?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Biometric measurement provide crucial information on fish growth and aquatic habitat well-being. This study offers the first comprehensive elucidation on biometric indices and reproductive biology of Cyprinus carpio var. communis in Lake Arekit (Ethiopia). A total of 209 fish were collected (ranging between 8.8 and 51.3 cm in total length (TL), while 16.99–2,498.3 g total weight (TW)) using gillnets during June 2022 to May 2023. The overall sex ratio (male, M; female, F) was 1:0.66 and significantly varied from the hypothetical sex ratio (1:1) (χ2 = 8.85, p < 0.05). The length-weight relationship of C. carpio var. communis was TW = 0.0392 × TL2.792 with a ‘b’ value of 2.792, which indicated a negative allometric growth pattern with r2 values of 0.99. The condition factor of C. carpio showed significant variations between sex and months, with a mean value of 4.01 ± 0.49 (p < 0.05) while the Fulton condition factor showed no significant difference between sexes and months. Fecundity variation was better correlated with length (AF = 152.06 × TL1.879, r2 = 0.795; p < 0.05) than with weight (AF = 1541.2 × TW0.651, r2 = 0.6523; p < 0.05). The fecundity ranged between 46,214 and 210,271 eggs/fish, with a mean of 127,516.6 ± 53,447.1 eggs/fish. Breeding activity peaks in March and June, coinciding with a rise in water temperature. The size at first maturity (Lm50) was found to be 32.28 cm and 28.83 cm of total length for females and males, respectively. Our findings would be very beneficial in planning the sustainable management of C. carpio in Lake Arekit and nearby ecosystems.
1. Introduction
The common carp (Cyprinus carpio), a freshwater fish, has been successfully introduced into freshwaters worldwide (Yaqoob, Citation2021). The C. carpio, first introduced to Ethiopian waterways in 1936 for aquaculture (Welcome, Citation1988), has shown fantastic adaptation in various reservoirs and natural lakes, and it currently constitutes an important fishery (Kebede et al., Citation2018). The widespread dispersion and success of C. carpio introductions are largely attributable to their adaptability to various environmental circumstances (Tesfaye & Wolff, Citation2014). Even though C. carpio has been widely introduced into Ethiopian waterbodies, there is scanty knowledge of its growth pattern and reproductive biology in natural waterbodies (Mekonnen et al., Citation2019; Tessema et al., Citation2020).
According to Tessema et al. (Citation2020), the relationship between length and weight is crucial to fish biology, physiology, and ecology. Tesfaye and Wolff (Citation2014) claim that it is possible to compare fish life cycles across geographic locations, species, and populations using information on length and weight. According to Syed et al. (Citation2020) and El-Aiatt (Citation2021), fisheries biologists frequently employ an index to gauge the ‘well-being’ of fish. This index may be calculated using length-weight relationships. According to Tesfaye and Wolff (Citation2014), an essential aspect of fishery biology utilized to evaluate the general health of populations is fish condition. The condition factor also provides information when contrasting two populations that are living in specific feeding, density, and climatic conditions; when determining the period of gonadal maturation; and when monitoring a species’ level of feeding activity to determine whether it is effectively utilizing its feeding source (Mekonnen et al., Citation2019; Tessema et al., Citation2020). To maximize fishery production, it is crucial to discover the environmental determinants of this variance because fish conditions might vary both within and between populations.
In order to manage fisheries effectively and to bridge the knowledge gap in fisheries basic sciences, it is crucial to understand fish reproductive biology (Tessema et al., Citation2020; Wagaw et al., Citation2022). Since these traits may differ regionally, it is crucial to identify the reproductive traits of fish in each environment for effective fisheries management (Dereli et al., Citation2022). Fish species differ greatly in terms of fecundity, even among individuals of the same species, size, and range (Shinkafi & Ipinjolu, Citation2012). This may be due to the population’s members’ varying success at feeding before spawning (Tessema et al., Citation2020), as well as the release of the eggs in batches (Shafat et al., Citation2016). Fecundity can vary depending on species (Mohamad et al., Citation2020), body size, gonadal weight, age classes, and other factors (Kebede et al., Citation2018). Fecundity and gonado somatic index, two crucial demographic traits necessary for comprehending a species’ life history, are critical components of reproduction (Shafat et al., Citation2016).
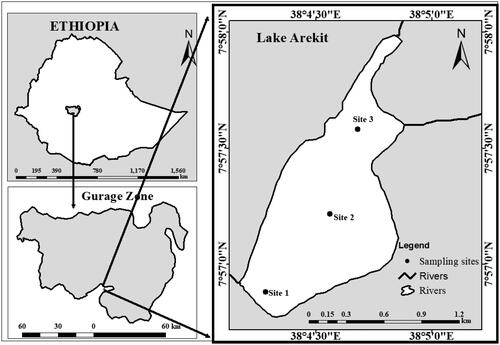
Several studies on the reproductive biology of C. carpio have been undertaken in several water bodies in Ethiopia, including Hailu (Citation2013) in Amerti Reservoir, Abera et al. (Citation2015) in Lake Ziway, Mekonnen et al. (Citation2019) in Lake Lugo, and Tessema et al. (Citation2020) in Lake Hayq. Cyprinus carpio is introduced in Lake Arekit, situated in the Gurage Zone, Ethiopia (). There has never been research done on the biometric index and reproductive biology of C. carpio, yet this lake is a significant habitat and fishing location for the species. In order to enable sustainable fishery exploitation, this study sought to provide knowledge on a few aspects of reproductive biology and environmental factors related to the reproductive pattern for the species of C. carpio in Lake Arekit, Ethiopia, including the length-weight relationship, sex ratio, Fulton’s condition factor, fecundity, and seasons of spawning.
2. Materials and methods
2.1. Study area
Lake Arekit is situated at 370°53’30" and 380°10’00" E and 70°59’30" and 80°16’00" N in Ethiopia. It is found at an elevation of 2820–2950 meters above sea level. The lake’s surface area is 130 ha, and its average depth is 2.5 m (). This lake is located in the Central Ethiopia Regional State, 220 km from Addis Ababa and 70 km from Wolkite. The lake doesn’t have an outlet and receives its water from adjacent watersheds and direct rainfall. According to Enawgaw and Wagaw (Citation2023), Lake Arekit has an alkaline pH range of 7.29–11.31, dissolved oxygen concentrations of 6.8–16.7 mg/L, a temperature range of 25.8–29.8 °C, turbidity readings of 154 NTU to 317 NTU, and a water transparency range of 4.3–16.1 cm. The lake also has significant levels of inorganic nutrients, including phosphate, which ranges from 2.12 mg/L to 5.26 mg/L, and nitrate, which varies from 2.19 mg/L to 10.64 mg/L (Enawgaw & Wagaw Citation2023).
The lake provides irrigation services to the community (Tilahun et al., Citation2022). Lake Arekit also supports phytoplankton, dominated by Cyanophyta and Bacillariophyta such as Microcystis aeruginosa, Cylindrospermopsis raphidiopsis, Anabaena spiroides, Pediastrum duplex, Aulacoseira granulate, Navicula schroeteri, and Nitzschia palea (Enawgaw & Wagaw, Citation2023). The lake is also known for its outstanding avifauna diversity, with mainly Blue-Winged Goose (Cyanochen cyanoptera) (endemic), White-collared Pigeon (Columba albitorques), Thick-billed Raven (Corvus crassirostris), and Wattled Ibis (Bostrychia carunculata) (endemic to both Ethiopia and Eritrea) inhabiting the lake (Tilahun et al., Citation2022). In Lake Arekit, the sole fish species present is the common carp (Cyprinus carpio var. communis).
2.2. Sample collection and data analysis
For this study, 209 fish specimens were collected monthly from three different sites between June 2022 and May 2023 in Lake Arekit. Fish samples were transported to the Wolkite University Biology Laboratory for the measurement of total length (TL) to the nearest 0.1 cm and total weight (TW) to the nearest 0.1 g. The Cyprinus carpio var. communis sex determination was made by macroscopic gonadal examination according to the shape and color of gonads (Bagenal, Citation1978).
2.2.1. Sex ratio
The sex ratio was determined, in accordance with Geffroy and Wedekind (Citation2020), by contrasting the proportions of the two sexes in the fish population.
Where X = sex ratio; M = number of male fish; F = number of female fish.
The difference in population sex ratio was examined using chi-square (χ2) analysis.
Where Oi is the observed value and Ei is the expected value.
2.2.2. Length-weight relationship
The allometric equation TW = a × TLb (Ricker, Citation1975) was used to determine the relationship between total length (TL) and total weight (TW) for the entire sample and separately for each sex. This equation can be translated into logarithmic form as follows: Log TW = Log a + b × Log TL, where TW and TL are the total weight and length, respectively, and ‘a’ and ‘b’ are the regression constants.
A Student’s t-test was used to analyze the b value. According to b values, the relationship adopts an isometric growth pattern (b = 3.0), a negative allometric growth pattern (b < 3.0), or a positive allometric growth pattern (b > 3.0) (Tesfaye & Wolff, Citation2014).
2.2.3. Condition factor (CF) and fulton condition factor (FCF)
Each fish’s Condition Factor (CF) was determined using the formula CF = TW/TLb × 100, where CF stands for condition factor, TW for total weight (g), TL for total length (cm), and ‘b’ for the coefficient of allometry value determined using the length-weight equation (Bagenal Citation1978). The formula used to compute the Fulton Condition Factor (FCF) is as follows: FCF = TW/TL3 × 100, where FCF stands for Fulton Condition Factor, TW stands for Total Weight (g), TL stands for Total Length (cm), and ‘3’ is for ideal fish growth (isometric). It is important to highlight that this parameter was assessed every month for the entire population and separately for each sex. The analysis of variance (ANOVA) test was used to statistically test the condition factor (CF) and Fulton condition factor (FCF) with a 95% confidence level.
2.2.4. Spawning season and fecundity
The stages of gonad development were categorized as follows: stage 0-immature; stage 1-resting; stage 2-developing; stage 3-ripe; stage 4-running; stage 5-spent; and stage 6-recovering (Bagenal, Citation1978). The spawning season is determined following the monthly fluctuations of the gonad somatic index (GSI), calculated using the following formula:
(Peña-Mendoza et al., Citation2005)
2.2.5. Length at sexual maturity (Lm50)
The length at which 50% of both sexes reached maturity (Lm50) was determined by modelling the proportion of mature individuals to their respective length classes based on the following logistic function (Echeverria, Citation1987):
Where P = percentage of mature fish by length class, L = total length class, a and b are model coefficients. This analysis allows the estimation of the 95% confidence intervals for Lm50. The Lm50 was estimated using the equation:
Data were analyzed using ‘Solver’ in the Microsoft Excel program (Tokai, Citation1997).
3. Results
3.1. Sex ratio
During the sampling period, 209 Cyprinus carpio var. communis were caught, ranging from 8.8 to 51.3 cm in total length and 16.99 to 2,498.3 g in total body weight, with 126 males (60.29%) and 83 females (39.71%). The male-to-female sex ratio (1:0.66) differed significantly from the ideal male-to-female sex ratio (1:1) (χ2 = 8.85, p < 0.05) ().
Table 1. Summary of monthly sex ratio (males: females) of Cyprinus carpio var. communis in Lake Arekit.
3.2. Length-weight relationships
The values of 0.99 for the determinant coefficients (r2) for combined sexes, females and males, were highly significant. A b-value less than 3 in the length-weight regression equation for C. carpio var. communis in Lake Arekit indeed indicates negative allometric growth for the combined sexes (2.792), females (2.794), and males (2.789) (; ).
Figure 2. Length-weight relationship of female (a), male (b) and combined sexes (c) of Cyprinus carpio var. communis in Lake Arekit.
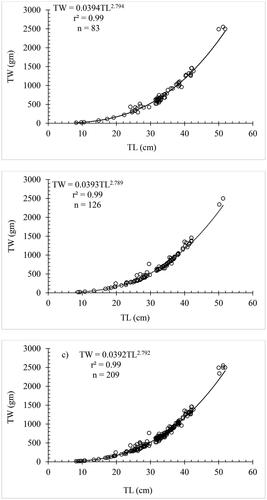
Table 2. Length-weight relationship of Cyprinus carpio var. communis from Lake Arekit from June 2022 to May 2023.
3.2. Condition factor (CF)
The variance in the mean CF of C. carpio var. communis in Lake Arekit is shown in . With a mean of 4.01, the CF of C. carpio var. communis ranged from 3.29 to 6.47. The CF for males and females ranged from 3.29 to 5.80 and 3.38 to 6.47, respectively (). The CF in C. carpio var. communis exhibited significant variations between males and females (p < 0.05). For male C. carpio var. communis, the Fulton Condition Factor (FCF) values ranged from 1.63 to 3.18, with a mean value of 1.89. The FCF values for female C. carpio var. communis ranged from 1.59 to 3.11, with a mean value of 1.93. The mean FCF value for C. carpio var. communis’s combined sexes was 1.91 ± 0.2. The difference in mean FCF between male and female C. carpio var. communis in Lake Arekit was not statistically significant (p > 0.05).
Table 3. The mean Condition Factor and Fulton Condition Factor of C. carpio var. communis from Lake Arekit from June 2022 to May 2023.
The results of the analysis of variance (ANOVA) between the monthly Condition Factor (CF) mean values revealed a significant difference (p = 0.014) (). shows that the mean monthly condition factor for C. carpio var. communis in Lake Arekit varies from 3.77 to 4.38. However, the mean monthly values of the Fulton Condition Factor (FCF) were not significantly different (ANOVA, p > 0.05) and varied from 1.84 ± 0.13 in June to a peak of 2.04 ± 0.44 in December ().
Table 4. Temporal mean Condition Factor (CF) and Fulton Condition Factor (FCF) of C. carpio var. communis from Lake Arekit from June 2022 to May 2023.
3.3. Gonado somatic index (GSI)
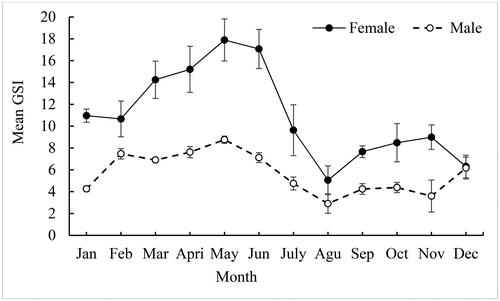
Male and female C. carpio var. communis followed roughly the same pattern, according to monthly fluctuations in the Gonado-Somatic Index (GSI) (). The mean monthly GSI values in females ranged from 5.05 to 17.07 and displayed significant monthly variations (p < 0.05). Female GSI values peaked between March and the end of June (). This suggests that C. carpio var. communis breeds in the lake during the minor rainy season and up until the start of the major rainy season. While the GSI value of male C. carpio var. communis ranged from 3.6 to 8.76 with a mean of 5.68 ± 4.01 () and exhibits significant variations between months (p < 0.05).
3.4. Fecundity
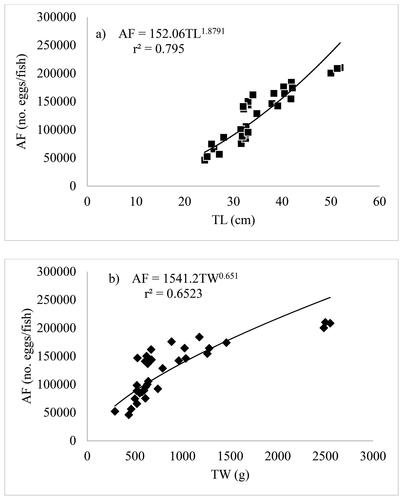
Thirty-four (34) mature females of C. carpio var. communis were examined for fecundity. The total length of the measured C. carpio var. communis’s varied from 24.1 cm to 51.9 cm, and its weight ranged from 290.5 g to 2,498.5 g. The total fecundity varied between 46,214 and 210,271 eggs/fish, with a mean absolute fecundity (AF) of 127,516.6 ± 53,447.1 eggs/fish. The relationship between total body length, weight, and absolute fecundity was positively correlated; the fecundity-length relationship was AF = 152.06 × TL1.879 (r2 = 0.795), and the fecundity-total weight relationship was AF = 1541.2 × TW0.651 (r2 = 0.652) ().
3.5. Length at sexual maturity (Lm50)
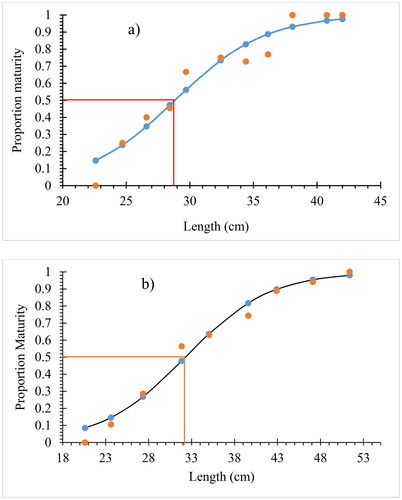
The size at first maturity of the fish in the current study showed a significant difference between sexes (p < 0.05). Male C. carpio var. communis reached sexual maturity at a size of 28.83 cm, whereas females did so at a size of 32.28 cm (). The smallest male with ripe gonads was 21.5 cm and weighed 196.8 g, whereas the comparable female was 24.1 cm and weighed 438.4 g.
4. Discussion
4.1. Sex ratio
This study, which compared the findings with those of the same fish species in other regions, is regarded as the first to examine the biological traits of C. carpio var. communis in Lake Arekit (Ethiopia). The results of the current investigation revealed that there were significantly more male C. carpio var. communis than females, with a sex ratio of 1:0.66 (Male: Female). The findings concur with Nyaboke et al. (Citation2014) who report a sex ratio of 1.63:1 (Male: Female) for C. carpio in Lake Naivasha (Kenya). According to Mert and Bulut (Citation2014), C. carpio captured at Turkey’s Damsa Dam Lake had a sex ratio that was skewed in favors of males. However, populations of the same species in Amerti Reservoir (Hailu, Citation2013), Lake Ziway (Abera et al., Citation2015), and Lake Langeno (Kebede et al., Citation2018) (Ethiopia) had been noted to have more females than males. The imbalance in the ratio of male to female may result from behavioral differences between the sexes, which make one sex easier to catch than the other, or from differences in habitat preference brought on by variations in sexual maturity stages over the spawning season (Wagaw et al., Citation2022). Additionally, there are a number of other potential causes for differences in sex ratio distribution, including temperature-dependent sex determination (Mert & Bulut, Citation2014), the presence of hormone-like substances in the environment (Bohlen & Ritterbusch, Citation2000), sex-selective mortality based on predation (Quinn & Buck, Citation2001), or intersexual differences in life span or behavior (Passos et al., Citation2014).
4.2. Length-weight relationships (LWR)
The LWR for C. carpio var. communis in the current study has a stronger correlation coefficient (r2 = 0.99), which suggests that length increases with weight. This finding is consistent with previous research on C. carpio from lakes and reservoirs in Ethiopia (Hailu, Citation2013; Kebede et al., Citation2018; Mekonnen et al., Citation2019; Tessema et al., Citation2020). Since the ‘b’ values of C. carpio populations residing in various localities in Ethiopia and some other countries ranged from 1.9455 to 3.423 (), the parameters of the length-weight relationship of C. carpio var. communis in Lake Arekit are comparable to those of the earlier research (). According to our findings, the exponent ‘b’ of the length-weight relationship for male, female, and both sexes of C. carpio exhibited negative allometry growth (b < 3), which deviated significantly from the ideal isometric growth pattern (b = 3). Previous research indicates that C. carpio showed a similar negative allometric growth pattern in Amerti Reservoir (Hailu, Citation2013) and Lake Ardibbo (Asnake & Mingist, Citation2018) (Ethiopia), with ‘b’ values of 2.92 and 2.82, respectively. But El Moata et al. (Citation2005) and Karataş et al. (Citation2007) noted positive allometric growth for C. carpio, reporting ‘b’ values of 3.423 and 3.32, respectively. The variations in growth pattern (b-value) could be related to differences in age, maturity, sex, stomach fullness, disease, parasite infestation and associated environmental factors such as seasonality (Wagaw et al., Citation2022).
Table 5. Length-weight relationship for C. carpio of the previous studies.
4.3. Condition Factor and Fulton Condition Factor (FCF)
According to Ricker (Citation1975), the fish population’s Condition Factor (CF) values alter depending on factors such as sex, gonad development, age, growth, seasonal fluctuations, and feeding conditions. In the current investigation, there were statistically significant differences in the CF between the sexes and months (p < 0.05, t-test) ( and ). For females, the mean CF was computed as 4.010 ± 0.49 (ranged from 4.33 to 6.47) and for males, 3.80 ± 0.36 (ranged from 3.29 to 5.80) (). In terms of mean CF, females outperformed males, and the differences were statistically significant (p < 0.05, ). The higher condition factor for the females could be attributed to high gonad maturity and high fat accumulation. Further, metabolic stress during maturation or spawning, as well as changes in feeding activity, may be responsible for the difference in CF values between females and males (Jonsson & Jonsson, Citation2014). In numerous lakes, C. carpio has been shown to have a similar condition (Karataş et al., Citation2007; Hailu, Citation2013; Tessema et al., Citation2020). The changes in the mean CF between the months in the current investigation were statistically significant (p < 0.05, ). In December and May, the maximum mean condition factor was computed as 4.38 and 4.33, respectively (). Similar results were observed by Sara et al. (Citation2016) and Sahtout et al. (Citation2017), who discovered that the winter and summer seasons had the greatest values of the condition factor. These temporal variations in the CF of C. carpio var. communis in Lake Arekit may be related to the availability of food consumed during various months, the degree of gut fullness, and other environmental factors (Tessema et al., Citation2020; Wagaw et al., Citation2022).
It is evident that the mean FCF of the C. carpio var. communis in Lake Arekit is greater than the FCF at the Foum El-khanga Dam (FCF = 1.57) (Sahtout et al., Citation2017), Damsa Dam Lake (FCF = 1.58) (Mert & Bulut, Citation2014), Lake Hayq (FCF = 1.22) (Tessema et al., Citation2020), and Amerti Reservoir (FCF = 1.22) (Hailu, Citation2013). The average FCF value, however, was less than that reported for C. carpio in the Guadalquivir River in southwest Spain (FCF = 1.94) (Fernández-Delgado, Citation1990). The mean condition factor has been employed as a crucial instrument in signaling health, growth, and feeding intensity (Bagenal, Citation1978; Froese, Citation2006). This has contributed to the understanding that Lake Arekit offers favourable conditions for the growth of the C. carpio var. communis population.
Between sexes and months, the Fulton Condition Factor (FCF) did not show any statistically significant differences in the current investigation (p > 0.05) ( and ). The mean FCF for females (1.93 ± 0.26) was higher than that for males (1.89 ± 0.20), but the differences between sexes were not significant (p > 0.05, ). The population’s FCF was assessed to be 1.91 ± 0.2 and ranged between 1.84 ± 0.13 (in June) and 2.04 ± 0.244 (in December). Between-month variations in the mean FCF were not statistically significant (p > 0.05, ). The present findings are supported by those of Tessema et al. (Citation2020), who found no statistically significant difference in the Fulton Condition Factor (FCF) of C. carpio between sexes and months in Lake Hayq. However, this condition factor may be impacted by factors such as sex, age, species type, maturity, body fat buildup, and environmental factors (Wagaw et al., Citation2022).
4.4. Gonado Somatic Indexes (GSI)
Gonado-Somatic Indexes (GSI) demonstrated in the current investigation that C. carpio var. communis reproduces year-round in Lake Arekit. According to Kebede et al. (Citation2018), the tropics often have very little seasonal variation in water temperature and photoperiod, which makes it easier for many fish species to spawn throughout the year. All fish species, however, have distinct peak times for mating throughout the year that are largely influenced by food supply and water temperature (Durant et al., Citation2007). In the present study, the gonado somatic index of C. carpio var. communis from Lake Arekit showed a significant variation during seasonal fluctuations (p < 0.05). According to Sahtout et al. (Citation2017), the gonado somatic index is considered an indirect tool for estimating a species’ spawning season. The GSI was low in both males and females of C. carpio var. communis throughout the late summer months of July, August, September, October, November, and December. Due to their larger gonads, females had higher GSI values than males. Males have lower gamete production energy expenditure than females, which accounts for their lower GSI values (Tessema et al., Citation2020).
Cyprinus carpio spawning starts when the water temperature reaches about 18 °C (Ouellet et al., Citation2021). Higher GSI values were seen for both males and females in the current study between the months of March and June. Males and females showed peak GSI values of 8.76 and 17.89, respectively, which were statistically significant (p < 0.05). According to Enawgaw and Wagaw (Citation2023), this may be related to Lake Arekit’s higher water temperature readings of 27.36 °C. Similar to this, it has been reported that the peak breeding season for C. carpio occurs between February and April in Amerti Reservoir (Hailu, Citation2013), March and July in Fincha Reservoir (Degefu et al., Citation2012), and February and April in Lake Hayq (Tessema et al., Citation2020). However, the average water temperature in Lake Arekit, which varied from 25.8 °C to 29.8 °C throughout the study period, seems to favor year-round spawning of C. carpio var. communis. Other Ethiopian lakes and reservoirs have recorded C. carpio breeding all year round (Degefu et al., Citation2012; Hailu, Citation2013; Kebede et al., Citation2018; Tessema et al., Citation2020).
4.5. Fecundity
The fecundity of C. carpio var. communis in Lake Arekit ranged from 46,214 to 210,271 mature eggs/fish, with a mean absolute fecundity (AF) of 127,517 ± 53,447 eggs/fish, corresponding to fish total lengths of 24.1 and 51.9 cm. This finding deviates from earlier research on the fecundity of C. carpio in different Ethiopian water bodies. According to Hailu (Citation2013), C. carpio fecundity ranged from 36,955 to 318,584 and had a mean of 170,937 eggs/fish in Amerti Reservoir, and for C. carpio in Lake Ziway, fecundity ranged from 75,645 to 356,745 with a mean of 210,538 eggs/fish (Abera et al., Citation2015). The estimated fecundity, however, is greater than that found in earlier studies for the same species in Lake Langano (Kebede et al., Citation2018) and Lake Hayq (Tessema et al., Citation2020). According to Smith (Citation2004), depending on their body size, C. carpio can produce between 500,000 to 3 million eggs per individual spawning. Significant variations in fecundity between individuals of the same species in various bodies of water frequently indicate various reproductive methods. Fecundity may differ within a species due to various environmental habitat adaptations (Blanck & Lamouroux, Citation2007). According to Usman et al. (Citation2015), fecundity is better correlated with temperature change. Additionally, the variation in fecundity may be explained by the varying food abundances among the population’s members (Peña-Mendoza et al., Citation2005). Also, Tsadik and Bart (Citation2007) noted that fecundity increased with higher feeding levels. Fecundity variation in the current study had a stronger correlation with overall length (r2 = 0.795, p < 0.05) than with total weight (r2 = 0.652, p < 0.05). The absolute fecundity increased in Lake Langano (Kebede et al., Citation2018) and Dal Lake (Mohamad et al., Citation2020) in a direct proportion to increases in the weight and length of C. carpio.
4.6. Length at sexual maturity (Lm50)
Males of the C. carpio var. communis species matured earlier than females in the current study. Other studies also support the idea that males can mature at smaller sizes than females (Degefu et al., Citation2012; Mutethya et al., Citation2020; Tessema et al., Citation2020) (). In the present study, 50% of C. carpio var. communis were sexually mature at lengths of 32.28 cm for females and 28.83 cm for males. According to Degefu et al. (Citation2012) and Abera et al. (Citation2015), respectively, 50% of C. carpio individuals from Fincha reservoir and Lake Ziway had total lengths of 24.5 and 37.5 and 27.2 and 28.7 cm for males and females, respectively (). But in Lake Marmara, individuals mature sexually (Lm50) at a total length of 39.6 cm (male) and 43.7 cm (females) (Dereli et al., Citation2022), 54.0 cm (males), and 49.0 cm (females) (Mutethya et al., Citation2020). According to Wagaw et al. (Citation2022), the typical abiotic as well as biotic variables in these areas, such as water temperature, salinity, photoperiod, or amount of available food, can be responsible for these variances in Lm50 in different research areas. For instance, at higher temperatures than at lower ones, many organisms grow more quickly and reach maturity earlier (Wootton et al., Citation2022). The size at maturity should decrease with rising temperatures because higher temperatures accelerate the maximum rate of gonadal growth more than they accelerate the rate of mass-specific assimilation (Angilletta et al., Citation2004). Additionally, the shortened length of the first sexual maturity can point to a species’ vulnerability to overexploitation, which can have detrimental effects on population, recruitment, and preservation and lead to biological reactions like fish maturing at smaller sizes (Pörtner & Peck, Citation2010).
Table 6. Length at maturity (Lm50) of C. carpio as determined by different authors at different times.
5. Conclusion
The objective of the current study was to compile the first thorough baseline data on C. carpio var. communis from Lake Arekit in Ethiopia. Future research on this species’ morphometric relationships, spawning patterns, and reproductive behaviors in the region will benefit from these findings. According to this study, C. carpio var. communis in Lake Arekit showed length-weight relationships that followed a curvilinear pattern with a statistically significant negative allometric growth pattern (b = 2.79). In comparison to the predicted 1:1 hypothetical distribution, the sex ratio (1:0.66, M:F) of C. carpio var. communis in Lake Arekit was statistically significant. Both male and female C. carpio var. communis remained in good physical condition during the study period in Lake Arekit. The TL and TW of C. carpio var. communis were substantially associated with fecundity. Cyprinus carpio var. communis breeds in Lake Arekit between March and June, with a peak spawning period in May. Thus, fishing periods ought to end during these intense spawning months. Length at sexual maturity (Lm50) was 32.28 cm in females and 28.83 cm in males, respectively. So the present study forms an important database for the conservation and management of C. carpio var. communis in Lake Arekit.
Authors’ contributions
Solomon Wagaw and Yirga Enawgaw were responsible for the study’s design, project management, data collection, analysis, and interpretation, as well as manuscript writing. Ahmed Aba Bulgu, Ayalew Sisay and Assefa Wosnie participated in the data analysis, results interpretation, and manuscript writing. Ashenafi Teklemariyam and Demeke Tegod participated in sample collection and manuscripit writing. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
We would like to express our gratitude to the Lake Arekit fishermen for their assistance during specimen collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data that supports the findings of this study is available within the article.
Additional information
Notes on contributors
Solomon Wagaw
Solomon Wagaw obtained his PhD in Aquatic Science, Fisheries, and Aquaculture from Addis Ababa University, Ethiopia. With extensive experience and expertise in these areas, the author has made significant contributions to the comprehension and control of aquatic ecosystems and the sustainable growth of fisheries and aquaculture.
References
- Abera, L., Getahun, A., & Lemma, B. (2015). Some aspects of reproductive biology of the common carp (Cyprinus Carpio Linnaeus, 1758) in Lake Ziway, Ethiopia. Global Journal of Agricultural Research and Reviews, 3, 1–12.
- Angilletta, M. J., Jr, Steury, T. D., & Sears, M. W. (2004). Temperature, growth rate, and body size in ectotherms: Fitting pieces of a life-history puzzle. Integrative and Comparative Biology, 44(6), 498–509. https://doi.org/10.1093/icb/44.6.498
- Asnake, W., & Mingist, M. (2018). Freshwater fisheries resource potential estimation: The case of Lake Ardibo, Northern Ethiopia. Fisheries and Aquaculture Journal, 9(239), 2.
- Bagenal, T. (1978). Age and growth. In Methods for assessment of fish production in fresh waters (pp. 101–136). Wiley.
- Birecikligil, S. S., Çiçek, E., Öztürk, S., Seçer, B., & Celepoğlu, Y. (2016). Length-length, length-weight relationship and condition factor of fishes in Nevşehir Province, Kızılırmak River Basin (Turkey). Acta Biologica Turcica, 29(3), 72–77.
- Blanck, A., & Lamouroux, N. (2007). Large‐scale intraspecific variation in life‐history traits of European freshwater fish. Journal of Biogeography, 34(5), 862–875. https://doi.org/10.1111/j.1365-2699.2006.01654.x
- Bohlen, J., & Ritterbusch, D. (2000). Which factors affect sex ratio of spined loach (genus Cobitis) in Lake Müggelsee? Environmental Biology of Fishes, 59(3), 347–352. https://doi.org/10.1023/A:1007695703991
- Degefu, F., Tesfaye, G., & Tefera, F. (2012). Study on the adaptability status and reproductive success of Oreochromis niloticus L. (Pisces: Cichlidae) and carp (Cyprinus carpio L., 1758) in a tropic reservoir (Fincha, Ethiopia). International Journal of Aquaculture, 2(10), 65–71.
- Dereli, H., Şen, Y., Kebapçıoğlu, T., Erdoğuş, M., Ölçek, Z. S., Özdemir, M., & Ulman, A. (2022). Management recommendations for common carp fisheries in Turkey in light of their reproductivity and gear selectivity. Journal of Fisheries and Environment, 46(1), 141–156.
- Durant, J. M., Hjermann, D. Ø., Ottersen, G., & Stenseth, N. C. (2007). Climate and the match or mismatch between predator requirements and resource availability. Climate Research, 33(3), 271–283. https://doi.org/10.3354/cr033271
- Echeverria, T. W. (1987). Thirty-four species of California rockfishes: Maturity and seasonality of reproduction. Fishery Bulletin, 85(2), 229–250.
- El Moata, J., El Ghmari, A., Droussi, M., Latrache, H., Sajieddine, M., Omoregie, E., & El Bouadili, A. (2005). Growth performances of common carp fry (Cyprinus carpio) in semi arid and semi intensive conditions: Deroua Fisheries Station (Béni Mellal, Morocco). Journal of Aquatic Sciences, 20(1), 39–42. https://doi.org/10.4314/jas.v20i1.20037
- El-Aiatt, A. O. (2021). Length-weight relationship and condition factor of white grouper Epinephelus aeneus (Geoffroy Saint Hilaire, 1817) in the Mediterranean coast of Sinai, Egypt. Egyptian Journal of Aquatic Biology and Fisheries, 25(4), 573–586.
- Enawgaw, Y., & Wagaw, S. (2023). Phytoplankton communities and environmental variables as indicators of ecosystem productivity in a shallow tropical lake. Journal of Freshwater Ecology, 38(1), 2216244. https://doi.org/10.1080/02705060.2023.2216244
- Fernández-Delgado, C. (1990). Life history patterns of the common carp, Cyprinus carpio, in the estuary of the Guadalquivir River in south-west Spain. Hydrobiologia, 206, 19–28.
- Froese, R. (2006). Cube law, condition factor and weight–length relationships: History, meta‐analysis and recommendations. Journal of Applied Ichthyology, 22(4), 241–253. https://doi.org/10.1111/j.1439-0426.2006.00805.x
- Geffroy, B., & Wedekind, C. (2020). Effects of global warming on sex ratios in fishes. Journal of Fish Biology, 97(3), 596–606. https://doi.org/10.1111/jfb.14429
- Hailu, M. (2013). Reproductive aspects of common carp (Cyprinus carpio L, 1758) in a tropical reservoir (Amerti: Ethiopia). Journal of Ecology and the Natural Environment, 5(9), 260–264. https://doi.org/10.5897/JENE2013.0387
- Jonsson, B., & Jonsson, N. (2014). Early environment influences later performance in fishes. Journal of Fish Biology, 85(2), 151–188. https://doi.org/10.1111/jfb.12432
- Karataş, M., Çiçek, E., Başusta, A., & Başusta, N. (2007). Age, growth and mortality of common carp (Cyprinus carpio Linneaus, 1758) population in Almus Dam Lake (Tokat-Turkey). Journal of Applied Biological Sciences, 1(3), 81–85.
- Kebede, M. T., Getahun, A., & Lemma, B. (2018). Reproductive biology of commercially important fish species in lake Langeno, Ethiopia. Asian Fisheries Science, 31(4), 319–339. https://doi.org/10.33997/j.afs.2018.31.04.006
- Le Cren, E. D. (1951). The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). The Journal of Animal Ecology, 20(2), 201–219. https://doi.org/10.2307/1540
- Mekonnen, E., Berihanu, G., & Yitayew, T. (2018). Length-weight relationships, sex ratios and size at first maturity of fishes of Lake Ardibo, South Wollo, Ethiopia. Abyssinia Journal of Science and Technology, 3(1), 13–19.
- Mekonnen, E., Brehanu, G., & Yitayew, T. (2019). Biological aspects, catch and length distribution of African catfish, Clarias gariepinus and Common Carp, Cyprinus Carpio in in Lake Lugo, South Wollo, Ethiopia. Ethiopian Journal of Science and Technology, 12(3), 185–202. https://doi.org/10.4314/ejst.v12i3.1
- Mert, R., & Bulut, S. (2014). Some biological properties of carp (Cyprinus carpio L., 1758) introduced into Damsa Dam Lake, Cappadocia Region, Turkey. Pakistan Journal of Zoology, 46(2), 337–346.
- Mohamad, I., Bhat, F. A., & Bhat, B. A. (2020). Assessment of fecundity and its relation with body parameters of common carp. Cyprinus Carpio Var. Communis in Dal Lake, Kashmir. Human and Social Science, 54, 620.
- Mutethya, E., Yongo, E., Laurent, C., Waithaka, E., & Lomodei, E. (2020). Population biology of common carp, Cyprinus carpio (Linnaeus, 1758), in Lake Naivasha, Kenya. Lakes & Reservoirs: Science, Policy and Management for Sustainable Use, 25(3), 326–333. https://doi.org/10.1111/lre.12322
- Nyaboke, C. A., Kembenya, E. M., Ogello, E. O., Githukia, C. M., Yasindi, A., Outa, N., & Munguti, J. M. (2014). Length-weight relationship and condition factor of common carp, Cyprinus carpio in Lake Naivasha, Kenya. International Journal of Current Research, 6(9), 8286–8292.
- Ouellet, V., St-Hilaire, A., Secretan, Y., Mingelbier, M., Morin, J., & Dugdale, S. J. (2021). The importance of including water temperature simulations in a 2D fish habitat model for the St. Lawrence River. Water, 13(13), 1736. https://doi.org/10.3390/w13131736
- Passos, C., Tassino, B., Reyes, F., & Rosenthal, G. G. (2014). Seasonal variation in female mate choice and operational sex ratio in wild populations of an annual fish, Austrolebias reicherti. PloS One, 9(7), e101649. https://doi.org/10.1371/journal.pone.0101649
- Peña-Mendoza, B., Gómez-Márquez, J. L., Salgado-Ugarte, I. H., & Ramirez-Noguera, D. (2005). Reproductive biology of Oreochromis niloticus (Perciformes: Cichlidae) at Emiliano Zapata dam, Morelos, Mexico. Revista de Biologia Tropical, 53(3–4), 515–522. https://doi.org/10.15517/rbt.v53i3-4.14666
- Pörtner, H. O., & Peck, M. A. (2010). Climate change effects on fishes and fisheries: Towards a cause‐and‐effect understanding. Journal of Fish Biology, 77(8), 1745–1779. https://doi.org/10.1111/j.1095-8649.2010.02783.x
- Quinn, T. P., & Buck, G. B. (2001). Size-and sex-selective mortality of adult sockeye salmon: Bears, gulls, and fish out of water. Transactions of the American Fisheries Society, 130(6), 995–1005. https://doi.org/10.1577/1548-8659(2001)130<0995:SASSMO>2.0.CO;2
- Ricker, W. E. (1975). Computation and interpretation of biological statistics of fish populations. Fisheries Research Board of Canada Bulletin, 191, 1–382.
- Sahtout, F., Boualleg, C., Khelifi, N., Kaouachi, N., Boufekane, B., Brahmia, S., Mouaissia, W., & Bensouilah, M. (2017). Study of some biological parameters of Cyprinus carpio from Foum El-khanga Dam, Souk-Ahras, Algeria. Aquaculture, Aquarium, Conservation & Legislation, 10(4), 663–674.
- Sara, B., Barour, C., Sameh, A., Boualleg, C., & Mourad, B. (2016). Environmental parameters and parasitism in common carp (Cyprinus carpio Linnaeus, 1758) caught from Oubeira Lake (North-East of Algeria). Research Journal of Fisheries and Hydrobiology, 11, 1816–9112.
- Shafat, S., Bhat, F. A., Balkhi, M. H., Najar, A. M., & Mudasir, H. (2016). Reproductive traits of Schizothorax niger Heckel, 1838 in Dal Lake, Kashmir. SKUAST Journal of Research, 18(2), 138–145.
- Shinkafi, B. A., & Ipinjolu, J. K. (2012). Gonadosomatic index, fecundity and egg size of Auchenoglanis occidentalis (Cuvier and Valenciennes) in River Rima, North-western Nigeria. Nigerian Journal of Basic and Applied Sciences, 20(3), 217–224.
- Smith, B. B. (2004). Carp (Cyprinus carpio L.) spawning dynamics and early growth in the lower River Murray, South Australia/Benjamin B. Smith [Doctoral dissertation]. The University of Adelaide.
- Syed, N., Shah, T. H., Balkhi, M. H., Bhat, F. A., Abubakr, A., Wani, G. B., Bhat, B. A., Mohd, I., Wali, A., & Wani, I. F. (2020). Length-weight relationship and condition factor of Cyprinus carpio var. communis in Manasbal Lake, Kashmir. Journal of Pharmacognosy and Phytochemistry, 9(2), 1539–1544.
- Tesfaye, G., & Wolff, M. (2014). The state of inland fisheries in Ethiopia: A synopsis with updated estimates of potential yield. Ecohydrology & Hydrobiology, 14(3), 200–219. https://doi.org/10.1016/j.ecohyd.2014.05.001
- Tessema, A., Getahun, A., Mengistou, S., Fetahi, T., & Dejen, E. (2020). Reproductive biology of common carp (Cyprinus carpio Linnaeus, 1758) in Lake Hayq, Ethiopia. Fisheries and Aquatic Sciences, 23(1), 10. https://doi.org/10.1186/s41240-020-00162-x
- Tilahun, B., Hailu, A., Abie, K., Kidane, T., & Alemkere, A. (2022). Avifauna diversity and conservation challenges in Lake Arekit, Southern Ethiopia. Israel Journal of Ecology and Evolution, 68(1-4), 74–83. https://doi.org/10.1163/22244662-bja10032
- Tokai, T. (1997). Maximum likelihood parameter estimates of a mesh selectivity logistic model through SOLVER on MS-Excel 1997. Bulletin of the Japanese Society of Fisheries Oceanography, 61, 288–298.
- Tsadik, G. G., & Bart, A. N. (2007). Effects of feeding, stocking density and water-flow rate on fecundity, spawning frequency and egg quality of Nile tilapia, Oreochromis niloticus (L.). Aquaculture, 272(1–4), 380–388. https://doi.org/10.1016/j.aquaculture.2007.08.040
- Usman, I., Auta, J., & Abdullahi, S. A. (2015). Effect of monthly variation in water temperature on artificial breeding of common carp (Cyprinus carpio L.) in Zaria, Nigeria. International Journal of Fisheries and Aquatic Studies, 3, 353–356.
- Wagaw, S., Mengistou, S., & Getahun, A. (2022). Aspects of the growth and reproductive biology of Oreochromis niloticus (Linnaeus, 1758) in a tropical Soda Lake, Lake Shala, Ethiopia. Fisheries and Aquatic Sciences, 25(7), 380–389. https://doi.org/10.47853/FAS.2022.e34
- Welcome, R. L. (Ed.). (1988). International introductions of inland aquatic species (Vol. 294). Food & Agriculture Org.
- Wootton, H. F., Morrongiello, J. R., Schmitt, T., & Audzijonyte, A. (2022). Smaller adult fish size in warmer water is not explained by elevated metabolism. Ecology Letters, 25(5), 1177–1188. https://doi.org/10.1111/ele.13989
- Yaqoob, S. (2021). A review of structure, origin, purpose & impact of common carp (Cyprinus carpio) in India. Annals of the Romanian Society for Cell Biology, 25(6), 34–47.
- Yüce, S., Gündüz, F., Demirol, F., Çelik, B., Alpaslan, K., Çoban, M., Aydin, R., & Şen, D. (2016). Some population parameters of mirror carp (Cyprinus carpio L., 1758) inhabiting Atatürk Dam Lake. Journal of Limnology and Freshwater Fisheries Research, 2(1), 31–31. https://doi.org/10.17216/LimnoFish-5000149481