Abstract
Rice is a very important cereal crop almost all over the world, but in recent years, its production and productivity have been hindered by several limiting factors. Fungi, bacteria, viruses, insects, and nematodes have caused problems in rice crops all over the world. This review looks at the data on major diseases and pests of rice in Ethiopia and makes a few suggestions for future research work. Brown spot, Downy mildew, Kernel smut, Leaf blast, Node and Neck blast, Panicle blast, Sheath blight, Sheath brown rot, Sheath rot, Rice Yellow Mottle Virus, Bacterial leaf strike, and Nematodes are just some of the diseases affecting rice in Ethiopia, and their prevalence ranges from 7% in sheath blight in the Amhara region to 100% in leaf and panicle blast in the Benishangul-Gumuz region and from the Southern Nations, Nationalities’, and Peoples’ Regional State (SNNPRS). Termites and rice stem borers are, moreover, the most common rice pests described in Ethiopia. Even though there is a rich source of literature on the management options of rice diseases across the world using fungicides, resistance varieties, germplasms, botanicals, and bioagents, only a few management practices, such as the use of fungicides, resistant varieties, and germplasms, have been studied in Ethiopia, but there is not much data available. There needs to be more research on the occurrence, distribution, current status, and yield losses of these diseases and pests, and more investigation into management options such as biological control, botanicals, and cultural practices.
REVIEWING EDITOR:
1. Introduction
Rice is categorized under the grass family Poaceae. It’s a major source of nutrition around the world, especially in African countries like Ethiopia (African Growth & Technology Foundation, Citation2013; Liu et al., Citation2013). Growing food demand is really tough for African countries, and it’s even more important since it plays a crucial role in providing security and self-sustenance for African and some Asian countries that have a lot of people who don’t have enough to eat (FAO, Citation2012). Roughly half of the world’s population relies on rice as a staple in their diet, and it’s popular in hotter, densely populated areas. Many Asians get the majority of their calories from rice, which is an important ingredient in most of their dishes (FAO, Citation2004).
Rice is essential for food security in Africa, especially for sub-Saharan countries struggling with poverty. It’s more important than other agricultural crops (Salih et al., Citation2013). The intake of rice is going up at a faster rate than any other major grain in this region due to the quick population growth and the rising urbanization in developing countries (Seck et al., Citation2013). On top of that, rice is getting more popular in Africa since it’s easy to make, tastes great, and can be used to create all sorts of dishes (Belayneh & Tekle, Citation2017).
Although rice isn’t as popular in Ethiopia as Teff is, it still has a huge economic impact as a food, income, fodder, and even a way to get foreign currency (Halos-Kim, Citation2015; Teshome & Dawit, Citation2011). Straw can also be used for animal feed and to build roofs (Wolie & Admassu, Citation2016). Now, most of the rice produced is used for food, and what’s left is sold unaltered on the local market. Before, producers cooked traditional dishes like ‘fermented bread’ and ‘dough’ mixed with sorghum or just on its own,’shorba’, ‘enjera’, and ‘kinche’ (Tilahun, Citation2019). They also use it to make their own alcoholic beverages like ‘Tella’ and ‘Areka’ (Mesfin & Zemedu, Citation2015; MoARD, Citation2010). It’s a great way for farmers to make use of their resources, like swampy and waterlogged areas. Basically, around 85% of young workers have jobs thanks to this, and it makes up 90% of foreign exchange and over 45% of the nation’s Gross Domestic Product (GDP) (Belayneh & Tekle, Citation2017).
Ethiopian farmers have seen a significant improvement in their rice production over the past few decades, with an average of three tons per hectare (Alemu et al., Citation2018; Tadesse & Tadesse, Citation2019). This is a huge jump from the 23 tons they were producing before, and they now produce a total of 353,998 tons of rice (Belayneh & Tekle, Citation2017).
The rice crop is really important to the economy, but there are a lot of things holding back production and productivity (Abera, Citation2017; Ou, Citation1985). Fungi, bacteria, viruses, insects, and nematodes have caused problems in rice crops all over the world. Grass-like weeds are another issue that can reduce rice production (Eucord, Citation2012). In addition, the extreme weather and high salt levels are causing a lot of problems for rice crops (Ansari et al., Citation2015; Mahmood-Ur-Rahman et al., Citation2015). Common fungal issues that affect rice are sheath blight, leaf scale, grain rot, brown spot, and blast from bottom to top (John & Fielding, Citation2014). Not much research has been done in this area, and the existing reports about diseases of this crop are not organized. This paper reviews and brings together the current information on fungal diseases and other pests of rice in Ethiopia and ways to manage them. For this purpose, articles and information from other sources were collected, reviewed, and compiled.
1.1. Rice production trend in Ethiopia and in the rest of the world
About 90% of the world’s rice is fundamentally cultivated by China, India, Japan, Korea, Southeast Asia, and the adjacent Pacific islands (Poehlman, Citation2013). China is the top producer of rice, with 206.5 million tons, followed by India (157.2 million tons), Indonesia (70.8 million tons), Bangladesh (52.3 million tons), Vietnam (45.0 million tons), Thailand (32.6 million tons), and Myanmar (26.4 million tons) (FAOSTAT, Citation2017). Outside of Asia, Brazil and the U.S. deliver the biggest sum; however, their combined production is <5% of the overall world rice production. In 2008/2009, lands utilized for rice cultivation expanded from 157.8 million hectares to 161.1 million hectares by the year 2016/2017 (FAO, Citation2018). According to FAO (Citation2015), 741.3 million tons of paddy rice were collected from 164 million hectares within the world in 2014, with China and India accounting for roughly 50%. Over 75% of the 54 African nations and their regions are also growing rice by more than 800 million farmers, and Africa produces around 26.4 million tons of rice for their nourishment and employment (Africa Rice Centre, Citation2009; FAO, Citation2018). In sub-Saharan Africa (SSA), crop productivity is critical to poverty reduction and food security issues. It has the same importance as other grains grown in the region (Balasubramanian et al., Citation2007; World Bank, Citation2008). Rice cultivation in Sub-Saharan Africa has expanded from 18.4 million metric tons (Mt) in 2010 to 46.8 million metric tons (Mt) by 2020, and this increase may be a result of better imputed supply and innovation enhancements (African Rice Center, Citation2011). Becker and Johnson (Citation2001) proved that nearly 90% of rice in Asia is grown under paddy field conditions; in contrast, 60% of rice in SSA is grown in upland ecosystems.
Rice can be grown in a variety of agroecologies and elevations. It can be grown from sea level to 3000 m above sea level and requires a climate of sufficient rainfall, sustained sunshine, heat, and humidity (Belayneh & Tekle, Citation2017). As Dilnesaw et al. (Citation2019) report, highland rain-fed rice can be grown in Ethiopia at altitudes ranging from 1000 to 2000 m.
Rice farming in Ethiopia is assumed to have started in 1957 around Metahara, taking after the adjacent areas of the Awash River. From 1968 to 1988, its agronomy was expanded to Fogera, Melkaworer, Debrezeit, and Arbaminch by the government and non-governmental organizations (Traoré et al., Citation2006). Rice production and utilization in Ethiopia may be a modern phenomenon (Altieri, Citation2018; Traoré et al., Citation2006), but there is a developing tendency to cultivate the development and generation of rice.
According to Sendeku (Citation2005), the Amhara region alone is estimated to have 201,955 ha in North Shewa, 166,500 ha in the Tana Basin, 137,326 ha in South Wollo, 65,476 ha in Armachiho-Metema, 29,050 ha in Quara-Metema, 18,694 ha in Dangila and Jawi, 10,500 ha in Bichena, and 1,580,499 ha elsewhere, which are considered suitable for rice cultivation.
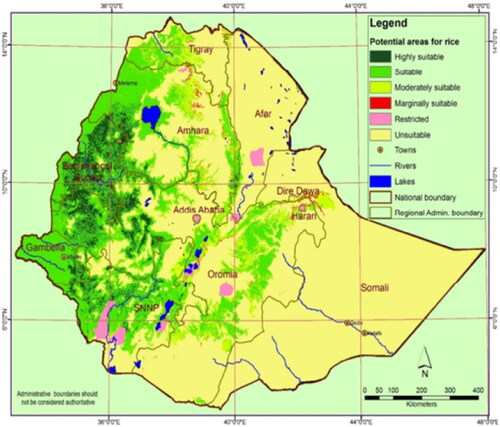
Several suitable areas for rice farming have already been identified and well-documented in various regions of the country () (MoARD, Citation2010). The most important rice-producing areas in Ethiopia are the Fogera plains adjacent to Lake Tana and the provinces of Gambella and Benishangul-Gumuz (Abera, Citation2017; Martinelli et al., Citation2015). Ethiopia is estimated to have 30 million hectares of land suitable for rice cultivation (Negussie & Alemu, Citation2011; Wolie & Admassu, Citation2016).
Figure 1. Agro-ecological suitability map for rain-fed rice production of Ethiopia (MoA, Citation2010; MoARD, Citation2010).
The total planted area nationwide increased from 57,575.72 ha in 2019/20 to 85,288.87 ha in the 2020/2021 growing season, an increase of 48.13% year-on-year, but there are significant regional differences. Thus, production increased from 1,706,301.01 quintals in 2019/2020 to 2,682,235.14 quintals in 2020/2021, up again by 6.10%, while productivity increased from 29.64 quintals in 2019/2020 to 31.45 quintals per hectare (Akasa, Citation2023). This may be due to the increased use of approved breeds, advanced machinery, and best management strategies (Dessie et al., Citation2018). With the introduction and licensing of rice varieties in the country, production is improving from time to time. To date, about 35 cultivars have been registered in Ethiopia, of which 21 highland and eight lowland cultivars are both NERICA and sativa types (Dessie, Citation2020).
1.2. Rice production constraints in Ethiopia
The basic troubles confronting rice agriculture all over the world are biotic and abiotic limitations. Biotic variables like fungal, bacterial, and viral diseases have been reported. The foremost commonly occurring rice diseases and insect pests incorporate rice yellow mottle infection, rice stem borer, rice leaf hopper, stalked eyed flies, rice mealy bugs, rice weevils, rice blast, leaf scaled, grain rot, brown spot, sheath blight, brown spot, bacterial blight, and others (Jamal-U-Ddin et al., Citation2012). Bacterial leaf strike, bacterial panicle blight, brown spot, sheath blight, leaf blast, panicle blast, rice yellow mottle infection (RYMV), and sheath spot on rice crops were distinguished as imperative diseases of rice in Ethiopia (Bewket, Citation2018).
In addition, rice cultivation often faces various biological factors, including pests such as aphids, cicadas, grasshoppers, jacids, mallards, pink stem borers, stem borers, and yellow stem borers, which adversely affect yield per unit area. Termites and rice stem borers are common rice pests in Ethiopia (Bewket, Citation2018). According to Hagos (Citation2015) in the Tselemet region of Ethiopia, ‘termites’ and ‘rodents’ are pests of rice in the area, causing 21.3 and 31.3% of damage, respectively. Rodents, mainly squirrels and rats, are considered to be the major obstacles resulting in significant yield and weight loss. Similarly, some farmers have had termite problems in their fields, especially during periods of moisture stress. ‘Disease incidence’ was also mentioned by a rare informant (15.3%). According to Hagos and Zemedu (Citation2015) around 47% of the agriculturalists responded that pests and insects are the major biotic limitations of rice production. Similarly, 53% of the sources answered that disease may be a primary deterrent to rice growth. Furthermore, they also reported that soil fertility, flooding, insect pests, and diseases are some of the challenges confronting agriculturalists who are producing rice within the Fogera area of Ethiopia. The emergence of disease is a new phenomenon that requires the attention of researchers and experts in the field. It is also proven that insect pests and grass weeds are one of the limiting factors affecting rice production in Ethiopia (Eucord, Citation2012).
1.3. Losses and impacts of rice diseases in Ethiopia and some other countries
Due to many restrictions, rice production is gradually declining. It follows global wheat production, with 90% of Asians and about 50% of the world’s population relying on rice as a food source (Fahad et al., Citation2019). Disease is the main limiting factor, causing 100% yield loss, along with other limiting factors. Among the major diseases, rice blast is the most devastating, causing annihilation in seasons favorable for disease outbreaks (Bewket, Citation2018). Similarly, in Bangladesh, Khatun et al. (Citation2021), reported up to 98% yield loss by rice blast; Hossain et al. (Citation2017) also showed a 65.4 and 56.9% loss due to blast. Similarly, Simon (Citation2016) observed a yield loss of 80–100% in South Africa because of a blast in rice. Moreover, the International Rice Research Institute (Citation2018) reported a yield loss of up to 100% due to the rice blast. Furthermore, Malicdem and Fernandez (Citation2015) noticed that a 50–85% yield loss has been seen in the Philippines due to the rice blast. Malicdem and Fernandez (Citation2015) also proved that yield losses up to 100% were due to rice blast disease. In Kenya, Kihoro et al. (Citation2013) reported 60–100% yield losses due to rice blasts, whereas Neupane and Bhusal (Citation2020) reported rice blasts cause an 80% yield loss in Nepal. Another major disease that farmers are facing is sheath blight, which is caused by Rhizoctonia solani Kuhn and causes around 25–50% yield losses (Malicdem & Fernandez, Citation2015).
1.4. Losses of rice due to other biological factors in Ethiopia and elsewhere
In practically every African country where rice is grown, stem borers are thought to be the main issue (Nwilene et al., Citation2013). Deuteragonista thoracica Smith has been shown to produce yield losses in densely infested areas that range from 60 to 100% (Heinrichs & Barrion, Citation2004). When treatment was not implemented, the combined field and post-harvest damage caused by vertebrate pests resulted in a loss of almost 40% of the possible crop yield (Funmilayo & Akande, Citation1997). Befikadu (Citation2018) indicated that 12% of post-harvest losses in rice are caused by rodents, molds, and weevils if the seeds are not sufficiently dried. In addition, an average 2.4% loss was recorded due to poor storage. Birds are another cause of post-harvest losses (Mustofa & Gondar, Citation2017). Rats, weevils, and other home pets are also responsible for pre-, during-, and post-harvest rice losses. Moreover, Yalew et al. (Citation2021) also reported that improper weed management reduces yield by 90%. Weeds will grow if proper weed management is not carried out. The largest loss in rice seed yield was caused by weed pests (37.02%), which were followed by insect pests (27.3%) and disease pests (15.6%) (Mondal et al., Citation2017).
1.5. Occurrence and distribution of rice diseases in Ethiopia
Vulnerable hosts, aggressive infectors, and a favorable climate are the three epidemic factors responsible for disease outbreaks (John & Fielding, Citation2014). The disease can alter host functional activity. It can lead to a decrease in both the quality and quantity of crop production (Allen et al., Citation2017).
According to the results of Hagos and Zemedu (Citation2015), in the Fogera area of the Amhara region, about 53% of farmers said disease is the main obstacle to crop production, especially rice. This means that farmers who embrace technology adaptation can be confident that their seed quality will produce crops that are free from infection, or at least minimally affected by disease. Dessie (Citation2018) reported the following important rice diseases and pests in Ethiopia: brown spot [Cochliobolus miyabenus (Cochliobolus miyabenus (Ito & Kuribayashi) Drechs.ex Dastur)], leaf blight [Sarocladium oryzae (Sawada) W. Gams & D. Hawksworth)], leaf blight [Thanatephorus cucumber (A.B. Frank) Donk], rice blast [Pyricularia oryzae (Cavara)], rice flies (Diopsis thoracic Westwood), termites, and stem borers (Pyralidae), which are important pests in rice cultivation. In addition, Yalew et al. (Citation2021), in his report indicated that the following fungal pathogens: Fusarium spp.; Pythium spp. and Sarocladium spp. which are attacking and identified from rice in Ethiopia. He has also reported other rice diseases caused by bacteria like Xanthomonas spp., Pseudomonas spp., and nematodes like Hoplolaimus spp., Tylenchorhynchus spp., and Helicotylenchus spp. More recently, Gudisa and Tesfaye (Citation2021) also reported the following rice fungal diseases with the indicated mean prevalence of sheath rot (52.79%), sheath brown rot (86.11%), panicle blast (100%), brown spot (100%), and blast (100%) (). In Ethiopia, a survey of the major rice-growing areas of the country found the following rice diseases: brown spot, downy mildew, kernel smut, leaf blast, node and neck blast, panicle blast, sheath blast, sheath brown rot, and sheath rot (Asfaha et al., Citation2015; Wubneh & Bayu, Citation2016; Zeleke et al., Citation2019). The overall means of reported disease’s prevalence, incidence, and severity in all surveyed areas of Ethiopia showed that the highest prevalence (100%) was observed in Southern Nations, Nationalities, and Peoples Region (SNNPRS) by Leaf blast () (Asfaha et al., Citation2015); Panicle blast and leaf blast (100%) at heading followed by 80% by Leaf blast at vegetative growth stage in Benishangul-Gumuz region (, ) Wubneh and Bayu (Citation2016), sheath rot (69%), in the Amhara region () (Zeleke et al., Citation2019), sheath blight (62%), at the heading in Benishangul-Gumuz, sheath brown rot (60%), and sheath blight (56.75%) at the vegetative growth stage in Benishangul-Gumuz, respectively (, ) (Wubneh & Bayu, Citation2016).
Figure 2. Different stages of rice stem borer identified from rice in Ethiopia (Bewket, Citation2018).
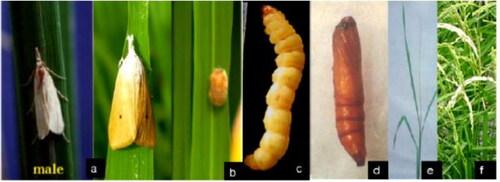
Figure 3. Distribution of rice diseases in Pawi and Jawi districts during 2020 main cropping season (Gudisa, Citation2020).
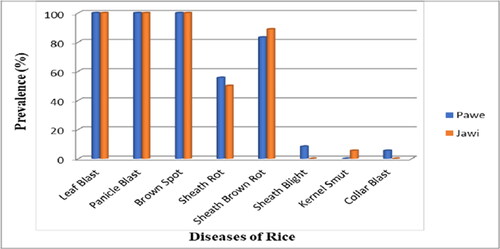
Table 1. The overall disease prevalence, incidence, and severity of rice diseases in different administrative districts of Ethiopia.
The overall trend of incidence observed in the surveyed areas was as follows: The highest incidence recorded was 96% during physiological maturity and 75% at seedling by sheath blast, followed by brown spot (74%) and sheath blight (69.25%) at vegetative growth stage both in Benishangul-Gumuz region (, ) (Wubneh & Bayu, Citation2016); leaf blast (65.68%) in SNNPRS () (Asfaha et al., Citation2015) and lastly, sheath rot (35%) in Amhara region () (Zeleke et al., Citation2019). Similarly, the maximum rate of disease severity was recorded by Leaf blast (47.15%) in SNNPRS () (Asfaha et al., Citation2015) followed by Sheath rot (28%), Sheath brown rot (18%), and Panicle blast (16%) all in Amhara region () (Zeleke et al., Citation2019) and lastly by Panicle blast (10%) and Leaf blast (7%) at maturity and Leaf blast (5%) at seedling stage in Benishangul-Gumuz region (, ) (Wubneh & Bayu, Citation2016) ().
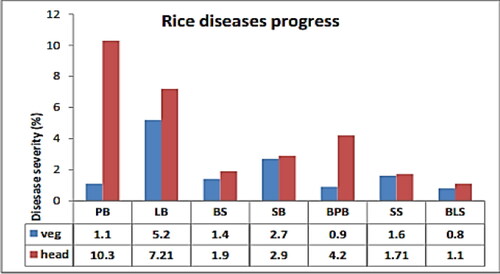
Figure 4. The disease severity level in vegetative and heading growth stages of rice at Pawe district (Wubneh & Bayu, Citation2016). Where: veg: vegetative growth stage; head: head setting growth stage; PB: panicle blast; LB: leaf blast; BS: brown spot; SB: sheath blight; BPB: bacterial panicle blight; SS: sheath rot; BLS: bacterial leaf strike.
The occurrence, dissemination, and extent of the detailed diseases escalated may be related to climate conditions reasonable for the diseases’ outbreak and may be related to the mode of spore dispersal of the respected pathogens and other related aggravating components polished within the country ().
Figure 5. Symptoms of sheath rot (a), sheath brown (b), and brown spot (c) disease in a rice field in Ethiopia. Source: Zeleke et al. (Citation2019).

At Pawe, leaf blast disease showed the highest prevalence (80.08%), incidence (75%), and severity (5.2%) at the seedling growth stage relative to the rest of the disease rates (, ) (Wubneh & Bayu, Citation2016). However, at physiological maturity, leaf blast scored the maximum disease severity of 7.21 percent next to panicle blast (10.3%) (, ) (Wubneh & Bayu, Citation2016). This could be due to the weather conditions in that area getting cold and humid due to heavy rain. As a result, asexual spores (conidium) are going to be produced and start to infect new plants, specifically by sticking themselves to foliage and panicles (African Rice Center, Citation2011). In addition, when the host becomes old, it loses its immunity on the other side, and the pathogen becomes aggressive to infect new plants, causing severe symptom development (Wubneh & Bayu, Citation2016) ().
Figure 6. Symptoms of panicle blast (A), neck blast (B), node blast (C), and leaf blast (D) diseases in rice field in Ethiopia. Source: Zeleke et al. (Citation2019).

Similarly, in a survey on the occurrence and spread of sheath blight disease of rice in major rice-growing areas of the Chhattisgarh region in India, the occurrence of the disease was reported (Parshuram et al., Citation2017). Moreover, Mwakasege (Citation2015) in the Morogoro area of Tanzania reported the following rice diseases, which consist of brown spots, leaf blasts, and sheath blight. In India, a survey on sheath blight disease of rice in the Tamil Nadu area by Neha et al. (Citation2017) indicated that the maximum PDI of 36.5% was recorded in Naduthittu, followed by Vadakkumangudi (32.4%), Vallampadugai (26.5%), and Muttlur (21.4%), and the least level of incidence was noticed in Ramapuram (10.5%). In Bangladesh, Khatun et al. (Citation2021) also reported 22 rice diseases that are caused by the funguses. A survey in Burkina Faso by Valentin et al. (Citation2015) indicated yellow mottle disease (YMVD), bacterial leaf streak (BLS), leaf blast, and brown spot of rice. Adego et al. (Citation2017) reported a 54–96% incidence of rice yellow mottle disease. In Nigeria, Odedara et al. (Citation2016) reported RYMV with a 7.7% incidence. Mdangi et al. (Citation2019) also reported more cricket, stalked eyed fly, army warm, cutworm, rice grass hopper, and rice leaf folder. Mwakasege (Citation2015) reported rice yellow mottle virus (RYMV), rice sheath blight (RSB), rice blast (RB), and rice bacterial leaf blight (RBLB) diseases with different severity levels in Tanzania. In Uganada, Stella et al. (Citation2020) reported rice yellow mottle virus (RYMV), leaf blast (LB), brown spot (BS), sheath rot (ShR), panicle blast (PB), and bacterial leaf blight (BLB) diseases in rice crops. Amayo et al. (Citation2020) also reported 72.61% rice blast prevalence. According to Hossain et al. (Citation2017), blast, bacterial leaf blight, sheath blight, and more recently, false smut are the heavy epidemic rice diseases in Bangladesh ().
Figure 7. Symptoms of sheath blight (a) and rice yellow motile viruses (b) diseases in rice filed. Source: Zeleke et al. (Citation2019).

1.6. Disease diagnosis and identification techniques
Plant diseases have an impact on crop productivity and production, and they are one of the main limiting factors that interfere with crops’ normal physiological growth and yield (Abade et al., Citation2021). Disease assessment and intervention strategies should be coordinated with disease outbreaks through routine crop monitoring, followed by precise disease diagnosis and identification activities by plant pathologists, to minimize yield loss and maintain crop productivity, and early action should be taken (Altieri, Citation2018). For farmers to stop disease outbreaks and select effective management measures, early disease detection is essential. Unexpected weather circumstances, for instance, can have detrimental effects on crops, altering both the host’s vulnerability and the pathogen’s aggressiveness. In certain cases, this can result in significant disease outbreaks, which can lower crop yields and cost farmers money (Savary et al., Citation2012).
The majority of fungal and bacterial detection methods have historically relied on morphological techniques based on culture (Capote et al., Citation2012). This means that the sample must be cultured on a certain medium, and the possible pathogens must be allowed to develop under controlled conditions. This might take days or even weeks, and the process can be particularly challenging in the case of biotrophic pathogens (Goud & Termorshuizen, Citation2003). Additionally, morphological pathogen identification requires considerable taxonomic expertise (Donoso & Valenzuela, Citation2018). One of the biggest challenges in crop production is accurately identifying plant diseases affecting crops (Mohanty et al., Citation2016). This is because most identification operations are traditionally done visually with the naked eye or with a simple magnifying lens. To make this tedious and time-consuming operation less of a burden, several molecular approaches have been employed to identify plant diseases more quickly, accurately, and sensitively. The most widely used of these are methods based on polymerase chain reaction (PCR), such as multiplex PCR, isothermal PCR, nested PCR, reverse transcription (RT)-PCR, real-time PCR, PCR, and PCRELISA (Capote et al., Citation2012). Immunological techniques are also widely used; however, they are typically more expensive than PCR-based technologies, and the shell life of kits based on immunological techniques is more constrained. Examples of these techniques include multiplex immunodetection, immunofluorescence, and enzyme-linked immunosorbent assay (ELISA) (plate-trapped antigen-ELISA and double antibody sandwich-ELISA) (Martinelli et al., Citation2015). Advanced techniques of serological and molecular testing are not routinely used, especially in developing countries like Africa (Barbedo, Citation2016). DNA-based and serological methods have transformed traditional disease detection methods that were based on symptom visualization (Martinelli et al., Citation2015).
Recently, significant advancements have been made in automatic systems for identifying and diagnosing rice disease and insect lesions. To categorize a picture into one of ten prevalent rice diseases, Lu et al. (Citation2017) created a modest, manually constructed CNN network model. However, this model was unable to identify the precise position and number of rice diseases and pests. To detect rice diseases, Zhou et al. (Citation2019) proposed combining faster R-CNN with FCM-Km. This technique addressed several issues with rice disease images, including noise, fuzzy image edges, complicated background interference, and low detection accuracy. To identify rice illnesses and pests in videos, Li et al. (Citation2020) developed a deep learning and bespoke backbone video detection architecture. To precisely find the target place for producing candidate regions, Bari et al. (Citation2021) applied the RPN structure to the Faster R-CNN algorithm. This had an excellent detection effect on three diseases on rice leaves of a single plant. A deep neural network model was suggested by Daniya and Vigneshwari (Citation2021) to identify rice diseases. Initially, the photos of rice diseases are cleaned up of their background noise. After that, segmentation will be done using the SegNet network. For detection, statistical, CNN, and texture characteristics will be retrieved. However, due to the following primary issues, these techniques have not been extensively employed in paddy fields for the detection and identification of diseases and pests: Rather than on paddy fields, some photos are taken in laboratories. In actuality, in intricate agricultural settings, the appearance of disease-related and pest-related lesions differs. The lab-derived models were unable to provide a strong identifying effect in fields. In certain investigations, the disease and pest lesions were only found on a single leaf. It is not possible to use these results to directly identify several lesions from multiple rice plants in a single image. The majority of image-capturing devices required manual operation. We created an intelligent monitoring system for spotting and identifying lesions of disease and pest on rice canopy to address the aforementioned issues and enhance the monitoring intelligence of pests and diseases on rice canopy in paddy fields.
1.7. Management options for rice disease
Diseases in rice can be controlled by sowing resistant cultivars and applying cultural, biological, botanical, and chemical control strategies individually or by combining one or more methods at a time. All of the above disease management strategies have their own strengths and weaknesses. Chemical control and the use of resistant cultivars are commonly used measures worldwide to control plant diseases (Kumar et al., Citation2013).
Common cropping methods used to treat crop diseases include mulching, cover crops, maintaining plant density, adjusting planting dates, crop rotation, cover crops, zoning, and selection of high-quality seeds, soil solarization, warm water treatments, and appropriate amounts of soil amendments. Implement fertilizer and water management separately or in combination. In developing countries, such control practices may be the only method available to control plant diseases in some crops (Khoury & Makkouk, Citation2010).
Cultural management methods not only help promote healthy plant growth but also directly reduce the inoculum potential (pruning, transplanting, crop rotation, tillage, etc.) and reduce hostile soil microbial biology. It is also effective in increasing the physical activity of the soil (Khoury & Makkouk, Citation2010).
For decades, fungicides have played an important role in disease control. In the 1960s, systemic fungicides replaced older, higher-quality, more specific non-systemic insecticides in disease control. After some time, triazole fungicides captured the largest share of the pesticide market (Oliver & Hewitt, Citation2014). However, older products, such as mancozeb and chlorothalonil, as well as copper and sulfur-based products, are still used by crop growers, especially in low-income countries, due to their low cost. More recently, new classes of fungicides have been formulated, including anilinopyrimidines, phenoxyquinolines, oxazolidinediones, spiroketalamines, phenylpyrroles, strobilurins, and more effective systemic resistance activators (Kumar et al., Citation2017).
The use of immune, resistant, or tolerant cultivars is the most cost-effective and environmentally friendly way to treat rice diseases, especially devastating diseases like blasts (Khan et al., Citation2002; Haq et al., Citation2002). Rice cultivars resistant to major rice diseases, such as late blight (Sere et al., Citation2004), bacterial late blight (Mew et al., Citation1992), rice tungro (Azzam & Chancellor, Citation2002), and brown spot (Waller, Citation1987) are widespread. However, resistance is easily disrupted with the emergence of new or more virulent strains or types of pathogens (Ghazanfar et al., Citation2009). Therefore, there is a need to regularly accelerate breeding programs to develop rice cultivars resistant to blasts and other serious, economically important diseases (Kumar et al., Citation2013).
Biological control of plant pathogens has been recognized as a potential control strategy in recent years, as chemical control poses environmental problems that can lead to serious ecological problems. Organic management methods are an effective and environmentally friendly approach to sustainable agricultural practices (Nega, Citation2014). Fungi of the genus Trichoderma and bacteria, such as Pseudomonas and Bacillus subtilis Ehrenberg, are the most promising biocontrol agents against a single plant pathogen under different ecological conditions (Bashar et al., Citation1970). Combating plant diseases with biological control agents inhibits pathogen growth through a variety of mechanisms of action (Gurung, Citation2016).
Plants can produce a range of aromatic secondary compounds, such as phenols, phenolic acids, quinones, flavones, flavonoids, flavonols, tannins, and coumarins. These groups of secondary metabolites exhibit antagonism and serve as a plant defense strategy against plant pathogens (Evans & Cowan, Citation2016).
The concept of integrated disease management (IDM) for combating rice diseases is becoming more popular among farmers and researchers, incorporating different control methods and practices to control the disease. IDM minimizes the use of pesticides and offers options for other control methods, such as host plant resistance, cultural practices, and biological control (Khoury & Makkouk, Citation2010).
2. Management works done to rice diseases in Ethiopia
2.1. Fungicide control
Among the many approaches to controlling plant diseases is chemical control, which is widely practiced in many countries, especially for precise and rapid treatment (Mariappan et al., Citation1995). The composition, amount, timing, and method of application of fungicides depend on the prevalence and intensity of the disease (Bewket, Citation2018).
A significant number of works have been done in different corners of the world to manage rice diseases using fungicides. Just to mention some of them: In India, Varaprasada and Anil (Citation2018) reported integrated management of rice blasts by seed treatment with carbendazim followed by spraying of tricyclazole. According to Singh et al. (Citation2015), among the fungicides evaluated, fenoxanil 5% + isoprothiolane 30% was found to be the most promising in reducing the incidence of neck blast, followed by metominostrobin. In Nepal, Magar et al. (Citation2015) studied the effect of different fungicides, viz., Tricyclazole 22% + Hexaconazole 3% SC, Streptomycin 5% + Thiophanate Methyl 50% WP, Prochloraz 25% EC, Kasugamycin 2% WP, Hexaconazole 4% + Zineb 68% WP, and Udaan (Hexaconazole 3% SC) against rice blast. Among them, Tricyclazole 22% + Hexaconazole 3% SC was found to be the most effective on leaf and neck blast severity, followed by Prochloraz 25% EC and Udaan (Hexaconazole 3% SC). Jamal-U-Ddin et al. (Citation2012), reported that mancozeb and garlic completely inhibited the mycelial growth of M. oryzae, and maximum mycelial inhibition was provided by Pseudomonas lilacinu. In Nepal, Rachana et al. (Citation2021) studied different fungicides against rice blast. From the results, Tricyclazole 75% WP (TRIP) was more effective among other fungicides, followed by Biomycin. Similarly, in Nepal, Magar et al. (Citation2015) indicated that tricyclazole (22%) and hexaconazole (3% SC) are the most effective on leaf and neck rice blast severity. Neupane and Bhusal (Citation2020) reported the most usual approaches for the management of rice blast disease are management in fertilizers and irrigations, plantation of resistant varieties, and application of fungicides. Prasad et al. (Citation2020) reported that Hexaconaxoke 5% EC is highly effective in reducing the severity of the sheath blight disease in rice. Parshuram et al. (Citation2017) indicated that the combined fungicide TAQAT 75% WP (Captan 70% + Hexaconazole 5%) was found effective against sheath blight, sheet rot, and stem rot diseases of rice. Prasad et al. (Citation2020) studied the management of sheath blight disease of rice by combined application of Propiconazole 13% + Difenconazole 13.9% SC (Tespa), Propiconazole 25% EC (Tilt), Tebuconazole 50% + Trifloxystrobin 25% WG (Nativo), Azoxystrobin 23% EC (Amistar), Validamycin 5% L (Vamcin), Captan 70% + Hexaconazole, WB (Takat), Hexaconazole 5% EC (Contaf), and Carbendazin 50 WP (Bavestin), among all Hexaconaxoke 5% EC found highly effective in reducing the sheath blight disease severity. Out of the different fungicides screened against the sheath blight disease by Neha et al. (Citation2017), the new chemical ICF 310 completely inhibited the growth of Rhizoctonia solani Kuhn, causing the sheath blight disease of rice. In India, Parshuram et al. (Citation2017), also studied a new combined fungicide, TAQAT 75% WP (Captan 70% + Hexaconazole 5%), against rice sheath blight (ShB), sheath rot (ShR), and stem rot (StR) diseases. The combined fungicide TAQAT 75% WP (Captan 70% + Hexaconazole 5%) was found effective against the mentioned diseases.
When we look at the Ethiopian case, limited reports are available on the management of rice fungal diseases using fungicides. For example, Muluadam et al. (Citation2020) indicated that Proseed plus 63 WS (carboxin + thiram + imidacloprid); Joint 246 FS (imidacloprid + tebuconazole); and Imidalm T450 WS (imidacloprid 250 g/kg) fungicides were effective against rice sheath rot disease, respectively. They also found that seed treatments were better than foliar sprays. The effectiveness of these fungicides may be explained by targeting specific enzymes or proteins made by fungi, and they do not damage untargeted plant tissue. Thus, they can penetrate and move inside plant tissues, resulting in a cure and leading to maximum protection by moving towards infecting tissues (McGrath, Citation2016). Similarly, treating seeds with fungicides had a direct effect on the panicle length, since the pathogen mainly infects the uppermost flag leaf sheaths that enclose the emerging young panicle during the boot stage and causes an empty head. Moreover, pre-sowing treatments were effective in enhancing plant height since they inhibited panicle exertion (Yalew et al., Citation2021). Similarly, Horo and Gudisa (Citation2021) evaluated the effectiveness of seven chemical fungicides, namely Amistar Xtra 280 SC, Artea 330 EC, Contaf Max 350 SC, Fungozeb 80 WP, Matco, Rex® Deo, and Tilt 250 EC, for the control of rice blast disease under field conditions. The result revealed that all the test fungicides have considerably inhibited disease development. However, Contaf Max 350 SC had superior disease reduction by more than 80% and gave the maximum grain yield. Similar results on the role of fungicides in controlling different rice fungal diseases in different parts of the world are also reported by Bhandari et al. (Citation2017), Diaz-Soltero (Citation2022), Goswami et al. (Citation2012), Ilyas and Iftikhar (Citation1997), Kandhari (Citation2007), Kindo and Tiwari (Citation2015), Latif et al. (Citation2011), Magar et al. (Citation2015), Chauhan et al. (Citation2017), Prasad et al. (Citation2006, Citation2020), and so many other researchers.
2.2. Botanical and biological control
In India, Prasad et al. (Citation2020) reported that the bulb extract of garlic and the rhizome extract of ginger suppressed the mycelial growth of the sheath blight disease of rice, followed by neem leaf extract, bulb extract of onion, and tulsi leaf extract, respectively. Hashim et al. (Citation2018) also studied different plant extracts, oil cakes, and antagonistic organisms against Bipolaris oryzae Haan (Cochliobolus miyabeanus), the causal agent of brown spot disease in rice. The results of the in vitro studies indicated that two leaf extracts, Nerium oleander and Pithecolobium dulce, showed the maximum inhibition of mycelial growth and spore germination in B. oryzae. Among the four oil cake extracts tested, neem cake extract showed the highest inhibition percent for mycelial growth and spore germination of the pathogen. The author also reported that Trichoderma viride (Thakur and Norris) was significantly effective in inhibiting the mycelial growth and spore germination of the pathogen. In Nigeria, Agbowuro et al. (Citation2021) studied aqueous plant leaf extracts of five plants against rice blast disease (Magnaporthe oryzae Shiva) in vitro and in vivo. The result revealed that the apple of Sodom (Calotropis procera) is the most efficient in inhibiting the growth of the pathogen because it is low-cost and eco-friendly. In Tanzania, Hubert et al. (Citation2015) studied the effect of aqueous extracts of Aloe vera, Allium sativum, Annona muricata, Azadirachta indica, Bidens pilosa, Camellia sinensis, Chrysanthemum coccineum, processed Coffee arabica, Datura stramonium, Nicotiana tabacum, and Zingiber officinalis on the control of rice blast disease [Pyricularia grisea (Cooke.) Sacc.] in vitro and in vivo. The results showed that processed C. arabica had the highest inhibitory effect against P. grisea. According to Kumar et al. (Citation2021), the following extracts inhibited mycelial growth of Rhizoctonia solani: neem leaf extract (64.63%), onion bulb extract (63.52%), and tulsi leaf extract (61.52%). These extracts were found to be 10% concentrated. According to research by Harish et al. (Citation2008), post-infectional spraying of rice plants with neem cake extract, Nerium oleander L. leaf extract, and Trichoderma viride dramatically decreased the incidence of brown spots on rice by 66, 52, and 45%, respectively, in glasshouse tests. Hubert et al. (Citation2015) found that the highest inhibitory effects against Pyricularia grisea were observed in processed Coffea arabica L. at 10 and 25% (v/v), which were followed by an aqueous extract from Nicotiana tabacum L. at 10% concentration (80.35%), and finally extracts from 25% Aloe vera L. (79.45%) and 25% Cynomorium coccineum L. flower (78.83%). These extracts did not exhibit any phytotoxic effects on seed germination, shoot height, root length, dry weight, seedling growth, or seedling vigor index. The ethanolic leaf extracts of Datura metel and Jatropha curcas L. have been shown by Durgeshlal et al. (Citation2019) to have the strongest antifungal activity against isolated pathogens that cause sheath blight, with 98.611 ± 1.589 and 98.588 ± 1.589% mycelial inhibition, respectively, at 100% concentration. On the other hand, Jatropha curcas L and Ruellia tuberosa L had the strongest antifungal properties against rice blast, with respective values of 97.436 ± 0.555 and 97.115 ± 0.96%, respectively. Kala et al. (Citation2015) reported that rice bacterial leaf blight was significantly inhibited by the ethanol extract, hexane extract, and aqueous extract of Morinda coreia Buch.-Ham., Datura innoxia Mill, Ocimum tenuiflorum L., Calotropis gigantea L. Dryand, Vitex negundo L., and Pongamia pinnata L. Pierre, as well as the fruit extracts of Morinda coreia and Datura innoxia against the bacteria Xanthomonas oryzae pv. oryzae isolated from paddy fields ().
Figure 8. Few medicinal plants [Kulkual (Euphorbia abyssinica) J. Gmel] (a), Eret (Aloe sp.) (b), Nechbahirzaf (Eucalyptus globulus Labill.) (c), and Nechshinkuret (Allium sativum L.) (d) are used to treat different diseases, including rice, in Ethiopia. Source: Mekonnen et al. (Citation2022).
![Figure 8. Few medicinal plants [Kulkual (Euphorbia abyssinica) J. Gmel] (a), Eret (Aloe sp.) (b), Nechbahirzaf (Eucalyptus globulus Labill.) (c), and Nechshinkuret (Allium sativum L.) (d) are used to treat different diseases, including rice, in Ethiopia. Source: Mekonnen et al. (Citation2022).](/cms/asset/6479bfed-5119-4de2-8fe7-634cae933faa/oafa_a_2300558_f0008_c.jpg)
Rice blast and sheath blight can be effectively managed by trichoderma species (Yadav et al., Citation2018). Raju et al. (Citation2020) also reported that soil application of Pseudomonas fluorescens at 2 kg/ac was found to be most effective in managing sheath blight incidence and promoting growth. At the same time, he also discovered that aqueous neem leaf extract (5 ml per liter) was better at controlling the occurrence of stem borer. Hashim et al. (Citation2018) indicated the management of rice blast disease with Trichoderma asperellum, Bacillus subtilis, and the fungicide Linkimil 72 WP. Kumar et al. (Citation2021) reported the bulb extract of garlic and ginger rhizome extract of ginger suppressed the mycelial growth of sheath blight disease of rice at 10% concentration, followed by neem leaf extract, bulb extract of onion, and tulsi leaf extract. Jacqueline et al. (Citation2021) examined mycelial, crude, lyophilized, and mycelial mass extracts of Waitea circinata (Warcup & Talbot), a mycorrhizal fungus that is antagonistic to orchids and has the potential to be used as a biocontrol agent against rice diseases. The rice pathogens Cochliobolus miyabeanus (Ito and Kurib.) Drechsler ex Dastur, Monographella albescens (Thumen) Parkinson, Sivanesan and C. Booth, Sarocladium oryzae (Sawada) W. Gams & D. Hawksw, and Magnaporthe oryzae B.C. Couch were inhibited by the mycelial mass extract, whereas C. miyabeanus and M. albescens were inhibited by the crude and lyophilized extracts, respectively. It has been demonstrated that numerous kinds of diseases, parasitoids, and predators target rice insect pests. Before stem borers enter the stem of the rice plant, a variety of predatory animals prey on their eggs and small larvae. Ants and a dozen other predators feed on stem borer larvae, including coccinellid beetles like Micraspis crocea (Mulsant), Harmonia octomaculata (Fabricius), and carabid beetles like Ophionea spp. (Bewket, Citation2018). Compared to larval or pupal parasitoids, egg parasitoids are more significant, with up to 90% of eggs being parasitized during harvest. The following natural enemies of the rice stem borer, as described by Rahaman et al. (Citation2016), are also found in the rice field: the Earwig (Forficula auricularia L.), Carabid beetle [Ophonea indica (Thunberg)], Ladybird beetle (Coccinella septempunctata L.), Micraspis discolor (Fabricius), Spiders (Araneae spp.), Damselfly (Zygoptera spp.), and Dragonfly [Anisoptera Berthold (Heinrich)]. In the rice fields, the following was the rank order of the relative abundance of natural enemies: ladybird beetles (49.95%), spiders (17.82%), damselflies (6.26%), carabid beetles (5.81%), green mirid bugs (4.94%), dragonflies (2.30%), and earwigs (2.21%).
2.3. Progress research on evaluation of rice genotypes and varieties against rice diseases
Rice is considered a ‘millennial crop’ and is expected to contribute to ensuring domestic food security. Therefore, recently, rice was introduced to this country, and very little research has been done on this crop. Research activities conducted in-house focus on cultivar development, with little research activity on plant pest management (Tariku, Citation2011).
Using resistant cultivars to control plant diseases in general and rice in particular is a viable and environmentally friendly approach (Rijal & Devkota, Citation2020). So far, 20 improved varieties have been officially approved for mass production. These include the seven varieties of NERICA rice developed by African rice researchers, and the NERICA rice varieties have spread to various countries in Africa, leading to the green rice revolution (Tariku, Citation2011). The New Rice for African (NERICA) varieties combines the genes of African rice varieties with Asian rice varieties; it was developed by the African Rice Center (ARC) as an inter-specific hybridization of Oryza glaberrima and O. sativa. These cultivars combined the hardiness of the African rice with the productivity of the Asian rice to develop NERICA (Jones et al., Citation1997), which has provided a window of opportunity in Sub-Saharan Africa to reduce hunger due to their ecological suitability, disease resistance, and increasing yield (MoA, Citation2009).
In addition to rice cultivation, farmers are also focusing on breed development and have developed two varieties, one upland rice variety and one paddy rice variety, through conventional breeding. The two varieties selected by the farmers (‘Demwoze and Nechu Ruz’) are widely cultivated in the Fogera (rainfed) region of the Amhara region and the Guraferda region of the Southern Peoples, Nationalities, and Peoples Region and have mostly moderate resistance to the rice disease (Tariku, Citation2011).
A screening study of blast resistance in Ethiopian rice cultivars grown in different ecosystems was performed by Taddesse et al. (Citation2020) on landraces collected from the Amhara, SNNPR, Benshangul-Gumize, and Gambela regions. The majority of germplasm from Ethiopia revealed resistance to most blast isolates, but some showed susceptibility to some blast isolates. From the 57 accessions checked (32 improved and 25 landraces), the majority showed resistance or moderate resistance to all the blast isolates tested (Tadesse & Tadesse, Citation2019). In addition, Fetene et al. (Citation2020) investigated the host response based on their mean percent severity index (PSI) value according to the Sharma et al. (Citation2013) rating scale on sheath rot of Sarocladium oryzae, and the results of their work indicated that the tested genotypes had a different reaction to the disease. Among eighty germplasms, three genotypes were immune, twenty-seven were resistant, thirty-five were moderately resistant, thirteen were moderately susceptible, and two germplasms were susceptible. Moreover, Zeleke et al. (Citation2019) also observed the reactions of different rice varieties. The highest sheath rot severity (46%), incidence (43%), sheath brown rot severity (38%), and incidence (46%), diseases were recorded on the X-jigna cultivar than Gumara. Therefore, cultivars X-jigna and Gumera were susceptible to all identified diseases, and the NERICA-4 cultivar was identified as resistant to all diseases except brown spot, sheath brown rot, and sheath rot. Furthermore, Asfaha et al. (Citation2015) also observed in their rice disease field survey that NERICA cultivars had good resistance to rice blast disease relative to other varieties. More recently, a study conducted by Yalew et al. (Citation2021) on the screening of brown spot disease caused by Bipolaris oryzae-resistant lowland rice (Oryza sativa L.) genotypes showed that of the 49 lines screened, sixteen genotypes showed highly resistant reactions, and six of them, including susceptible checks, were highly susceptible. The result of a field experiment on the evaluation of Upland NERICA rice (Oryza sativa L.) varieties for grain yield and disease resistance by Seyoum et al. (Citation2011) revealed that NIERICA-4 and NIERICA-3 gave the highest grain yield and moderate disease resistance. He added that the reason why X-Jigna, a single rice cultivar, dominated lowland rice production in Ethiopia is because of its good disease reaction, especially panicle blast and brown spot, and its good performance in other agronomic parameters. But it is less resistant to the rice sheath rot disease.
Similarly, plenty of screenings of rice genotypes and varieties against rice diseases have been reported outside Ethiopia. For example, out of 143 scented rice genotypes screened by Singh et al. (Citation2015) against rice blast, 32 genotypes, namely HKR 06-42, HKR 07-2, HKR 07-9, HKR 07-14, HKR 07-23, HKR 07-24, HKR 07-41, HKR 08-15, HKR 08-17, HKR 08-23, HKR 08-25, HKR 08-26, HKR 08-35, HKR 08-40, HKR 08-51, HKR 09-3, HKR 09-7, HKR 09-8, HKR 09-10, HUBR 10-9, HUR 98, HUR-PB-7M, MAUB 192, MEPH 108, NDR 6110, Pusa 1509-3-3-9-5, RP 3392-132-8-3-3, RP 3392-179-45-18-8, SJR 80, SJR 81, UPR 3506-7-11, and UPR 3506-15-1-1 were found consistently resistant to leaf blast. Similarly, out of 123 non-scented inbred and hybrid genotypes screened by Singh and Sunder (Citation2015) on the same disease, 20, 91, and 12 genotypes were found to be resistant, intermediate, and susceptible. However, only nine genotypes, namely, HKR 05-10, HKR 05-22, HKR 07-95, HKR 07-239, HKR 08-12, HKR 08-17, HKR 08-71, HKR 08-110, and HKR 08-118, showed consistent resistance. Neupane and Bhusal (Citation2020) also reported that the use of resistant cultivars, such as Khumal-1, Khumal-2, Khumal-3, Radha-12, Chandannath-1, Chandannath-3, Sabitri, and Palung-2, for the management of rice blasts is a sustainable and eco-friendly approach.
Resistant species may contain resistance genes (R genes), and pathogens may contain avirulent genes that activate host defense responses. In addition, it may involve the synthesis of plant hormones that trigger the synthesis and activation of host or pathogen enzymes to effectively activate and defend pathogens. Other defense mechanisms, such as structural reactions and hypersensitivity reactions, may be involved in the defense process (Singh & Singh, Citation2018; Yalew et al., Citation2021). Similar results on the role of resistant varieties to control rice fungal diseases have been reported in other countries by Adorada et al. (Citation2013), Bhandari et al. (Citation2017), Fang et al. (Citation2017), Kalboush (Citation2019), Latif et al. (Citation2011), Lore et al. (Citation2015), Moldenhauer et al. (Citation1992), Sedeek and Elwahsh (Citation2015), Srinivasachary et al. (Citation2011), and Wang et al. (Citation2007, Citation2009).
An integrated management strategy for rice blast disease has also been suggested by Bewket (Citation2018) focusing on a multidimensional control approach including resistance cultivars, healthy seeds, fertilizer management, and cultural systems.
3. Conclusion
This review has confirmed severe outbreaks of rice disease and pests in the northwest, west, and south of Ethiopia, with a significant number of yield losses. Fungal diseases, such as sheath rot, sheath brown rot, and blat (head and leaf), are the most common diseases. And termites and rice stemborers are also common rice pests in Ethiopia. The prevalence, incidence, and severity of these diseases and pests varied from mild to severe depending on agroecological and environmental conditions. Fungicide control with Contaf Max, Tilt 250 EC, Proseed Plus 63 WS (carboxin + thiram + imidacloprid), Joint 246 FS (imidacloprid + tebuconazole), and ImidalmT450 WS (imidacloprid 250 mg/kg) was effective against rice disease and was shown to be valid. Seed dressing fungicides were superior to foliar fungicides in sheath rot rice disease. The immune genotype "scrid014-1-1-1" is a recently introduced high-yielding and resistant strain, and cultivars like Gumara and NERICA-4 show moderate resistance to rice disease infection. It proved to be a higher yield than the check of the local cultivar Wanzaye is well suited for the development of resistant cultivars, as this genotype does not produce a disease response. Taken together, the above studies suggest that further research in screening and breeding will lead to the release of more varieties with good yields and better disease resistance, and consequently, the country will need more rice to feed its growing population. It sends a big message that can increase production. This also applies to neighboring countries, as Ethiopia is rich in potential areas suitable for rice cultivation and agroecology.
4. Recommendations and future research prospects
As rice cultivation is a recent phenomenon in Ethiopia, little research has been done on the epidemiological and management aspects of rice diseases and pests. Therefore, more research needs to be done on the occurrence and distribution of the diseases and pests, as well as on the favorable weather conditions for pest development, including in areas that have not yet been studied. Furthermore, future efforts should be directed toward integrating multiple disease and pest management options, including developing resistant varieties, improving farming practices, raising awareness among farmers and professionals, identifying natural enemies and biological control agents, identifying effective fungicides and botanicals, etc. A quarantine and regulatory control system for rice pests is mandatory. The yield loss caused by each pathogen type should be studied and quantified for that crop. In addition, the effectiveness of disease control options has been tested elsewhere, like the efficacy of new and novel fungicides against different rice diseases reported by various authors. The need for short-term strategies and the prioritization of prudent and viable treatment options will help control this disease. Since scientific knowledge is universal, farmers should get support from extension agents and should apply management options tested somewhere else that could be available and easily accessed. Policymakers should ratify appropriate policies and regulations to guide disease management options, their utilization, and their practicability. Because disease and pest outbreaks are highly related to environmental factors, future studies should consider these aspects of disease epidemiology.
Disclosure statement
The authors declare that they have no conflicts of interest.
Additional information
Notes on contributors
Melkamu Andargie
Melkamu  Andargie Dagnew is a plant pathology PhD student working at Bahir Dar University, Ethiopia. His area of research interest is plant disease epidemiology and management, focusing on host pathogen interaction, host resistance, and applications of botanicals and biological control.
Merkuz Abera
Merkuz Abera is a professor of plant pathology at Bahir Dar University. His area of research interest is crop protection and integrated management of different crop diseases.
Adane Tesfaye
Adane Tesfaye is a professor of agricultural entomology at Bahir Dar University. His area of research interest is crop protection and integrated management of economically important agricultural insect pests.
Esuyawkal Demis
Esuyawkal Demis is a senior researcher at Fogera National Rice Research Institute. His research focuses on storage insect pests, plant entomology, and pathology, especially stored product pests.
References
- Abade, A., Ferreira, P. A., & De Barros Vidal, F. (2021). Plant disease recognition on images using convolutional neural networks: A systematic review. Computers and Electronics in Agriculture, 185, 1. https://doi.org/10.1016/j.compag.2021.106125
- Abera, M. (2017). Agriculture in the Lake Tana Sub-basin of Ethiopia. In Social and ecological system dynamics: characteristics, trends, and integration in the Lake Tana Basin, Ethiopia (pp. 375–319). Berlin: Springer International Publishing.
- Adego, A., Were, H., Muoma, J., Mukoye, B., Hebrard, E., Pinel-Galzi, A., Poulicard, N., & Fargette, D. (2017). Current status of rice yellow mottle disease in western Kenya. Research Journal of Agriculture, 4, 1–12.
- Adorada, D. L., Stodart, B., Cruz, C. V., Gregorio, G., Pangga, I., & Ash, G. (2013). Standardizing resistance screening for Pseudomonas fuscovaginae and evaluation of rice germplasm at seedling and adult plant growth stages. Euphytica, 192(1), 1–16. https://doi.org/10.1007/s10681-012-0804-z
- Africa Rice Centre (2009). African Rice Center annual report 2008. Responding to the rice crisis. Author.
- African Growth and Technology Foundation (2013). Nitrogen use efficiency, water use efficiency, and salt-tolerant rice projects. Author.
- African Rice Center (2011). Boosting Africa’s Rice Sector Research for Development Strategy 2011–2020. Author.
- Agbowuro, G. O., Aluko, M., Salami, A. E., & Awoyemi, S. O. (2021). Evaluation of different aqueous plant extracts against the rice blast disease fungus (Magnaporthe oryzae). Ife Journal of Science, 22(3), 193–202. https://doi.org/10.4314/ijs.v22i3.17
- Akasa, L. U. (2023). Trends in oat production area, productivity, and utilization status in Ethiopia. American Journal of Bioscience and Bioinformatics, 2(1), 14–19. https://doi.org/10.54536/ajbb.v2i1.1459
- Alemu, D., Tesfaye, A., Assaye, A., Addis, D., Tadesse, T., & Thompson, J. (2018). A historical analysis of rice commercialization in Ethiopia: The case of the Fogera Plain. Agricultural Policy Research In Africa.
- Allen, T. W., Bradley, C. A., Sisson, A. J., Byamukama, E., Chilvers, M. I., Coker, C. M., Collins, A. A., Damicone, J. P., Dorrance, A. E., Dufault, N. S., Esker, P. D., Faske, T. R., Giesler, L. J., Grybauskas, A. P., Hershman, D. E., Hollier, C. A., Isakeit, T., Jardine, D. J., Kelly, H. M., Wrather, J. A. (2017). Soybean yield loss estimates due to diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Progress, 18(1), 19–27. https://doi.org/10.1094/PHP-RS-16-0066
- Altieri, M. A. (2018). Agroecology: The science of sustainable agriculture. CRC Press.
- Amayo, R., Teddy, O., Jimmy, L., Silue, D., Richard, E., & Geoffrey, T. (2020). Rice blast prevalence in smallholder rice farmlands in Uganda. Journal of Agricultural Science, 12(10), 105–115. https://doi.org/10.5539/jas.v12n10p105
- Ansari, M. U. R., Shaheen, T., Bukhari, S., & Husnain, T. (2015). Genetic improvement of rice for biotic and abiotic stress tolerance. Turkish Journal of Botany, 39, 911–919. https://doi.org/10.3906/bot-1503-47
- Asfaha, M. G., Selvaraj, T., & Woldeab, G. (2015). Assessment of disease intensity and isolates characterization of blast disease (Pyricularia oryzae CAV.) from South West of Ethiopia. International Journal of Life Sciences, 3, 271–286.
- Azzam, O., & Chancellor, T. C. (2002). The biology, epidemiology, and management of rice tungro disease in Asia. Plant Disease, 86(2), 88–100. https://doi.org/10.1094/PDIS.2002.86.2.88
- Balasubramanian, V., Sie, M., Hijmans, R., & Otsuka, K. (2007). Increasing rice production in sub-Saharan Africa: Challenges and opportunities. Advances in Agronomy, 94, 55–133.
- Barbedo, J. G. A. (2016). A review on the main challenges in automatic plant disease identification based on visible range images. Biosystems Engineering, 144, 52–60. https://doi.org/10.1016/j.biosystemseng.2016.01.017
- Bari, B. S., Islam, M. N., Rashid, M., Hasan, M. J., Razman, M. A. M., & Musa, R. M. (2021). A real-time approach of diagnosing rice leaf disease using deep learning-based faster R-CNN framework. PeerJ Computer Science, 7, e432. https://doi.org/10.7717/peerj-cs.432
- Bashar, M., Hossain, M., Rahman, M., Uddin, M., & Begum, M. (1970). Biological control of sheath blight disease of rice by using antagonistic bacteria. Bangladesh Journal of Scientific and Industrial Research, 45(3), 225–232. https://doi.org/10.3329/bjsir.v45i3.6529
- Becker, M., & Johnson, D. E. (2001). Cropping intensity effects on upland rice yield and sustainability in West Africa. Nutrient Cycling in Agroecosystems, 59(2), 107–117. https://doi.org/10.1023/A:1017551529813
- Befikadu, D. (2018). Postharvest losses in Ethiopia and opportunities for reduction: A review. International Journal of Sciences: Basic and Applied Research, 38, 249–262.
- Belayneh, T., & Tekle, J. (2017). Review on adoption, trend, potential, and constraints of rice production to livelihood in Ethiopia. International Journal of Research, 5(6), 644–658. https://doi.org/10.29121/granthaalayah.v5.i6.2017.2097
- Bewket, G. B. (2018). Review on integrated pest management of important disease and insect pest of rice (Oryzae sativa L.) (pp. 184–196). World Scientific News.
- Bhandari, D. R., Khanal, M. P., Joshi, B. K., Acharya, P., & Ghimire, K. H. (2017). Rice science and technology in Nepal (pp. 719–734). Government of Nepal.
- Capote, N., Pastrana, A. M., Aguado, A., & Sánchez-Torres, P. (2012). Molecular tools for detection of plant pathogenic fungi and fungicide resistance. Plant Pathology, 7, 151–202.
- Chauhan, R. S., Yadav, N. K., & Ravinder, C. (2017). Management of sheath rot of rice caused by Sarocladium oryzae (Sawada) Gams and Hawksworth. Annals of Biology, 33(1), 108–112
- Daniya, T., & Vigneshwari, S. (2021). Deep neural network for disease detection in Rice Plant using the texture and deep features. The Computer Journal, 65(7), 1812–1825. https://doi.org/10.1093/comjnl/bxab022
- Dessie, A. (2018). Cereal crops research achievements and challenges in Ethiopia. International Journal of Research Studies in Agricultural Sciences, 4, 23–29.
- Dessie, A. (2020). Rice breeding achievements, potential and challenges in Ethiopia. International Journal of Research Studies in Agricultural Sciences, 6, 35–42.
- Dessie, A., Zewdu, Z., Worede, F., & Bitew, M. (2018). Yield stability and agronomic performance of rainfed upland rice genotypes by using GGE bi-plot and AMMI in North West Ethiopia. International Journal of Research and Review, 5, 123–129.
- Diaz-Soltero, H. (2022). Global identification of invasive species: The CABI invasive species compendium as a resource. In Invasive species and global climate change. CABI GB.
- Dilnesaw, Z., Ebrahim, M., Getnet, B., Fanjana, F., Dechassa, F., Mequaninnet, Y., Hagose, H., Alemaw, G., Adane, A., & Negi, T. (2019). Evaluation of rice (Oryza sativa L.) variety adaptation performance at Omo Kuraz sugar development project Salamago district South Omo Zone, SNNPR state, Ethiopia. International Journal of Advanced Research in Biological Sciences, 6, 78–85.
- Donoso, A., & Valenzuela, S. (2018). In-field molecular diagnosis of plant pathogens: recent trends and future perspectives. Plant Pathology, 67(7), 1451–1461. https://doi.org/10.1111/ppa.12859
- Durgeshlal, C., Sahroj Khan, M. S., Prabhat, S. A., & Prasad, Y. A. (2019). Antifungal activity of three different ethanolic extract against isolates from diseased rice plant. Journal of Analytical Techniques and Research, 1(1), 047–063. https://doi.org/10.26502/jatri.007
- Eucord (2012). Rice sector development in East Africa, a desk study prepared for the Common fund for commodities.
- Evans, S. M., & Cowan, M. M. (2016). Plant products as antimicrobial agents. In Cosmetic and drug microbiology. CRC Press.
- Fahad, S., Adnan, M., Noor, M., Arif, M., Alam, M., Khan, I. A., Ullah, H., Wahid, F., Mian, I. A., & Jamal, Y. (2019). Major constraints for global rice production. In Advances in rice research for abiotic stress tolerance. Elsevier.
- Fang, X., Snell, P., Barbetti, M., & Lanoiselet, V. (2017). Rice varieties with resistance to multiple races of Magnaporthe oryzae offer opportunities to manage rice blast in Australia. Annals of Applied Biology, 170(2), 160–169. https://doi.org/10.1111/aab.12324
- FAO (2004). Production yearbook (Vol. 50). Author.
- FAO (2012). FAO rice market monitor, trade and markets division (Vol. XV). Author.
- FAO (2015). Rice market monitor (Vol. 18). Author.
- FAO (2018). World Food and Agriculture: Statistical Pocketbook. 254 pp. Rome.
- FAOSTAT (2017). FAO statistical database for agriculture. Food and Agriculture Organization of the United Nations. Retrieved from http://faostat.fao.org
- Fetene, D. Y., Birhan, M., & Zeleke, T. (2020). Screening of rice germplasms for their resistance against sheath rot disease (Sarocladium oryzae) at Fogera, Ethiopia. Journal of Plant Pathology & Microbiology, 11, 518.
- Funmilayo, O., & Akande, M. (1997). Vertebrate pests of rice in southwestern Nigeria. PANS, 23(1), 38–48. https://doi.org/10.1080/09670877709412395
- Ghazanfar, M. U., Habib, A., & Sahi, S. (2009). Screening of rice germplasm against Pyricularia oryzae the cause of rice blast disease. Pakistan Journal of Phytopathology, 21, 41–44.
- Goswami, S., Thind, T., Kaur, R., & Kaur, M. (2012). Management of sheath blight of rice with novel action fungicides. Indian Phytopathology, 65, 92–93.
- Goud, J., & Termorshuizen, A. (2003). Quality of methods to quantify microsclerotia of Verticillium dahliae in soil. European Journal of Plant Pathology, 109(6), 523–534. https://doi.org/10.1023/A:1024745006876
- Gudisa, W., & Tesfaye (2021). Survey of the status of major upland rice (Oryza sativa L.) fungal diseases and evaluation of genotypes resistance in Metekal and Awi Zone, Northwest Ethiopia. Ambo University.
- Gurung, S. (2016). Screening of endophytes for the control of root rot pathogens of pepper (Capsicum annum). Tennessee State University.
- Hagos, A., & Zemedu, L. (2015). Determinants of improved rice varieties adoption in Fogera district of Ethiopia. Science, Technology and Arts Research Journal, 4(1), 221–228. https://doi.org/10.4314/star.v4i1.35
- Hagos, H. (2015). Production of upland rice and constraints faced by the farmers in Tselemti district, Northern Ethiopia. Journal of Poverty, Investment and Development, 19, 30–35.
- Halos-Kim, L. (2015). Value-addition, agro-enterprises, partnerships & market access. African Journal of Food, Agriculture, Nutrition and Development, 15(5), 1–20.
- Haq, I., Fadnan, M., Jamil, F., & Rehman, A. (2002). Screening of rice germplasm against Pyricularia oryzae and evaluation of various fungitoxicants for control of disease. Pakistan Journal of Phytopathology, 14, 32–35.
- Harish, S., Saravanakumar, D., Radjacommare, R., Ebenezar, E. G., & Seetharaman, K. (2008). Use of plant extracts and biocontrol agents for the management of brown spot disease in rice. BioControl, 53(3), 555–567. https://doi.org/10.1007/s10526-007-9098-9
- Hashim, I., Mamiro, D., Mabagala, R. B., & Tefera, T. (2018). In vitro and in vivo evaluation of microbial agents for management of rice blast disease in Tanzania. World Journal of Agricultural Sciences, 14, 108–117.
- Heinrichs, E. A., & Barrion, A. T. (2004). Rice-feeding insects and selected natural enemies in West Africa: Biology, ecology and identification (E. A. Heinrichs, A. T. Barrion, & G. P. Hettel, Eds.). International Rice Research Institute and WARDA-The Africa Rice Center.
- Horo, J. T., & Gudisa, T. (2021). Efficacy of different fungicides for the control of rice blast (Pyricularia oryzae) disease under field conditions at Pawe, Northwest Ethiopia. International Journal of Phytopathology, 10(3), 215–224. https://doi.org/10.33687/phytopath.010.03.3649
- Hossain, M., Ali, M. A., & Hossain, M. D. (2017). Occurrence of blast disease in rice in Bangladesh. American Journal of Agricultural Sciences, 4, 74–80.
- Hubert, J., Mabagala, R. B., & Mamiro, D. P. (2015). Efficacy of selected plant extracts against Pyricularia grisea, causal agent of rice blast disease. American Journal of Plant Sciences, 6(5), 602–611. https://doi.org/10.4236/ajps.2015.65065
- IRRI (2018). International Rice Research Institute: The International Rice Genebank.
- Ilyas, M., & Iftikhar, K. (1997). Screening of rice germplasm and fungitoxicants against bakanae disease of rice. Pakistan Journal of Phytopathology, 9, 67–73.
- Jacqueline, C., Amanda, A., Kellen, C., Denise, C., Marta, C., & K, L. (2021). Efficiency of a new Waitea circinata extract against rice pathogens. Pesquisa Agropecuária Tropical, 51, e66916.
- Jamal-U-Ddin, H., Mubeen, L., Mumtaz, A., Pathan, M., Ali, K., & Serwar, S. G. (2012). In-vitro evaluation of fungicides, plant extracts and bio-control agents against rice blast pathogen Magnaporthe oryzae Couch. Pakistan Journal of Botany, 44, 1775–1778.
- John, A., & Fielding, M. (2014). Rice production constraints and ‘new’ challenges for South Asian smallholders: Insights into de facto research priorities. Agriculture & Food Security, 3(1), 16. https://doi.org/10.1186/2048-7010-3-18
- Jones, M. P., Dingkuhn, M., Aluko, G. K., & Semon, M. (1997). Interspecific Oryza sativa L. × Oryza glaberrima Steud. Progenies in Upland Rice Improvement, 94, 237–246.
- Kala, A., Soosairaj, S., Mathiyazhagan, S., & Raja, P. (2015). Isolation and identification of Xanthomonas oryzae pv. oryzae the causal agent of rice bacterial leaf blight and its activities against of six medicinal plants. Asian Journal of Plant Science and Research, 5, 80–83.
- Kalboush, Z. (2019). Resistance of rice genotypes to the blast fungus and the associated biochemical changes. Egyptian Journal of Agricultural Research, 97(1), 39–55. https://doi.org/10.21608/ejar.2019.68552
- Kandhari, J. (2007). Management of sheath blight of rice through fungicides and botanicals.
- Khan, J., Jamil, F., Cheema, A., & Gill, M. (2002). Screening of rice germplasm against blast disease caused by Pyricularia oryzae Cav. integrated plant disease management. In Proceedings of 3rd National Conference of Plant Pathology, NARC, Islamabad, 1–3 October 2001 (pp. 86–89). Pakistan Phytopathology Society.
- Khatun, M. T., Nessa, B., Salam, M. U., & Kabir, M. S. (2021). Strategy for rice disease management in Bangladesh. Bangladesh Rice Journal, 25(1), 23–36. https://doi.org/10.3329/brj.v25i1.55177
- Khoury, W. E., & Makkouk, K. (2010). Integrated plant disease management in developing countries. Journal of Plant Pathology, 94, S35–S42.
- Kihoro, J., Njoroge, J. B., Hunja, M., Elijah, A., & Daigo, M. (2013). Investigating the impact of rice blast disease on the livelihood of the local farmers in greater Mwea region of Kenya. Springer Plus, 2, 308.
- Kindo, D., & Tiwari, P. (2015). Efficacy of fungicides for management of sheath rot disease in rice under field conditions. Plant Archives, 15, 119–120.
- Kumar, D., Khilari, K., Kumar, N., & Jain, S. K. (2017). Integrated disease management of rice root knot nematode (Meloidogyne graminicola) through organic amendments, Trichoderma spp. and Carbofuran. Journal of Pharmacognosy and Phytochemistry, 6, 2509–2515.
- Kumar, M. P., Gowda, D. S., Moudgal, R., Kumar, N. K., Gowda, K. P., & Vishwanath, K. (2013). Impact of fungicides on rice production in India. In Fungicides-showcases of integrated plant disease management from around the world (pp. 77–98). IntechOpen Book Series.
- Kumar, Y. V., Choudhary, V. P., Singh, S. K., Kumar, M. M., Vishwakarma, S. P., Prasad, R., Yadav, J., & Akash Singh, A. (2021). In vitro efficacy of botanicals against Rhizoctonia solani Kuhn inciting sheath blight of rice. The Pharma Innovation Journal, 10, 1144–1147.
- Latif, M., Badsha, M., Tajul, M., Kabir, M., Rafii, M., & Mia, M. (2011). Identification of genotypes resistant to blast, bacterial leaf blight, sheath blight and tungro and efficacy of seed treating fungicides against blast disease of rice. Scientific Research and Essays, 6, 2804–2811.
- Li, D., Wang, R., Xie, C., Liu, L., Zhang, J., Li, R., Wang, F., Zhou, M., & Liu, W. (2020). A recognition method for Rice Plant diseases and pests video detection based on deep convolutional neural network. Sensors, 20(3), 578. https://doi.org/10.3390/s20030578
- Liu, L., Waters, D. L., Rose, T. J., Bao, J., & King, G. J. (2013). Phospholipids in rice: Significance in grain quality and health benefits: A review. Food Chemistry, 139(1–4), 1133–1145. https://doi.org/10.1016/j.foodchem.2012.12.046
- Lore, J., Jain, J., Hunjan, M., Gargas, G., Mangat, G., & Sandhu, J. (2015). Virulence spectrum and genetic structure of Rhizoctonia isolates associated with rice sheath blight in the northern region of India. European Journal of Plant Pathology, 143(4), 847–860. https://doi.org/10.1007/s10658-015-0736-2
- Lu, Y., Yi, S. J., Zeng, N. Y., Liu, Y. R., & Zhang, Y. (2017). Identification of rice diseases using deep convolutional neural networks. Neurocomputing, 267, 378–384. https://doi.org/10.1016/j.neucom.2017.06.023
- Magar, P. B., Acharya, B., & Pandey, B. (2015). Use of chemical fungicides for the management of rice blast (Pyricularia grisea) disease at Jyotinagar, Chitwan, Nepal. International Journal of Applied Sciences and Biotechnology, 3(3), 474–478. https://doi.org/10.3126/ijasbt.v3i3.13287
- Mahmood-Ur-Rahman, Tayyaba, S., Shazia, A., & Tayyab, H. (2015). Genetic improvement of rice for biotic and abiotic stress tolerance. Turkish Journal of Botany, 39, 911–919.
- Malicdem, A., & Fernandez (2015). Rice blast disease forecasting for northern Philippines. WSEAS Transactions on Information Science and Applications, 12, 120–129.
- Mariappan, V., Rajeswari, E., & Kamalakannan, A. (1995). Management of rice blast, Pyricularia oryzae by using neem (Azadirachta indica) and other plant products. In V. Mariappan (Ed.), Neem for the management of crop diseases (pp. 3–10). New Delhi, India: Associated Publishing Co.
- Martinelli, F., Scalenghe, R., Davino, S., Panno, S., Scuderi, G., Ruisi, P., Villa, P., Stroppiana, D., Boschetti, M., Goulart, L. R., Davis, C. E., & Dandekar, A. M. (2015). Advanced methods of plant disease detection. A review. Agronomy for Sustainable Development, 35(1), 1–25. https://doi.org/10.1007/s13593-014-0246-1
- McGrath (2016). What are fungicides? The Plant Health Instructor Cornell University, 16. https://doi.org/10.1094/PHI-I-2004-0825-01
- Mdangi, M., Muhamba, D., Massawe, A. W., & Mulungu, S. L. (2019). Rice insect pest management in selected rice irrigation schemes in Morogoro Region, Tanzania. African Journal of Agricultural Research, 14(15), 698–704. https://doi.org/10.5897/AJAR2017.12309
- Mekonnen, A. B., Mohammed, A. S., & Tefera, A. K. (2022). Ethnobotanical study of traditional medicinal plants used to treat human and animal diseases in Sedie Muja District, South Gondar, Ethiopia. Evidence-Based Complementary and Alternative Medicine: eCAM, 2022, 1–22. https://doi.org/10.1155/2022/7328613
- Mesfin, A. H., & Zemedu, L. (2015). Improved rice seed production and marketing: challenges and opportunities; The case of Fogera district of Ethiopia. Journal of Agriculture and Environmental Sciences, 1, 1–21.
- Mew, T., Vera Cruz, C., & Medalla, E. (1992). Changes in race frequency of Xanthomonas oryzae pv. oryzae in response to rice cultivars planted in the Philippines. Plant Disease, 76(10), 1029–1032. https://doi.org/10.1094/PD-76-1029
- MoA (2009). National Rice Development Strategy, 2008–2018. Author.
- MoA (2010). National Rice Research and Development Strategy of Ethiopia. Author.
- MoARD (2010). National Rice Research and Development Strategy (Vol. 48). Author.
- Mohanty, S. P., Hughes, D. P., & Salathé, M. (2016). Using deep learning for image-based plant disease detection. Frontiers in Plant Science, 7, 1419. https://doi.org/10.3389/fpls.2016.01419
- Moldenhauer, K. A., Bastawisi, A., & Lee, F. (1992). Inheritance of resistance in rice to races IB-49 and IC-17 of Pyricularia grisea rice blast. Crop Science, 32(3), 584–588. https://doi.org/10.2135/cropsci1992.0011183X003200030003x
- Mondal, D., Ghosh, A., Roy, D., Kumar, A., Shamurailatpam, D., Bera, S., Ghosh, R., Bandopadhyay, P., & Majumder, A. (2017). Yield loss assessment of rice (Oryza Sativa L.) due to different biotic stresses under system of rice intensification (SRI).
- Muluadam, B., Desalegn, Y., & Tekalgn, Z. (2020). Evaluation of fungicides efficacy against rice sheath rot disease (Sarocladium oryzae) in rain fed low land rice (Oryzae sativa L.) in Fogera hub. International Journal of Agriculture and Biosciences, 9, 221–225.
- Mustofa, A. M., & Gondar, E. (2017). College of Agriculture and Rural Transformation Department of Plant Science.
- Mwakasege, L. D. (2015). Assessment of rice diseases and yield under system of rice intensification (SRI) in Morogoro, Tanzania [Dissertation]. Sokoine University of Agriculture.
- Nega, A. (2014). Review on concepts in biological control of plant pathogens. Journal of Biology, Agriculture and Healthcare, 4, 33–54.
- Negussie, T., & Alemu, D. (2011). An overview of the national rice research and development strategy and its implementation. In challenges and opportunities of rice in Ethiopian agricultural development (pp. 1–16). Ethiopian Institute of Agricultural Research.
- Neha, K., Naveenkumar, R., Balabaskar, P., & Manikandan, P. (2017). Evaluation of fungicides against sheath blight of rice caused by Rhizoctonia solani (Kuhn.). Oryza, 54(4), 470–479. https://doi.org/10.5958/2249-5266.2017.00064.9
- Neupane, N., & Bhusal, K. (2020). A review of blast disease of rice in Nepal. Journal of Plant Pathology and Microbiology, 11, 528.
- Nwilene, F. E., Nacro, S., Tamò, M., Menozzi, P., Heinrichs, E. A., Hamadoun, A., Dakouo, D., Adda, C., & Togola, A. (2013). Managing insect pests of rice in Africa. In Realizing Africa’s rice promise (pp. 229–240). CAB International.
- Odedara, O. O., Ademolu, K. O., & Ayo-John, E. I. (2016). Prevalence of rice yellow mottle virus (RYMV) on rice plants grown in selected farms in Ogun state: Preliminary results. Nigerian Journal of Biotechnology, 31(1), 96–102. https://doi.org/10.4314/njb.v31i1.13
- Oliver, R. P., & Hewitt, H. G. (2014). Fungicides in crop protection. CABI.
- Ou, S. (1985). Rice diseases (2nd ed., p. 380). Commonwealth Mycological Institute, Kew.
- Parshuram, R., Yadav, S. C., Awadhiya, G., Prasad, M. S., & Prakasam, V. (2017). Survey and occurrence of sheath blight of rice in major rice growing areas of Chhattisgarh. International Journal of Pure & Applied Bioscience, 5(4), 838–845. https://doi.org/10.18782/2320-7051.5442
- Poehlman, J. M. (2013). Breeding field crops. Springer Science & Business Media.
- Prasad, N., Singh, N., Avinash, P., & Tiwari, P. (2020). Efficacy of new fungicides against sheath blight disease management of rice caused by Rhizoctonia solani under field condition. International Journal of Chemical Studies, 8(3), 216–220. https://doi.org/10.22271/chemi.2020.v8.i3c.9228
- Prasad, P., Naik, M., & Nagaraju, P. (2006). Screening of genotypes, fungicides, botanicals, and bio-agents against Rhizoctonia solani, the incident of sheath blight of rice. International Journal of Plant Protection, 10, 247–251.
- Rachana, M., Anjeela, A., Sagar, K., Ashbin, K. D., Basistha, A., Darbin, J., & Krishna, A. (2021). Evaluation of different chemical fungicides against rice blast in field conditions. Journal of Agriculture and Natural Resources, 4, 295–302.
- Rahaman, M. M., Islam, K. S., Jahan, M., & Mamun, M. A. A. (2016). Relative abundance of stem borer species and natural enemies in rice ecosystem at Madhupur, Tangail, Bangladesh. Journal of the Bangladesh Agricultural University, 12(2), 267–272. https://doi.org/10.3329/jbau.v12i2.28681
- Raju, M., Visalakshmi, V., & Ramanamurthy, K. (2020). Evaluation of aqueous botanical extracts and bio-agents against biotic stresses in paddy. Journal of Pharmacognosy and Phytochemistry, 9, 330–333.
- Rijal, S., & Devkota, Y. (2020). A review on various management method of rice blast disease. Malaysian Journal of Sustainable Agriculture, 4(1), 29–33. https://doi.org/10.26480/mjsa.01.2020.29.33
- Salih, A., Sreewongchai, T., Sripichitt, P., & Parinthawong, N. (2013). Identification of blast-resistant varieties from landrace, improved and wild species of rice. Agriculture and Natural Resources, 47, 1–7.
- Savary, S., Ficke, A., Aubertot, J.-N., & Hollier, C. (2012). Crop losses due to diseases and their implications for global food production losses and food security. Food Security, 4(4), 519–537. https://doi.org/10.1007/s12571-012-0200-5
- Seck, P. A., Touré, A. A., Coulibaly, J. Y., Diagne, A., & Wopereis, M. C. (2013). Africa’s rice economy before and after the 2008 rice crisis. In Realizing Africa’s rice promise. CABI.
- Sedeek, S., & Elwahsh, S. (2015). Performance of some agronomic traits of selected rice breeding lines and its reaction to blast disease. Journal of Agricultural Research, 41, 167–180.
- Sendeku, W. (2005). Factors determining supply of rice: A study in Fogera district of Ethiopia [MSc thesis. School of Graduate Studies of Haramaya University.
- Sere, Y., Sy, A. A., Akator, S. K., Onasanya, A., & Zai, K. (2004). Screening strategy for durable resistance to rice blast disease at WARDA. In Rice blast in West Africa: Characterization of pathogen diversity, key screening sites and host resistance (pp. 38–43). The Africa Rice Center (WARDA).
- Seyoum, M., Alamerew, S., & Bantte, K. (2011). Evaluation of upland NERICA rice (Oryza sativa L.) genotypes for grain yield and yield components along an altitude gradient in Southwest Ethiopia. Journal of Agronomy, 10(4), 105–111. https://doi.org/10.3923/ja.2011.105.111
- Sharma, L., Nagrale, D., Singh, S., Sharma, K., & Sinha, A. (2013). A study on fungicides potential and incidence of sheath rot of rice caused by Sarocladium oryzae (Sawada). Journal of Applied and Natural Science, 5(1), 24–29. https://doi.org/10.31018/jans.v5i1.276
- Simon, M. (2016). [Increasing the resilience of elite rice cultivars to sheath rot (Sarocladium oryzae [(Sawada) W. Gams & D. Hawksw]) in Rwanda through breeding for resistance] [PhD dissertation]. University of KwaZulu-Natal Republic of South Africa.
- Singh, A., & Singh, I. K. (2018). Molecular aspects of plant-pathogen interaction. Springer.
- Singh, R., & Sunder, S. (2015). Identification of sources of resistance to blast and false smut their management with fungicides. Journal of Mycology and Plant Pathology, 45, 55–59.
- Singh, R., Sunder, R. S., & Dodan, D. S. (2015). Screening of scented rice genotypes and fungicides against blast and compatibility of pesticides used for the control of neck blast and plant hoppers. Plant Disease Research, 30, 1–5.
- Srinivasachary, Willocquet, L., & Savary, S. (2011). Resistance to rice sheath blight (Rhizoctonia solani Kühn)[(Teleomorph: Thanatephorus cucumeris (AB Frank) Donk.] disease: Current status and perspectives. Euphytica, 178(1), 1–22. https://doi.org/10.1007/s10681-010-0296-7
- Stella, E., Adur-Okello, Simon, A., Jimmy, L., Moses, E., & Michael, H. O. (2020). Farmers’ knowledge and management of rice diseases in Uganda. Journal of Agricultural Science, 12(12), 221–233. https://doi.org/10.5539/jas.v12n12p221
- Taddesse, L., Fukuta, Y., & Ishikawa, R. (2020). Genetic study of diversity and blast resistance in Ethiopian rice cultivars adapted to different ecosystems. Breeding Science, 70(3), 303–312. https://doi.org/10.1270/jsbbs.18198
- Tadesse, T., & Tadesse, Z. (2019). Review of rice response to fertilizer rates and time of nitrogen application in Ethiopia. International Journal of Applied Agricultural Sciences, 5(6), 129–137. https://doi.org/10.11648/j.ijaas.20190506.11
- Tariku, S. (2011). An overview of rice research in Ethiopia. In Challenges and opportunities of rice in Ethiopian agricultural development. Ethiopian Institute of Agricultural Research.
- Teshome, & Dawit, A. (2011). An overview of the national rice research and development strategy and its implementation. In Challenges and opportunities of rice in Ethiopian agricultural development. Empowering farmers’ innovation series (Vol. 2). Ethiopian Institute of Agricultural Research.
- Tilahun, Z. M. (2019). Effect of row spacing and nitrogen fertilizer levels on yield and yield components of rice varieties (pp. 180–193). World Scientific News.
- Traoré, O., Pinel, A., Hébrard, E., Dieudonné Gumedzoé, M. Y., Fargette, D., Traoré, A. S., & Konaté, G. (2006). Occurrence of resistance-breaking isolates of rice yellow mottle virus in West and Central Africa. Plant Disease, 90(3), 259–263. https://doi.org/10.1094/PD-90-0259
- Valentin, S. E. T., Bouma, J. N., Mouhameth, C., Vernon, G., Samuel, K. O., & Oumar, T. (2015). Farmers’ perception and impact of rice yellow mottle disease on rice yields in Burkina Faso. Journal of Agricultural Sciences, 6, 943–952.
- Varaprasada, R. C., & Anil, K. P. (2018). Integrated disease management of rice blast caused by Pyricularia grisea (Sacc.). International Journal of Current Microbiology and Applied Sciences, 7, 2952–2958.
- Waller, J. (1987). Rice diseases. By SH Ou Slough, UK: Commonwealth Agricultural Bureaux (1985) pp. 380, UK£38.00, USA $70, elsewhere£41.00. Experimental Agriculture, 23(3), 357–357. https://doi.org/10.1017/S0014479700017245
- Wang, Z., Jia, Y., Rutger, J., & Xia, Y. (2007). Rapid survey for presence of a blast resistance gene Pi-Ta in rice cultivars using the dominant DNA markers derived from portions of the Pi-Ta gene. Plant Breeding, 126(1), 36–42. https://doi.org/10.1111/j.1439-0523.2007.01304.x
- Wang, Z., Zuo, S., Li, G., Chen, X., Chen, Z., Zhang, Y., & Pan, X. (2009). Rapid identification technology of resistance to rice sheath blight in seedling stage. Acta Phytopathologica Sinica, 39, 174–182.
- Wolie, A. W., & Admassu, M. A. (2016). Effects of integrated nutrient management on rice (Oryza sativa L.) yield and yield attributes, nutrient uptake and some physico-chemical properties of soil: A review. Journal of Biology, Agriculture and Healthcare, 6, 20–26.
- World Bank (2008). World development report 2008: Agriculture for development. Author.
- Wubneh, W. Y., & Bayu, F. A. (2016). Assessment of diseases on rice (Oriza sativa L.) in major growing fields of Pawe district, Northwestern Ethiopia (pp. 13–23). World Scientific News.
- Yadav, R. N., Mishra, D., Zaidi, N. W., Singh, U. S., & Singh, H. B. (2018). Bio-control efficacy of Trichoderma spp. against the major diseases of rice (Oryzae sativa L.). International Journal of Agriculture, Environment and Biotechnology, 11(3), 543–548. https://doi.org/10.30954/0974-1712.06.2018.17
- Yalew, D., Getinet, A., & Berhan, M. (2021). Identification of newly emerging rice diseases in Fogera Plains, Ethiopia. International Journal of Recent Research in Life Sciences (IJRRLS), 8(3), 1–9.
- Zeleke, T., Birhan, M., & Ambachew, W. (2019). Survey and identification of rice diseases in South Gondar Zone, Amhara Region, Ethiopia. Journal of Agriculture and Crops, 5(58), 123–131. https://doi.org/10.32861/jac.58.123.131
- Zhou, G. X., Zhang, W. Z., Chen, A. B., He, M. F., & Ma, X. S. (2019). Rapid detection of rice disease based on FCM-KM and faster R-CNN fusion. IEEE Access, 7, 143190–143206. https://doi.org/10.1109/ACCESS.2019.2943454

