi-Hartenstein A, Ehlers U, Hartenstein V. Embryonic development of the nervous system of the rhabdocoel flatworm Mesostoma lingua (Abilgaard, 1789). J Comp Neurol. 2000;416(4):461–74.
- Younossi-Hartenstein A, Hartenstein V. Comparative approach to developmental analysis: the case of the dalyellid flatworm, Gieysztoria superba. Int J Dev Biol. 2000a;44(5):499–506.
- Younossi-Hartenstein A, Hartenstein V. The embryonic development of the polyclad flatworm Imogine mcgrathi. Dev Genes Evol. 2000b;210(8-9):383–98.
- Younossi-Hartenstein A, Jones M, Hartenstein V. Embryonic development of the nervous system of the temnocephalid flatworm Craspedella pedum. J Comp Neurol. 2001;434(1):56–68.
- Ramachandra NB, Gates RD, Ladurner P, et al. Embryonic development in the primitive bilaterian Neochildia fusca: normal morphogenesis and isolation of POU genes Brn-1 and Brn-3. Dev Genes Evol. 2002;212(2):55–69.
- Morris J, Nallur R, Ladurner P, et al. The embryonic development of the flatworm Macrostomum sp. Dev Genes Evol. 2004;214(5):220–39.
- Martín-Durán JM, Egger B. Developmental diversity in free-living flatworms. Evodevo. 2012;3:7.
- Morris J, Cardona A, De Miguel-Bonet Mdel M, Hartenstein V. Neurobiology of the basal platyhelminth Macrostomum lignano: map and digital 3D model of the juvenile brain neuropile. Dev Genes Evol. 2007;217(8):569–84.
- Rieger R, Salvenmoser W, Legniti A, et al. Phalloidin-rhodamine preparations of Macrostomum hystricinum marinum (Platyhelminthes): morphology and postembryonic development of the musculature. Zoomorphology. 1994;114:133–47.
- Baguna J, Romero R. Quantiative analysis of cell types during growth, degrowth and regeneration in the planarians Dugesia mediterranea and Dugesia tigrina. Hydrobiologia. 1981;84:181–94.
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23(6):251–8.
- Thompson CK, Brenowitz EA. Neurogenesis in an adult avian song nucleus is reduced by decreasing caspase-mediated apoptosis. J Neurosci. 2009;29:4586–91.
- González-Estévez C, Saló E. Autophagy and apoptosis in planarians. Apoptosis. 2010;15(3):279–92.
- Miller CM, Newmark PA. An insulin-like peptide regulates size and adult stem cells in planarians. Int J Dev Biol. 2012;56(1-3):75–82.
- Takeda H, Nishimura K, Agata K. Planarians maintain a constant ratio of different cell types during changes in body size by using the stem cell system. Zoolog Sci. 2009;26(12):805–13.
- Hill EM, Petersen CP. Wnt/Notum spatial feedback inhibition controls neoblast differentiation to regulate reversible growth of the planarian brain. Development. 2015;142(24):4217–29.
- Morris J, Ladurner P, Rieger R, et al. The Macrostomum lignano EST database as a molecular resource for studying platyhelminth development and phylogeny. Dev Genes Evol. 2006;216(11):695–707.
- Wasik K, Gurtowski J, Zhou X, et al. Genome and transcriptome of the regeneration-competent flatworm, Macrostomum lignano. Proc Natl Acad Sci U S A. 2015;112(40):12462–7.
- Grudniewska M, Mouton S, Simanov D, et al. Transcriptional signatures of somatic neoblasts and germline cells in Macrostomum lignano. Elife. 2016;5:e20607.

