Abstract
The present study aimed to evaluate the effects of rosemary (REO) and basil (BEO) essential oils (EO) on minced raw meat samples. The meat samples were subsequently treated with various concentrations: 0.5%, 1.0%, and 1.5% (v/w) of rosemary (MR0.5, MR1.0, and MR1.5) and basil (MB0.5, MB1.0, and MB1.5) EO, respectively, as well as meat sample without EO treatment was used as a control. Afterward, the samples were stored at 4 °C and monitored for pH, Hunter CIEL*a*b* color and bacterial counts at 0, 7, 14, and 21 days. Results indicated that EO treatment offered a lower pH, particularly for BEO treatment as compared to the control. Samples treated with BEO presented the higher CIEL*a*b* (lightness, redness and yellowness) values followed by REO treatments as compared to the control during the entire storage periods. The addition of both EO to minced meat resulted in a significant (p < 0.05) reduction of tested bacterial counts, whereas increased throughout storage in the control sample. The growth of total bacterial counts was delayed by 3, 4.82, 3.48, and 4.81 log CFU/g of meat in MR0.5%, MR1.0%, MB0.5%, and MB1.0%, respectively. Coliform and Salmonella counts were observed in MR1.0%, MB0.5%, and MB1.0% and fully inhibited at the end of storage. In general, BEO and REO treatments effectively maintained pH, color measured as CIEL*a*b* and prevent bacterial growth in minced meat during storage at 4 °C, with pronounced efficacy of BEO.
1. Introduction
Meat is one of the most perishable food products, so prolonging its shelf life is crucial for enhancing food system sustainability (Kondjoyan et al., Citation2022). Meat generally has a shorter shelf life due to its perishable nature. High protein, fat and moisture content in meat exposes it to microbial deterioration, lipid oxidation (off-odor), discoloration, and ultimate quality deterioration (Canto et al., Citation2016; Zhang et al., Citation2021).
Metabolic changes in meat constituents, particularly the oxidation of proteins and fats have a significant impact on postmortem meat quality. Color is one of the crucial quality criteria influencing consumer acceptance, as discoloration signifies spoiled meat and makes it less appealing (Guerrero et al., Citation2020). Oxidation of lipids and myoglobin pigment can be the main cause of meat discoloration (Wang et al., Citation2022).
Furthermore, postmortem minimal processing of meat such as cutting, chopping, and mixing may enhance and oxidation as well as microbial deterioration, especially in unsanitary conditions (Kondjoyan et al., Citation2022; Wang et al., Citation2022). Various preservation techniques are employed to safeguard meat from spoiling factors, including the use chemical preservatives (Wang et al., Citation2022), packaging (Cullere et al., Citation2018), EO-loaded packaging (Jaspal et al., Citation2021; Papazoglou et al., Citation2012), super chilling (Tian et al., Citation2022), EO-incorporated biodegradable films (Gómez-Estaca et al., Citation2010), and edible coating (Guerrero et al., Citation2020). Nevertheless, each technique has processing-related shortcomings of its own. For instance, it has been suggested that chemical preservatives can pose health hazards, especially to children (Seema, Citation2015). From this perspective, the demand for natural preservatives has increased along with consumer awareness of organic consumption. Given their prevalent acceptance as safe natural preservatives, EOs are among the viable alternatives in this regard (Murbach Teles Andrade et al., Citation2014). EOs are complex secondary metabolites that are often extracted from aromatic plants by cold pressing, steaming and hydro distillation (Hyldgaard et al., Citation2012). EOs play a significant role in plant defense as well as preservation due to their composition of terpenes, phenolics, and alcoholic components (Seema, Citation2015).
Furthermore, their hydrophobic nature and the presence of phenolic compounds like linalool, thymol, and carvacrol contribute to their antimicrobial and antioxidant properties (Gutierrez et al., Citation2008). Consequently, EOs are still be used in meat preservation because of their positive effects on health and, in some cases, potential synergistic preservative effects (Abdollahzadeh et al., Citation2014; Burt, Citation2004). Among EOs, rosemary and basil EO extracted from Rosmarinus officinalis and Ocimum basilicum have long been used as flavorings and preservatives.
The antimicrobial and antioxidant properties of EO derived from those plants drew substantial interest in the cosmetic industries in addition to food preservation (Hussain et al., Citation2008; Murbach Teles Andrade et al., Citation2014). In Ethiopia, these two aromatic plants are commonly used as seasonings, particularly rosemary leaf, which is frequently served with fried meat.
Rosemary and basil plants were mostly used to preserve meat, either as extract or EOs. The addition of REO and BEO to chicken meat has been reported to reduce Salmonella counts without compromising quality during storage at 4 °C and 18 °C (Stojanović-Radić et al., Citation2018). In addition, the use of BEO in fish fillets during repeated-frying has been reported to effectively reduce the oxidation process (Erol et al., Citation2022). Similarly, rosemary extract was employed as antioxidant in meat burgers (Hashemi Gahruie et al., Citation2017) and in porcine liver patties to lessen lipid oxidation (Doolaege et al., Citation2012).
The interest in using EO as natural preservative has surged since Food and Drug Administration (FDA) approved EO and their constituents as generally safe (GRAS) food additives (Vlaicu et al., Citation2023). Therefore, their extensive applications at various levels could assist in understanding the best concentration along with the nature of the food to be treated. The aim of this study was to evaluate the preservative effects of rosemary and basil EO on minced raw meat at various concentrations during storage at 4 °C.
2. Materials and methods
2.1. Materials
Fresh leaves of rosemary and basil were collected from experimental garden of Wondogenet Agricultural Research Center, Wondogenet, Ethiopia. A kilogram of fresh beef meat was purchased from a local market (butcher house) in Sebeta, Ethiopia, at 24 h post-mortem. Then, it was aseptically delivered to the lab, and red meat was selected for EO treatments.
2.2. Extraction of essential oils (EO)
Hydro distillation method was used to extract EO from rosemary and basil according to Tongnuanchan and Benjakul (Citation2014). The leaf samples were heated in a Clevenger-type apparatus for 3 h by fully immersing them in distilled water inside a boiling flask. EO was recovered by condensation from water vapor at the top. The obtained EO was poured into dark vails and kept at 4 °C until use. The composition of REO and BEO were reported in our previous work (Abdo et al., Citation2018; Mieso et al., Citation2022). The rosemary and basil EO were designated as REO and BEO throughout this study.
2.3. Meat sample preparation and treatments
The meat samples were prepared for EO treatments in accordance with the method followed by Fratianni et al. (Citation2010). All equipment was sterilized prior to use as well the necessary sanitary procedure was followed. The red meat was minced into uniform size using previously sterilized laboratory miller (HR7310/00, Philips). About 25 g of this portion were treated with 0.5%, 1.0%, and 1.5% (v/w) of REO and BEO, represented as MR 0.5%, MR 1.0%, and MR 1.5% and MB 0.5%, MB 1.0%, and MB 1.5%, respectively, and a sample without EO addition was used as a control. Then, each treatment was packed in a high-density polyethylene bag and stored for 21 days at 4 °C. The samples were analyzed every 7 days, starting immediately after treatment (0 days) until the end of storage (21 days). The choice of storage days was made in light of earlier research for a comprehensive evaluation of EO preservative effects on red meat (El Abed et al., Citation2014; Fratianni et al., Citation2010; Jayari et al., Citation2018).
2.4. Analysis of physical characteristics
2.4.1. PH measurement
The method followed by Radha Krishnan et al. (Citation2014) was used to measure pH of meat samples. Treated portion of meat sample (10 g) was mixed with 10 mL of distilled water. Then pH was measured using portable pH meter (model PH-016, USA) at room temperature. The pH meter was calibrated before measurement using standard solutions having a pH of 4, 7, and 10. The measurements were carried out in triplicate.
2.4.2. CIEL*a*b* color analysis
A deal-beam non-contact spectrophotometer (Aeros HunterLab, Reston, VA, USA) with D65 illumination, a 10° observer angle, and a 177.25 cm2 measurement area was used to measure the color of meat samples. Calibration was done with standard white tile and black glass prior to measurements. The measurements were carried out in triplicate using the CIEL*a*b* color scale, where L*, a*, and b* stands for lightness/darkness (100 white to 0 black), redness/greenness (+a* to -a*), and yellowness/blueness (+b* to -b*), respectively.
2.5. Microbial enumeration
EO preserved meat samples were evaluated for total bacterial count (TBC) using Nutrient Agar, total coliform count (TCC) using Violet Red Bile Lactose Agar (VRBL) and Salmonella using Xylose-Lysine Deoxycholate Agar (XLD) according to Krichen et al. (Citation2020). Sanitary procedure was followed during microbiological load evaluation, media was prepared according to written instructions on the label and every equipment was autoclaved using Vertical Steam Pressure sterilizer (LDZX-50KBS) at 121 °C for 15 min before used. About 25 mL of ready liquid media was poured on sterilized Petri dish inside laminar flow safety cabinet and left to solidify. One gram of minced raw meat samples was dispersed in 9 mL peptone water and then serially diluted (10−1 -10−5) in a test tube using sterilized pipet. One milliliter of each dilution was spread-plated on different selective media followed by incubation at 37 °C for 48 h. Next, the Petri dishes were examined for the growth of visible microbial colony. The analysis was carried out in three replications and the count of colonies was reported in log CFU/g.
2.6. Statistical analysis
The collected data were subjected to analysis of variance (ANOVA) using R software version 2021.4.0.5 (R Core Team, Austria). The results were reported as mean ± standard deviation and Tukey’s HSD test, at p < 0.05 significant mean separation. The correlations between pH, lightness (L*), redness (a*) yellowness (b*), total bacterial, coliforms and Salmonella counts were tested using Pearson’s correlation (r).
3. Results and discussion
3.1. pH
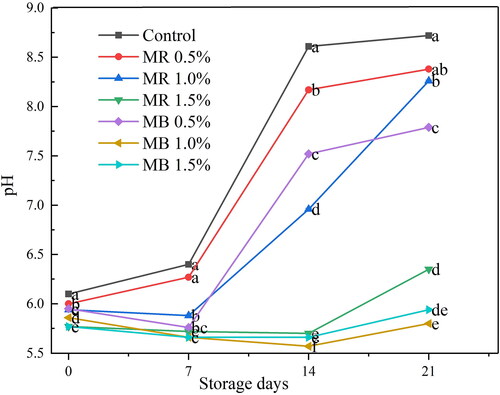
displays the effects of REO and BEO treatments on the pH of minced raw meat samples stored for 21 days at 4 °C. The pH value of the control minced meat sample was gradually increased from the initial day (6.10) to the 7th day, then sharply increased until the last day of storage (8.72).
Young et al. (Citation2004) proposed that pH ranges from 5.4 to 5.6 could be considered desirable characteristics of meat, but when reached to 6.9, it indicates the onset of darkness. As the pH values increased, the meat became darker because it transmitted more light into its depth than at low pH (Swatland, Citation2008). The pH in REO and BEO treatments similarly increased, with the lowest values for BEO treated samples than other treatments. Similar tendencies of pH results for raw chicken meat treated with various spice extracts have been reported earlier (Radha Krishnan et al., Citation2014). On the initial day of storage (day 0), MR1.5% and MB1.5% displayed the lowest pH values of 5.77, while MR0.5% had the highest (8.38) (). The current pH values were consistent with earlier studies on chilled meat, frozen lamp meat spiked with 0.05% thyme and garlic EO-loaded alginate edible coating, and minced meat treated with 0.06 to 0.32% lemon EO during storage at 4 °C (Ben Hsouna et al., Citation2017; Guerrero et al., Citation2020; Tian et al., Citation2022).
The pH values of treated meat decreased as the concentrations of EO increased, especially for BEO. This could be due to BEO constituents that contributed for better regulation of pH in meat during storage (Hussain et al., Citation2008). The treated meat samples exhibited lower pH values than untreated sample due to the delay in the growth of volatile basic nitrogen-generating microbes (Ben Akacha et al., Citation2023; Jaspal et al., Citation2021). However, pH values usually rise during prolonged storage because of buildup of basic compounds such as ammonia and trimethylamine produced by oxidation and microbial proliferation (Ijaz et al., Citation2020; Radha Krishnan et al., Citation2014).
3.2. CIEL*a*b* color parameters
The CIEL*a*b* color parameters of untreated and treated minced meat samples with REO and BEO are presented in . The addition of both REO and BEO significantly (p < 0.05) affected the L* (lightness) values of minced meat samples during storage at 4 °C. The L* values of untreated and treated samples were not significantly (p > 0.05) affected on the first day of storage. Starting from the 7th storage day, the L* values increased, but at a lower rate for the EO-treated compared to the untreated sample. Comparable results were found for minced meat treated with 2% rosemary, sage, and oregano EO (Ünal et al., Citation2014) and chicken meat treated with spice extracts during cold storage (Radha Krishnan et al., Citation2014). Conversely, some authors reported a decrease in L* values during storage in a study on minced meat treated with 0.5% to 2% cumin EO-loaded edible coating (Behbahani et al., Citation2020) and chicken breast meat treated with 0.2% oregano EO and 1.25% lactic acid (Jasbal et al., 2019). Variations in L* values could be attributed to types of meat, initial oxidation state, fat content, packaging, hygienic and storage conditions as well EO color and composition (Hernández et al., Citation2016; Radha Krishnan et al., Citation2014). After 21 days of storage at 4 °C, the L* values were lowest for MR0.5% (36.05) and highest for MB1.5% (46.11). These results are in agreement with Stojanović-Radić et al. (Citation2018), who found higher L* values for chicken meat treated with BEO than that treated with REO. The higher L* values in treated samples may be the result of alterations in light absorption or scattering properties caused by pigments present in EO. Moreover, the formation of light protein dispersion through meat structure degradation might be the cause of the increase in L* values over the storage period (Ijaz et al., Citation2020). There was a significant (p < 0.05) decline in the redness (a*) values of both untreated and treated minced meat samples during the entire storage period. The treated samples demonstrated a lower reduction in a* values, ranging from 11.33 to 42.33% compared to the control (42.98%) during 21 days of storage at 4 °C. The treatments MB1.0% and MR0.5% exhibited the lowest and highest reductions in a* values, respectively. A decline in a* values during storage at 4 °C was noticed earlier for minced meat wrapped in 4% REO-supplemented active packaging (Sirocchi et al., Citation2017) and treated with 0.1 and 1.5% pistachio-by-product EO (Krichen et al., Citation2020).
Table 1. Effect of REO and BEO treatments on color parameters (L*a*b*) of minced raw meat during storage at 4 °C for 21 days.
BEO-treated samples showed a lower decline in a* values than REO-treated samples during the entire storage period at 4 °C, indicating BEO can maintain meat redness more effectively than REO. According to Stojanović-Radić et al. (Citation2018), there was less change in a* values for BEO-treated chicken meat as compared to samples treated with REO and their combination. These could be due to the presence of antioxidant compounds in BEO such as phenols as they protect oxidative processes that can cause meat discoloration (Sirocchi et al., Citation2017). The presence of iron-containing myoglobin pigment is primarily responsible for the red color of meat, which is the most significant quality parameter. Therefore, myoglobin content and the oxidative states derived by oxygen are the main factors that determine the redness of meat during storage (Salueña et al., Citation2019). Myoglobin undergoes three oxidation states after exposed to oxygen, namely deoxymyglobin (DMb), oxymyoglobin (OMb), and metmyoglobin (MMb). These oxidation states in meat cause it to turn purplish red immediately after cutting, bright red when exposed to oxygen (Fe2+), and undesirable color caused by reduced myoglobin (Fe3+), respectively (Hernández et al., Citation2016). In fact, lipid oxidation affects the a* values, as it causes browning through degradation of myoglobin (Cullere et al., Citation2018; Ünal et al., Citation2014). The redness of meat is also influenced by the types of muscles. For instance, streaks of Longissimus lumborum (LL) had higher redness and metmyoglobin-reducing activity than those of Psoas major (PM) (Canto et al., Citation2016).
The b* values of both treated and untreated minced meat increased during storage at 4 °C for 21 days (). Compared to the untreated sample (53.70%), the treated samples presented a lower increase in b* values, which ranged from 6.64 to 29.28%. Comparable b* values were noted in previous study on chicken breast meat treated with 0.2% oregano EO and 1.2% lactic acid during storage in MAP and oxygen-permeable packaging at 4 °C (Jaspal et al., Citation2021). Similarly, meat preserved with 4% REO-enriched active packaging involving air, vacuum and high O2 experienced an increase in b* values during refrigerated storage (Sirocchi et al., Citation2017). Other authors reported constant b* values for chicken liver meat treated with 0.1% and 0.3% thyme EO throughout 12 days of storage at 4 °C in vacuum packing (Papazoglou et al., Citation2012). The yellow color of EO used in treatments likely contributed to the increase in b* values of meat. According to Jaspal et al. (Citation2021), the yellow color of oregano EO most likely led to the higher b* values in chicken meat. Based on multivariate analysis, Hernández et al. (Citation2016) suggested that oxymyoglobin (OMb) provided a more accurate description of meat b* values.
3.3. Microbial load
displays the microbial load of untreated and REO and BEO-treated minced meat during storage at 4 °C. During all 21 days of storage, the untreated sample displayed an increase in the counts of tested microbes. The REO and BEO treatments significantly reduced the counts of total bacteria, coliforms, and Salmonella (p < 0.05). The total bacterial count (TBC) in the untreated sample reached 8.27 log CFU/g after 7 days, which exceeded the microbial specification for fresh meat (7 log CFU/g) in accordance with previous findings (Zhang et al., Citation2021). However, EO treatment caused a TBC growth delay of 3, 4.82, 3.48, and 4.81 log CFU/g in MR0.5%, MR1.0%, MB0.5%, and MB1.0%, respectively, over a storage period of 21 days. These results were comparable with those of Karakosta et al. (Citation2022), who observed a reduction in total viable counts (TVC) of 3.2 log CFU/g at the 6th day in water buffalo meat treated with 1.25% chitosan + 1.25% Laurus nobilis EO during 18 days of storage at 4 °C.
Table 2. Effects of REO and BEO treatments on total bacteria, coliforms and Salmonella in minced meat during storage at 4 °C.
According to El Abed et al. (Citation2014), minced meat treated with 0.25% and 1.25% T. capitata EO reduced the counts of L. monocytogenes by 4 log CFU/g in just 3 days and by 1.45 and 1.13 log CFU/g, respectively, after 15 days. Furthermore, a significant reduction in TVC was noted after 18 days of storage at 4 °C in study on sliced meat treated with a Plantago major seed-based edible coating containing 1 to 1.5% Tarragon EO. Total coliform growth in the control sample increased from 2.9 to 4.47 log CFU/g over 0 to 21 storage days at 4 °C. On the other hand, coliform growth in MR0.5% was completely inhibited at the end of storage and delayed by 2 log CFU/g at day 14. At the beginning of storage, coliform counts in MR1.0%, MB0.5%, and MB1.0% were 1.62, 2.56, and 2.10 log CFU/g, respectively, and completely inhibited at the end of 21 days.
Comparable results were observed in minced meat preserved with 0.9% Salvia officinalis L. and Salvia sclarea, particularly their combined use, which reduced 1 to 2 log cycles of Enterobacteriaceae over 14 storage days at 4 °C (Ben Akacha et al., Citation2023). Additionally, in spiked minced meat with 1.5 to 2% cumin EO-loaded Lallemantia iberica seed mucilage, the load of coliforms was reduced during storage at 4 °C for 9 days. The growth of Salmonella was observed only on the first day in MR1.0%, MR0.5%, and MB1.0% with values of 27, 2.26, and 2.29 log CFU/g, respectively (). Minced meat treated with 1.5% EO showed absence of bacterial growth throughout the entire storage days except for Salmonella on the first day in MR1.5%.
In a study by Jiyari et al. (2018), minced meat treated with 1% T. capitatus and T. algereinsis and stored at 4 °C for 15 days displayed a reduction of S. typhimurium, which ranged from 4.39 to 4.77 log CFU/g that was initially inoculated at 107 CFU/g of meat. Similar authors reported that, 3% EO treatment completely inhibited the growth of S. typhimurium at day 6 for T. capitatus and at day 12 for T. algereinsis. The antimicrobial properties of EO are influenced by several factors, including their source, compositions, concentrations, nature of microbes and application methods (Ben Akacha et al., Citation2022; Citation2023; El Abed et al., Citation2014; Jiyari et al., 2018). Regarding origin, for instance, T. capitatus EO demonstrated a more pronounced effect than T. algereinsis EO when applied to minced meat at equal concentrations (Jiyari et al., 2018). This could be related with the presence of oxygenated monoterpenes constituents like thymol, carvacrol, and cinnamaldehyde, which possess stronger antimicrobial and antioxidant properties (Gaba et al., Citation2022).
Higher EO concentrations are believed to cause a remarkable damage to cell membranes, which could lead a higher rate of microbial death (El Abed et al., Citation2014; Gutierrez et al., Citation2008; Jiyari et al., 2018). This was evident in treatments MR1.5% and MB1.5% of this study with higher applied EO concentrations. The antimicrobial properties of EO also depend on the nature of microbes, with some having protective layers that limit EO diffusion (G-ve) and others being sensitive (G+) (Ben Akacha et al., Citation2023; El Abed et al., Citation2014; Jiyari et al., 2018). In terms of application, the use of a mixture of EOs and their incorporation in edible coatings may provide better microorganism protection than using separately (Ben Akacha et al., Citation2023). Overall, EO inhibits the growth of microbes by destroying cell structure through a process of diffusion, solubilization of cell membrane, dehydration and eventually death (Burt, Citation2004; Pateiro et al., Citation2021).
3.4. Correlation between parameters
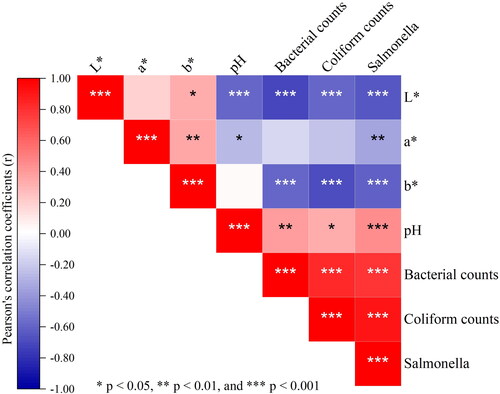
displays the correlation between pH, CIEL*a*b* color parameters, and tested microbial counts. The pH values were negatively associated with L* (r = -0.60) and a* (r = -0.28) values. Similar results were obtained in previous studies on minced meat spiked with an edible coating containing 0.5 to 1.5% tarragon EO and dark, firm and dry (DFD) meat quality attributes (Behbahani et al., Citation2017; Ijaz et al., Citation2020). Conversely, when the freshness indicator was used to monitor meat quality for 72 h at 20 °C, pH showed a significant positive correlation with L* (r = 0.72) and b* (r = 0.59) values (Lee & Shin, Citation2019). These variations could be attributed to treatments used, packaging, and storage conditions. Higher pH in meat enhances the breakdown of myofibrillar and sarcoplasmic protein structures, which makes meat appear darker due to more light transmission into its depth (Ijaz et al., Citation2020; Salueña et al., Citation2019; Swatland, Citation2008).
A positive correlation was obtained between pH values and the counts of total bacteria, coliforms and Salmonella, with r ranging from 0.34 to 0.45. A similar relationship was noted earlier for minced meat preserved with S. officinalis, S. scarlea, and Labularia maritima EOs, acetic acid and chitosan (Behbahani et al., Citation2017; Ben Akacha et al., Citation2023; Karakosta et al., Citation2022). Total bacteria, coliforms, and Salmonella counts were negatively correlated with L*a*b* values with r ranging from -0.35 to -0.71. Extended storage of meat facilitates lipid and protein oxidation, pigment degradation, microbial proliferation and ultimately causes meat discoloration (Behbahani et al., Citation2017). In line with previous work, there was a positive correlation determined between L*a*b* values as well as the tested microbials (Karakosta et al., Citation2022). Correlation analysis between variables may help to predict the properties of the other variables once one is determined.
4. Conclusion
REO and BEO treatments of minced raw meat effectively maintain pH, and color and prevent bacterial growth during 21 days of refrigerated storage. The rate of color changes was lower for essential oil treated samples as compared to the control. Basil essential oils were more successful than rosemary in all tested parameters. The results of Pearson correlation showed that pH had coherent relationships with tested microbial counts and was inversely associated with instrumental colors. The result presented here provides the opportunity to optimize the concentration of essential oil in minced meat preservation in parallel with desirable sensory characteristics. Moreover, in vivo antimicrobial verification and concentration-related applications at small and industrial scales should be subjects of future research.
Author’s contribution
Abdela Befa Kinki: Conceptualization, proposal development, supervision, and editing. Tamene Haile: Laboratory analysis and data management. Tegene Atlaw: Laboratory analysis, data management, formal analysis, original full write up, review and editing. Beriso Meiso: Laboratory analysis. Dessie Belay: Laboratory analysis. Legese Hagos: Methodology, microbial analysis, data management and editing. Fikadu Hailemichael: Microbial analysis. Junaid Abid: review and editing. Ahmed Elawady: Formal Analysis. Nida Firdous: Statically analysis and reviewing editing.
Consent to participate
Corresponding and all the co-authors are willing to participate in this manuscript.
Consent for publication
All authors are willing for publication of this manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
author biography.docx
Download MS Word (291.4 KB)Acknowledgment
The authors are thankful to Ethiopia Institute of Agricultural Research, Ethiopia for providing literature collection facilities, which helped in the study.
Disclosure statement
The authors declare that they have no conflict of interest.
Data availability statement
Even though adequate data has been given in the form of tables and figures, however, all authors declare that if more data is required then the data will be provided on a request basis.
Additional information
Funding
Notes on contributors

Abdela Befa Kinki
Abdela Befa Kinki Food Science and Nutrition Researcher II EIAR, Wondo Genet, Ethiopia. He is actively involved in various research activities related to food processing technology, food quality and nutrition, food safety, as well as medicinal and aromatic plants. Additionally, he serves as the Research Center Process Coordinator, providing laboratory services, training, and consulting.
Tegene Atlaw
Tegene Atlaw is a Food Science and Nutrition Researcher at EIAR, Wondo Genet, Ethiopia. He is engaged in different research activities focusing on food processing technology, food quality and nutrition, food safety, and medicinal and aromatic plants.
References
- Abdo, B. M., Asaminew, G., Mieso, B., & Sisay, W. (2018). Chemotypic characterization and antioxidant activities of Rosemarinus officinalis essential oil from Ethiopian cultivars. Medicinal & Aromatic Plants, 07(06), 1–10. https://doi.org/10.4172/2167-0412.1000325
- Abdollahzadeh, E., Rezaei, M., & Hosseini, H. (2014). Antibacterial activity of plant essential oils and extracts: The role of thyme essential oil, nisin, and their combination to control Listeria monocytogenes inoculated in minced fish meat. Food Control. 35(1), 177–183. https://doi.org/10.1016/j.foodcont.2013.07.004
- Behbahani, B. A., Noshad, M., & Jooyandeh, H. (2020). Improving oxidative and microbial stability of beef using Shahri Balangu seed mucilage loaded with Cumin essential oil as a bioactive edible coating. Biocatalysis and Agricultural Biotechnology, 24, 101563. https://doi.org/10.1016/j.bcab.2020.101563
- Behbahani, B. A., Yazdi, F. T., Shahidi, F., Mortazavi, S. A., & Mohebbi, M. (2017). Principle component analysis (PCA) for investigation of relationship between population dynamics of microbial pathogenesis, chemical and sensory characteristics in beef slices containing Tarragon essential oil. Microbial Pathogenesis, 105, 37–50. https://doi.org/10.1016/j.micpath.2017.02.013
- Ben Akacha, B., Ben Hsouna, A., Generalić Mekinić, I., Ben Belgacem, A., Ben Saad, R., Mnif, W., Kačániová, M., & Garzoli, S. (2023). Salvia officinalis L. and Salvia sclarea essential oils: Chemical composition, biological activities and preservative effects against Listeria monocytogenes inoculated into minced beef meat. Plants, 12(19), 3385. https://doi.org/10.3390/plants12193385
- Ben Akacha, B., Švarc-Gajić, J., Elhadef, K., Ben Saad, R., Brini, F., Mnif, W., Smaoui, S., & Ben Hsouna, A. (2022). The essential oil of Tunisian halophyte Lobularia maritima: A natural food preservative agent of ground beef meat. Life, 12(10), 1571. https://doi.org/10.3390/life12101571
- Ben Hsouna, A., Ben Halima, N., Smaoui, S., & Hamdi, N. (2017). Citrus lemon essential oil: Chemical composition, antioxidant and antimicrobial activities with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Lipids in Health and Disease, 16(1), 146. https://doi.org/10.1186/s12944-017-0487-5
- Burt, S. (2004). Essential oils: their antibacterial properties and potential applications in foods: A review. International Journal of Food Microbiology, 94(3), 223–253. https://doi.org/10.1016/j.ijfoodmicro.2004.03.022
- Canto, A. C. V. C. S., Costa-Lima, B. R. C., Suman, S. P., Monteiro, M. L. G., Viana, F. M., Salim, A. P. A. A., Nair, M. N., Silva, T. J. P., & Conte-Junior, C. A. (2016). Color attributes and oxidative stability of longissimus lumborum and psoas major muscles from Nellore bulls. Meat Science, 121, 19–26. https://doi.org/10.1016/j.meatsci.2016.05.015
- Cullere, M., Dalle Zotte, A., Tasoniero, G., Giaccone, V., Szendrő, Z., Szín, M., Odermatt, M., Gerencsér, Z., Dal Bosco, A., & Matics, Z. (2018). Effect of diet and packaging system on the microbial status, pH, color and sensory traits of rabbit meat evaluated during chilled storage. Meat Science, 141, 36–43. https://doi.org/10.1016/j.meatsci.2018.03.014
- Doolaege, E. H., Vossen, E., Raes, K., De Meulenaer, B., Verhé, R., Paelinck, H., & De Smet, S. (2012). Effect of rosemary extract dose on lipid oxidation, colour stability and antioxidant concentrations, in reduced nitrite liver pâtés. Meat Science, 90(4), 925–931. https://doi.org/10.1016/j.meatsci.2011.11.034
- El Abed, N., Kaabi, B., Smaali, M. I., Chabbouh, M., Habibi, K., Mejri, M., Marzouki, M. N., & Ben Hadj Ahmed, S. (2014). Chemical composition, antioxidant and antimicrobial activities of Thymus capitata essential oil with its preservative effect against Listeria monocytogenes inoculated in minced beef meat. Evidence-Based Complementary and Alternative Medicine, 2014, 1–11. https://doi.org/10.1155/2014/152487
- Erol, N. D., Erdem, Ö. A., Yilmaz, S. T., Kayalar, H., & Cakli, S. (2022). Effects of the BHA and basil essential oil on nutritional, chemical, and sensory characteristics of sunflower oil and sardine (Sardina pilchardus) fillets during repeated deep-frying. LWT, 163, 113557. https://doi.org/10.1016/j.lwt.2022.113557
- Fratianni, F., De Martino, L., Melone, A., De Feo, V., Coppola, R., & Nazzaro, F. (2010). Preservation of chicken breast meat treated with thyme and balm essential oils. Journal of Food Science, 75(8), M528–M535. https://doi.org/10.1111/j.1750-3841.2010.01791.x
- Gaba, A. B. M., Hassan, M. A., Abd EL-Tawab, A. A., Abdelmonem, M. A., & Morsy, M. K. (2022). Protective impact of chitosan film loaded oregano and thyme essential oil on the microbial profile and quality attributes of beef meat. Antibiotics, 11(5), 583. https://doi.org/10.3390/antibiotics11050583
- Gómez-Estaca, J., De Lacey, A. L., López-Caballero, M. E., Gómez-Guillén, M. C., & Montero, P. (2010). Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiology, 27(7), 889–896. https://doi.org/10.1016/j.fm.2010.05.012
- Guerrero, A., Ferrero, S., Barahona, M., Boito, B., Lisbinski, E., Maggi, F., & Sañudo, C. (2020). Effects of active edible coating based on thyme and garlic essential oils on lamb meat shelf life after long-term frozen storage. Journal of the Science of Food and Agriculture, 100(2), 656–664. https://doi.org/10.1002/jsfa.10061
- Gutierrez, J., Barry-Ryan, C., & Bourke, P. (2008). The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. International Journal of Food Microbiology, 124(1), 91–97. https://doi.org/10.1016/j.ijfoodmicro.2008.02.028
- Hashemi Gahruie, H., Hosseini, S. M. H., Taghavifard, M. H., Eskandari, M. H., Golmakani, M. T., & Shad, E. (2017). Lipid oxidation, color changes, and microbiological quality of frozen beef burgers incorporated with shirazi thyme, cinnamon, and rosemary extracts. Journal of Food Quality, 2017, 1–9. https://doi.org/10.1155/2017/6350156
- Hernández, B., Sáenz, C., Alberdi, C., & Diñeiro, J. M. (2016). CIELAB color coordinates versus relative proportions of myoglobin redox forms in the description of fresh meat appearance. Journal of Food Science and Technology, 53(12), 4159–4167. https://doi.org/10.1007/s13197-016-2394-6
- Hussain, A. I., Anwar, F., Sherazi, S. T. H., & Przybylski, R. (2008). Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chemistry, 108(3), 986–995. https://doi.org/10.1016/j.foodchem.2007.12.010
- Hyldgaard, M., Mygind, T., & Meyer, R. L. (2012). Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Frontiers in Microbiology, 3, 12. https://doi.org/10.3389/fmicb.2012.00012
- Ijaz, M., Li, X., Zhang, D., Hussain, Z., Ren, C., Bai, Y., & Zheng, X. (2020). Association between meat color of DFD beef and other quality attributes. Meat Science, 161, 107954. https://doi.org/10.1016/j.meatsci.2019.107954
- Jaspal, M. H., Ijaz, M., Haq, H. A. U., Yar, M. K., Asghar, B., Manzoor, A., Badar, I. H., Ullah, S., Islam, M. S., & Hussain, J. (2021). Effect of oregano essential oil or lactic acid treatments combined with air and modified atmosphere packaging on the quality and storage properties of chicken breast meat. LWT, 146, 111459. https://doi.org/10.1016/j.lwt.2021.111459
- Jayari, A., El Abed, N., Jouini, A., Mohammed Saed Abdul-Wahab, O., Maaroufi, A., & Ben Hadj Ahmed, S. (2018). Antibacterial activity of Thymus capitatus and Thymus algeriensis essential oils against four food-borne pathogens inoculated in minced beef meat. Journal of Food Safety, 38(1), e12409. https://doi.org/10.1111/jfs.12409
- Karakosta, L. K., Vatavali, K. A., Kosma, I. S., Badeka, A. V., & Kontominas, M. G. (2022). Combined effect of chitosan coating and Laurel essential oil (Laurus nobilis) on the microbiological, chemical, and sensory attributes of water Buffalo meat. Foods (Basel, Switzerland), 11(11), 1664. https://doi.org/10.3390/foods11111664
- Kondjoyan, A., Sicard, J., Cucci, P., Audonnet, F., Elhayel, H., Lebert, A., & Scislowski, V. (2022). Predicting the oxidative degradation of raw beef meat during cold storage using numerical simulations and sensors prospects for meat and fish foods. Foods (Basel, Switzerland), 11(8), 1139. https://doi.org/10.3390/foods11081139
- Krichen, F., Hamed, M., Karoud, W., Bougatef, H., Sila, A., & Bougatef, A. (2020). Essential oil from pistachio by-product: Potential biological properties and natural preservative effect in ground beef meat storage. Journal of Food Measurement and Characterization, 14(6), 3020–3030. https://doi.org/10.1007/s11694-020-00546-6
- Lee, E. J., & Shin, H. S. (2019). Development of a freshness indicator for monitoring the quality of beef during storage. Food Science and Biotechnology, 28(6), 1899–1906. https://doi.org/10.1007/s10068-019-00633-5
- Mieso, B., Befa, A., & Haile, T. (2022). Effect of drying methods and drying days on essential oil content and physicochemical properties of basil (Ocimum basilicum) varieties in Ethiopia. Trends Journal of Sciences Research, 1(1), 1–11. https://doi.org/10.31586/ujfst.2022.487
- Murbach Teles Andrade, B. F., Nunes Barbosa, L., da Silva Probst, I., & Fernandes Júnior, A. (2014). Antimicrobial activity of essential oils. Journal of Essential Oil Research, 26(1), 34–40. https://doi.org/10.1080/10412905.2013.860409
- Papazoglou, S., Tsiraki, M., & Savvaidis, I. N. (2012). Effect of thyme oil on the preservation of vacuum-packaged chicken liver. Journal of Food Science, 77(8), M473–480. https://doi.org/10.1111/j.1750-3841.2012.02823.x
- Pateiro, M., Munekata, P. E., Sant’Ana, A. S., Domínguez, R., Rodríguez-Lázaro, D., & Lorenzo, J. M. (2021). Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. International Journal of Food Microbiology, 337, 108966. https://doi.org/10.1016/j.ijfoodmicro.2020.108966
- Radha Krishnan, K., Babuskin, S., Azhagu Saravana Babu, P., Sasikala, M., Sabina, K., Archana, G., Sivarajan, M., & Sukumar, M. (2014). Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. International Journal of Food Microbiology, 171, 32–40. https://doi.org/10.1016/j.ijfoodmicro.2013.11.011
- Salueña, B. H., Gamasa, C. S., Rubial, J. M. D., & Odriozola, C. A. (2019). CIELAB color paths during meat shelf life. Meat Science, 157, 107889.
- Seema, P. (2015). A review of plant essential oils and allied volatile fractions as multifunctional additives in meat and fish-based food products. Food Additives & Contaminants: Part A, 32(7), 1049–1064.
- Sirocchi, V., Devlieghere, F., Peelman, N., Sagratini, G., Maggi, F., Vittori, S., & Ragaert, P. (2017). Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chemistry, 221, 1069–1076. https://doi.org/10.1016/j.foodchem.2016.11.054
- Stojanović-Radić, Z., Pejčić, M., Joković, N., Jokanović, M., Ivić, M., Šojić, B., Škaljac, S., Stojanović, P., & Mihajilov-Krstev, T. (2018). Inhibition of Salmonella Enteritidis growth and storage stability in chicken meat treated with basil and rosemary essential oils alone or in combination. Food Control. 90, 332–343. https://doi.org/10.1016/j.foodcont.2018.03.013
- Swatland, H. J. (2008). How pH causes paleness or darkness in chicken breast meat. Meat Science, 80(2), 396–400. https://doi.org/10.1016/j.meatsci.2008.01.002
- Tian, T., Kang, Y., Liu, L., & Wang, X. (2022). The effect of super-chilled preservation on shelf life and quality of beef during storage. Food Science and Technology, 42 https://doi.org/10.1590/fst.73222
- Tongnuanchan, P., & Benjakul, S. (2014). Essential oils: extraction, bioactivities, and their uses for food preservation. Journal of Food Science, 79(7), R1231–1249. https://doi.org/10.1111/1750-3841.12492
- Ünal, K., Babaoglu, A. S., & Karakaya, M. (2014). Effect of oregano, sage and rosemary essential oils on lipid oxidation and color properties of minced beef during refrigerated storage. Journal of Essential Oil Bearing Plants, 17(5), 797–805. https://doi.org/10.1080/0972060X.2014.956803
- Vlaicu, P. A., Untea, A. E., Panaite, T. D., Saracila, M., Turcu, R. P., & Dumitru, M. (2023). Effect of basil, thyme and sage essential oils as phytogenic feed additives on production performances, meat quality and intestinal microbiota in broiler chickens. Agriculture, 13(4), 874. https://doi.org/10.3390/agriculture13040874
- Wang, X., Ding, Y., Tian, T., & Liu, Y. (2022). Comparative efficacy of sodium lactate and Natamycin against discoloration and spoilage of fresh beef during chilled storage. Food Science and Technology, 42, e74421. https://doi.org/10.1590/fst.74421
- Young, O. A., West, J., Hart, A. L., & Van Otterdijk, F. F. H. (2004). A method for early determination of meat ultimate pH. Meat Science, 66(2), 493–498. https://doi.org/10.1016/S0309-1740(03)00140-2
- Zhang, B., Liu, Y., Wang, H., Liu, W., Cheong, K. L., & Teng, B. (2021). Effect of sodium alginate-agar coating containing ginger essential oil on the shelf life and quality of beef. Food Control. 130, 108216. https://doi.org/10.1016/j.foodcont.2021.108216