 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This field study aimed to investigate the potential of zinc oxide nanoparticles (ZnONPs) to enhance various aspects of bitter gourd plant germination, growth, phytochemical composition and yield. Bitter gourd seeds were subjected to soaking in aerated solutions of ZnONPs at concentrations of 50 and 100 ppm for 24 and 48 hours, with hydro-primed seeds serving as the control group. Priming bitter gourd seeds with 100 ppm ZnONPs for 48 hours significantly improved germination, increased the number of fruits per plant, enhanced fruit density, and showed improvements in elevating the plant’s phytochemical profile. Notably, seed priming with 100 ppm ZnONPs for 48 hours resulted in a 29% increase in germination percentage. Furthermore, treatment with 100 ppm ZnONPs for 48 hours exhibited significant enhancements in key parameters such as the number of fruits per plant, fruit density, fruit length and fruit diameter, demonstrating increases of 41%, 29%, 29%, and 42.3%, respectively, compared to the control group. The application of ZnONPs at concentrations of 100 ppm for both 24 and 48 hours to bitter gourd seeds also led to substantial elevations in the plant’s phytochemical profile. Specifically, total phenolic content saw increments of 12% and 27%, total flavonoid content saw increments of 16% and 30%, and total charantin content saw increments of 12% and 29%, respectively, compared to the hydro-priming treatment. The study underscores the operational benefits of employing ZnONPs for seed priming, which positively influences germination, growth, phytochemical composition, and ultimately the yield of bitter gourd plants.
Reviewing editor:
1. Introduction
Climate change poses a significant threat to global agricultural commodities, leading to frequent encounters with both abiotic and biotic stressors. These challenges ultimately result in reduced yields and compromised grain quality. Conventional agricultural practices, coupled with uneconomical landholdings and limited agricultural inputs, further exacerbate the vulnerability of agricultural commodities, particularly horticultural crops (Mazhar et al., Citation2021). The absence of crop rotation, improper land management, and excessive cultivation practices have depleted soil fertility, leading to diminished land performance and subsequent yield losses over time.
Given this context, it becomes imperative to expand the agricultural paradigm, particularly in light of population growth, with a primary focus on addressing food security. This expanded vision for agriculture should embrace eco-friendly technologies, such as nanotechnology, to enhance the performance of naturally bred varieties (Mazhar et al., Citation2022; Thakkar et al., Citation2010). Nanotechnology, involving the manipulation of materials at the nanoscale (one billionth of a meter), was first practiced by Norio Taniguachi in 1974 (Pirzadah et al., Citation2019). Materials at the nano-scale exhibit distinct properties, including heightened heat resistance, increased charge density, enhanced reactivity and strength, lowered melting points, and unique magnetic attributes. Leveraging these attributes, nanoparticles (NPs) exhibit enhanced interactions with seed coats, influencing embryo development, optimizing the release and management of pesticides and fertilizers, all while being environmentally benign (Thakkar et al., Citation2010; Mazhar et al., Citation2022).
Bitter gourd (Momordica charantia L.), a monoecious plant belonging to the Cucurbitaceae family, holds global prominence as a highly nutritious and medicinally valuable vegetable (Baig et al., Citation2020). Rich in minerals, vitamins, including ascorbic acid, and endowed with potent medicinal properties, bitter gourd is utilized in treating conditions such as diabetes, rheumatism, bronchial and haematological disorders and is a source of various Ayurvedic remedies. The escalating demand for bitter gourd is attributed to its pronounced medicinal and clinical significance (Baig et al., Citation2020).
The impact of plant nutrition on horticultural crops has long captivated researchers, with ongoing efforts to enhance growth, yield, and germination (Farooq et al., Citation2019). While optimal soil nutrition is paramount, achieving uniform and robust germination remains a prerequisite for favorable plant growth and agronomic performance (Rakshit and Singh, Citation2018). The hard seed coat of bitter gourd delays water absorption, resulting in slow germination even in seeds with high germination potential. Addressing this dormancy, seed priming has emerged as a favored strategy (Waqas et al., Citation2019; Sher et al., Citation2019).
Seed priming entails hydrating seeds in a solution containing water and biostimulants for a specific duration, followed by drying to enhance pre-germinative metabolism, while avoiding radical emergence in the priming mixture (Sadeghian & Yavari Citation2004). This approach ensures early and uniform germination through improved nutrient uptake and water efficiency (Farooq et al., Citation2007). Seed germination, a pivotal phase influenced by genetic, epigenetic, and environmental factors, benefits from pre-conditioning, as it softens the seed coat and promotes uniform emergence. Suitable pro-fertilizers used in priming break seed dormancy, deactivate growth inhibitors, and activate germination-related enzymes (Farooq et al., Citation2008). Enhanced uniform emergence and efficient germination bolster farmer profitability by optimizing biomass increase and yield attributes (Farooq et al., Citation2010).
Secondary metabolites or phytochemicals are valuable natural compounds with medicinal and practical applications, produced by plants in small quantities (Kabera et al., Citation2014; Rehman et al., Citation2023). Nanotechnology offers a platform to amplify phytochemical production. Strategies to optimize phytochemical yields are explored both in vivo and in vitro (Raei et al., Citation2014). Aloe vera-derived aloin, an important phytochemical, demonstrates increased production in response to nano TiO2 particles and AgNPs (Kosova et al., Citation2018). Manipulating Fe and ZnONPs has been shown to enhance the production of medicinal phytochemicals in Hypericum perforatum plants, used in managing depression (Sharafi et al., Citation2013). Environmental factors, including light, temperature, water availability, and pest interactions, contribute to variations in plant-produced secondary metabolites.
Zinc deficiencies in global croplands are a widespread concern that can adversely impact agricultural productivity and human nutrition (Rehman et al., Citation2018). Zinc is an essential micronutrient for plant growth and development. Zinc plays a fundamental role in the growth and development of plants, participating in a multitude of physiological and biochemical processes critical for their overall health (Rehman et al., Citation2015). One key function of zinc lies in its activation of enzymes crucial for metabolic pathways such as photosynthesis, respiration, and DNA synthesis. It is a vital component of chlorophyll, the green pigment essential for photosynthesis, thereby contributing directly to the plant’s ability to convert light energy into chemical energy. Additionally, zinc is pivotal in protein synthesis and the formation of ribosomes, essential components of the cellular machinery responsible for building and maintaining plant structures (Farooq et al., Citation2021). Beyond these functions, zinc also plays a role in stress response, influencing the plant’s ability to resist diseases and tolerate environmental stresses like drought and extreme temperatures. Zinc oxide nanoparticles (ZnONPs) priming is beneficial in zinc-deficient soils because it enhances the availability and uptake of zinc by plants. The nanoscale nature of ZnONPs provides a larger surface area, facilitating greater interaction with plant roots. This promotes efficient absorption of zinc, thereby addressing deficiencies in soils where conventional zinc sources may not be as effective. Additionally, the controlled application of ZnONPs through priming allows for targeted and optimal delivery of zinc to seeds, promoting seed germination, early seedling growth, and overall plant development (Rehman et al., Citation2018, Citation2022).
This study endeavours to fill a critical gap in the current literature by delving into the relatively unexplored realm of nano seed priming effects on horticultural crops, with a specific focus on bitter gourd plants. Our primary aim is to comprehensively understand how seed priming with ZnONPs can impact various facets of bitter gourd growth, including germination, overall plant development, yield, and phytochemical composition. The specific objectives encompass evaluating the influence of ZnONPs seed priming on germination and early seedling establishment, assessing its effects on vegetative and reproductive growth phases, exploring potential enhancements in yield-related parameters, and scrutinizing alterations in phytochemical profiles. Additionally, the study aims to devise a cost-effective and environmentally friendly seed pre-conditioning strategy using ZnONPs, aiming to complement or substitute existing commercial biostimulants commonly used in seed priming practices. Through these objectives, this research seeks to provide practical insights into the application of ZnONPs seed priming as a sustainable and effective approach in horticulture, potentially contributing to enhanced crop productivity and nutritional quality.
2. Materials and methods
2.1. Soil characteristics
Soil characteristics were appraised following Chapman and Pratt (Citation1962). The experimental soil was a sandy loam with an organic matter contents of 1.24% and saturation percentage of 34%. The electrical conductivity of the soil was 2.48 ds.m−1 with a pH of 7.6. The experimental soil had a zinc level of 1.2 mg/kg, with available nitrogen and phosphorus contents of 0.72% and 8.4 ppm, respectively. Traces of carbonates were present whereas sulphate and chloride contents were 1.99 and 8.76 meq L−1. The sodium and iron were also present at concentrations 0.035 and 3.02 meq L−1.
2.2. Experimental setup and analysis of germination attributes
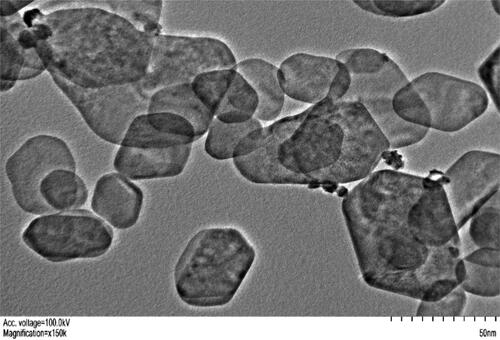
Germination experiment was performed under open field conditions with five treatments and three replicates per parameter studied. Experiment was performed in a split plot factorial pattern with one main plot divided in 5 subplots of 0.70 m × 0.35 m dimensions. 50 seeds were sown in each treatment plot (in 7 rows each with 7 plants and 1adjusted manually) with spacing 10 cm × 5 cm (Adhikari et al., Citation2021). The first subplot contained plants raised from conventional hydro priming using tap water soaking overnight before sowing. The second and third subplots of plants were raised through nano primed seeds with ZnONPs at concentration of 50 ppm and 100 ppm respectively for priming duration of 24 hours in both suplots. The fourth and fifth subplots of plants were raised through nano primed seeds with ZnONPs at concentration of 50 ppm and 100 ppm respectively for priming duration of 48 hours. The 50 ppm and 100 ppm priming solution were prepared by adding commercially available ZnO nano powder (obtained from Sigma-Aldrich Product No. 544906, Steinem, Germany) 50 mg and 100 mg in a litre of water respectively. The product was accessed with the identification number 544906 having a CAS Number of 1314-13-2 and an MDL (Molecular Design Limited) reference of MFCD00011300. Its chemical formula is ZnO, with a formula weight of 81.39 g/mol. The appearance of the product was described as white powder. The X-Ray Diffraction analysis confirms its adherence to a specific structure. The Complexometric Titration indicates a zinc content between 79.1% and 81.5%. The particle size is specified as being less than 100 nm for the average particle size and surface area within the range of 10 to 25 m2/g. Chemically, ZnONPs maintain the composition of bulk zinc oxide (ZnO), with a crystalline structure of zinc and oxygen atoms. Their increased reactivity, attributed to a higher surface area, makes them suitable for catalytic and photocatalytic applications. Surface functionalization further enhances stability and compatibility. displays a transmission electron micrograph image of the purchased ZnONPs. The mixtures were ultra-sonicated to achieve uniform dispersions. Continuous aeration was ensured in the priming solution using a common commercially available fish aquarium air pump. The temperature of priming solutions in all treatments was ensured at room temperature 25 °C (Saini et al., Citation2017). The germination percentage was calculated after 14 days of sowing using following formula:
where SL is shoot length and RL is root length
Figure 1. The transmission electron micrograph of ZnONPs depicting polygonal to quasi-spherical shapes. For imaging purposes, a FEI Tecnai 12 instrument equipped with a digital camera from Gatan was utilized. The nanoparticles were dried on a copper grid coated with a carbon layer.
Seedling vigour index II = Germination (%) × Seedling dry weight (g)
Seedling root and shoot were separated carefully to measure seedling shoot and root lengths (Abdul-Baki & Anderson, Citation1973; Rajasree & Pillai, Citation2012).
2.3. Growth and yield
The characteristics of bitter gourds were assessed across vegetative, reproductive, and fruit stages utilizing a randomized complete block design with three replications. During the vegetative state (28 days after sowing), parameters such as leaf area and the number of branches were examined. Reproductive traits encompassed the duration until the first flower appearance (45 days after sowing), the quantity of fruits per plant, total seed count per fruit, 1000-seed weight, as well as length, diameter, weight, and fruit density. The evaluation also involved the measurement of fresh fruit yield per plant, employing the methodology outlined by Palada et al., (Citation2017).
2.4. Determination of phytochemicals
For the determination of total flavonoids content, a mixture was prepared by combining 1 mL of freshly cut fruit extract, 0.5 mL of aluminium chloride, 0.5 mL of water, 0.5 mL of HCl, and 0.5 mL of sodium acetate. The mixture was allowed to stand at room temperature (25 ± 1 °C) for 10 minutes. Subsequently, absorbance at 425 nm was measured using a spectrophotometer (Shimadzu PC-1650, Kyoto, Japan) (Pekal and Pyrzynska, 2014). To determine total phenolic contents, 1 gram of freshly cut fruit was pulverized using a mortar and pestle. The pulverized sample was mixed with 20 mL of methanol and extracted for 2 hours in a water bath at 65 °C. After extraction, the mixture was centrifuged at 2775 g for 15 minutes and vacuum filtered through a Buchner funnel (Sulaiman and Balachandraan, Citation2012). The resulting extract was combined with Folin-Ciocâlteu (0.25 N), sodium carbonate (1 N), and distilled water. Following vortexing and a 2-hour incubation in the dark at room temperature (25 ± 1 °C), the absorbance of the solution was measured at 726 nm using a spectrophotometer (Shimadzu) (Horax et al., Citation2006).
For the assessment of charantin content, a major secondary metabolite with insulin-like properties, 1 gram of freshly harvested bitter gourd was subjected to two rounds of hexane treatment to remove fats. The defatted sample was then sonicated with pure methanol using a sonicator (Branson Ultrasonic Co., Danbury, CT, USA). The resulting extract was vacuum-filtered, dried at 40 °C using a rotary evaporator, and re-dissolved in 1 mL of HPLC-grade methanol. Charantin content was quantified using an HPLC system (NS-4000 model, Daejeon, South Korea) equipped with a UV detector set at 204 nm, following the method outlined by Kim et al., (Citation2014). Chlorophyll The chlorophyll content in the fresh-cut bitter gourd at different atmosphere and at storage periods were determined by using 80% acetone (Pekal and Pyrzynska, 2014).
2.5. Statistical analysis
The data were subjected to rigorous testing. The mean and standard error was evaluated using MS excel. The mean ± standard error bar charts were constructed in the excel sheet. XLSTAT version 2014 (Addinsoft, Paris France) was used for constructing heat map of the data, Spearman correlation matrix and principle component analysis. The analysis of variance was done using Co-STAT version 6.3 (developed by Cohort Software Berkley, CA, USA).
3. Results
The germination attributes were significantly increased upon different seed priming treatment regimens followed in the present research. An overall increase of 25.6% in germination percentage was observed following the treatment of bitter gourd seeds with 100 ppm ZnONPs for 24 hours, compared to the control group. Notably, there was a discernible upward trend in germination as the priming duration was extended. Remarkably, seed treatment with 100 ppm ZnONPs for 48 hours demonstrated the most substantial improvement, resulting in a 29% increase in germination percentage when compared to the control group, thereby emerging as the most effective treatment ((A)). To gain deeper insights into the impact of ZnONPs priming on bitter gourd seeds, seedling vigour indices were meticulously monitored, encompassing both seedling length (SVI-I) and weight (SVI-II). The data unequivocally indicated that seed priming across all concentration levels significantly bolstered the seedling vigour indices, encompassing both length and weight. Specifically, the treatment involving 100 ppm concentration for 24 hours exhibited a noteworthy enhancement in seedling vigour indices in terms of both length and weight. This trend persisted and even intensified with prolonged priming durations. Impressively, seed priming with a 100 ppm concentration for 48 hours led to a further substantial surge in seedling vigour indices, encompassing both length and weight ().
Figure 2. Germination attributes of bitter gourd plants as affected by various priming treatments of ZnONPs (Mean ± S. E. n = 3). 0 ppm is the control treatment with hydro priming with tap water overnight.
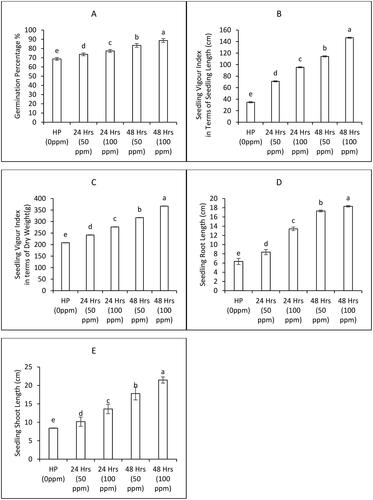
Furthermore, the lengths of the seedling roots and shoots were meticulously recorded to gauge the efficacy of seed priming. Intriguingly, a concentration of 100 ppm ZnONPs proved optimal for achieving maximal enhancement in both seedling shoot and root lengths, with a priming duration of 48 hours outperforming the 24-hour duration. Notably, when subjected to 100 ppm concentration and a 24-hour duration of priming, a substantial improvement was evident in seedling root length and shoot length, respectively. By maintaining the concentration at 100 ppm while extending the priming duration, the benefits grew even more pronounced, resulting in a remarkable 35% and 37% increase in seedling root length and shoot length, respectively, in comparison to the control treatment of hydro priming ().
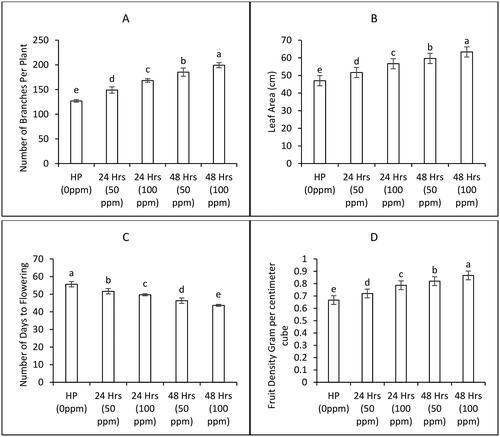
As the seedlings matured under open field conditions and the first flowers emerged, the number of branches and leaf areas were meticulously observed across three replicates. Additionally, the days taken for the first flower to appear were recorded. Notably, an escalating trend was observed in terms of the number of branches per plant as the priming duration and priming fertilizer concentration were elevated. ZnONPs applied at 100 ppm concentration for 24 hours and 48 hours to bitter gourd seeds increased number of branches by 10% and 21.9% respectively compared to hydro priming treatment ((A)). Data presented in (B) highlight increasing trend in leaf areas of bitter gourd plants. The leaf areas were increased from 13% to 25% by increasing priming duration from 24 hours to 48 hours compared to control at 100 ppm priming concentration ((B)). A decreasing trend to number of days to first flower in bitter gourd plants was seen by increasing priming fertilizer treatment and duration for priming ((C)).
Figure 3. Biomass and yield attributes of bitter gourd plants as affected by various priming treatments of ZnONPs (Mean ± S.E. n = 3). 0 ppm is the control treatment with hydro priming.
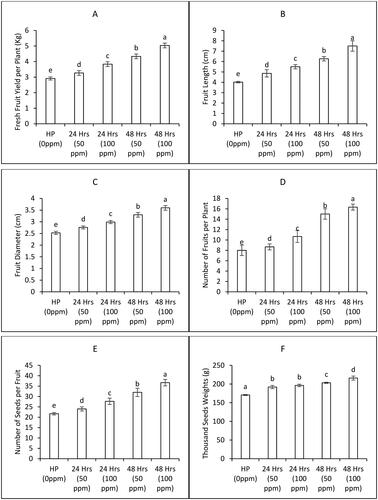
At harvest the agronomic profile of bitter gourd plants was compiled by observing several parameters such as number of fruits per plant, fruit density, fruit length, fruit diameter, fresh fruit weight or yield, number of seeds per fruit and thousand seeds weight. Furthermore, a consistent upward trend in the yield profile was evident as both the seed priming duration and the concentration of seed priming fertilizer (ZnONPs) were increased. Specifically, treating seeds with 100 ppm ZnONPs for 24 hours significantly improved several yield parameters. Notably, this treatment led to a remarkable 17% increase in fruit density, a 24% increase in fruit fresh yield, a 9% increase in fruit length, and an 18.3% increase in fruit diameter (, , respectively). Impressively, the application of ZnONPs (at a concentration of 100 ppm for 24 hours) also yielded a substantial increase of 29% in the number of fruits per plant, a 27% increase in the number of seeds per fruit, and a 7% increase in thousand seeds weight, as illustrated in .
Furthermore, the treatment involving 100 ppm ZnONPs for 48 hours exhibited even more pronounced enhancements in yield parameters. Specifically, this treatment resulted in a notable 29% increase in fruit density, a substantial 45% boost in fruit fresh yield per plant, a 29% elongation in fruit length, and an impressive 42.3% enlargement in fruit diameter (, , respectively). Similarly, the application of ZnONPs (at a concentration of 100 ppm for 48 hours) led to remarkable increases of 41% in the number of fruits per plant, 44% in the number of seeds per fruit, and 10% in the thousand seeds weight, as depicted in .
Figure 4. Biomass and yield attributes of bitter gourd plants as affected by various priming treatments of ZnONPs (Mean ± S.E. n = 3). 0 ppm is the control treatment with hydro priming.
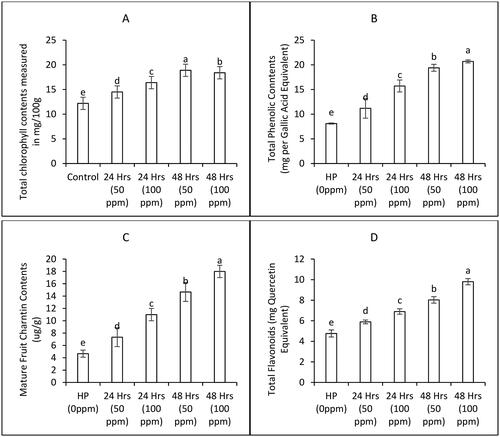
Data presented in show increasing trends in total chlorophyll contents with increasing priming concentration and duration. Data presented in shows the increasing trend observed upon increasing seed priming durations and concentrations for fruit total phenolic, fruit total flavonoids and fruit charantin contents. All the treatments proved highly significant in raising phytochemical profile (). ZnONPs applied at 100 ppm concentration for 24 hours and 48 hours to bitter gourd seeds increased total phenolic contents by 12% and 27%, respectively, total flavonoids contents by 16% and 30%, respectively and total charantin contents by 12% and 29%, respectively compared to hydro priming treatments.
Figure 5. Phytochemical of bitter gourd plants as affected by various priming treatments of ZnONPs (Mean ± S.E. n = 3). 0 ppm is the control treatment with hydro priming.
Significant correlations among various seed germination and growth attributes were adeptly illustrated through a Principal Component Analysis (PCA), as depicted in . The outcomes of the ANOVA analyses () and the Spearman correlation matrix () distinctly indicate the high significance of treatment results and the significant associations among the studied variables, collectively contributing to the enhancement of plant performance.
Figure 6. Loading plots of Principal Component Analysis (PCA) showing significant correlation among the studied variables of Bitter gourd plants. GP% = Germination Percentage, SVI-1 = Seedling Vigour Index in Terms of Seedling Length, SVI-II = Seedling Vigour Index in terms of Dry Weight (g), SRL (cm) = Seedling Root Length, SSL (cm) = Seedling Shoot Length, NOB/P = Number of Branches Per Plant, LA (cm) = Leaf Area, NODF = Number of Days to Flowering, NOF/P = Number of Fruits per Plant, F. De g/cm3 = Fruit Density Gram, FL(cm) = Fruit Length, FD(cm) = Fruit Diameter, FFY/P(Kg) = Fresh Fruit Yield per Plant, NOS/F = Number of Seeds per Fruit, 1000 SW(g) = Thousand Seeds Weights, TP mg GA eq. = Total Phenolic Contents, TF mg Q eq. = Total Flavonoids, T.C ug/g = Mature Fruit Charantin Contents.
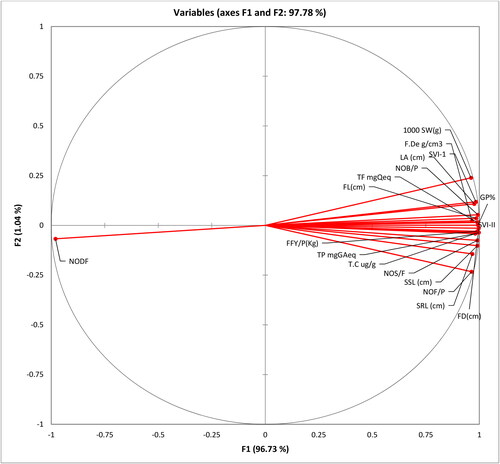
Table 1. Physiochemical parameters of bitter gourd seeds measured after seed priming using zinc Oxide NPs and analysed through ANOVA (with mean square and p values).
Table 2. Spearman correlation matrix showing significant correlation among germination, growth and yield parameters.
4. Discussion
In the current study, seed nano pre-conditioning using ZnONPs exhibited enhancements in various germination attributes, encompassing germination energy, seedling vigour indices, and seedling root and shoot lengths. These improved germination traits can be attributed to zinc’s participation in enzymatic pathways linked to auxin biosynthesis (Sedghi et al., Citation2013). Notably, zinc application has been reported to preserve membrane integrity and promote cell elongation, consequently influencing radical growth enhancement (Hassan et al., Citation2020; Rehman et al., Citation2015). This comprehensive effect, in turn, led to increased shoot and root biomass in the seedlings.
The study also observed that the duration of priming significantly impacted the germination behaviour of bitter gourd seeds (Dimkpa et al., Citation2012; Wojnarowicz et al., Citation2016). It is well recognized that the concentration of priming fertilizer and the duration of seed priming can vary among different plant species. The improved germination behaviour seen in bitter gourd seeds might be attributed to the application of zinc via nano priming in the form of ZnONPs (Wojnarowicz et al., Citation2018). The supplied zinc is thought to play a role in breaking seed dormancy, facilitating imbibition, and triggering enzymatic activation of various germination-associated biochemical events, including the hydrolysis of germination inhibitors (Rehman et al., Citation2018). Moreover, zinc is critical for the metabolism of carbohydrates and proteins stored within the endospermic food reserves, ultimately contributing to the elongation of shoot and root lengths in seedlings (Farooq et al., Citation2021; Hou et al., Citation2018). Furthermore, zinc plays a role in nurturing coleoptile and radical tissues, facilitating their improved emergence and germination. The application of ZnONPs is particularly intriguing due to the efficiency of nano carrier systems in providing a controlled and gradual release of fertilizer to plant tissues. ZnO nano ‘bullets’ can potentially interact with the seed coat by penetrating the nanopores present in the seed coats, thereby influencing the hard seed coat tissues and moderating water uptake by activating aquaporin (Faizan et al., Citation2018). These synergistic events likely contributed to the better germination percentage, enhanced seedling vigor indices, and improved root and shoot biomass observed in seedlings compared to the conventional hydro priming technique. Moreover, ZnONPs are implicated in the activation of the alpha-amylase enzyme, responsible for the mobilization of stored food reserves. All these findings strongly align with the results of the present study and provide a plausible mechanism of action underlying the observed enhanced germination patterns of bitter gourd seeds (Eisvand et al., Citation2018).
In addition to observing improved germination trends, the present study reports an increase in the biomass of bitter gourd plants, as indicated by the number of branches and leaf areas, through the use of seed priming with ZnONPs in comparison to hydro priming. Higher biomass mediated by ZnONPs has also been documented in various other crops, including carrot, rice, and tomato (Hassan et al., Citation2020). This augmented biomass can be attributed to the role of zinc in enhancing the photosynthetic machinery of plants. Specifically, zinc aids in increasing chlorophyll content, offering protection to chlorophyll, and facilitating the repair of photosystems (Atici et al., Citation2005). Existing literature supports the notion that zinc plays a role in safeguarding the sulfhydryl groups of chlorophyll pigments, thus promoting improved photosynthetic behavior that ultimately contributes to increased plant biomass (Chen and Arora, Citation2013; Dietz et al., Citation2016). Furthermore, zinc is instrumental in repairing and developing plastids, thereby promoting efficient photosynthesis. Notably, the proper functioning of photosystem-II closely correlates with the proper functioning of the D1 protein. Studies have demonstrated that zinc contributes to the repair of the D1 protein, solidifying its status as a valuable phyto-friendly pro-fertilizer (Chen and Arora, Citation2013; Dietz et al., Citation2016; Rehman et al., Citation2022).
The observed increase in biomass trends in our current experiment may stem from a robust interaction between the pro-fertilizer (in this case, ZnONPs) and the seed coat (Reed et al., Citation2012). The literature underscores the robust binding and adhesion properties of nanoparticles in comparison to other conventional priming agents. This attribute allows for the gradual release of active components such as zinc, providing the plant with more opportunities for enhanced growth and germination (Rizwan et al., Citation2019). Moreover, priming induces the activation of specific transcription factors, such as AGL-62, which intrinsically regulate the levels of phytohormones like ABA and GA. This regulation is vital for the endospermic auxin to travel towards the seed coats, loosening them through the acid growth theory mechanism. This interaction, coupled with GA, promotes superior seedling vigour, ultimately leading to enhanced growth and yield of the plants (Fang et al., Citation2008). Furthermore, the localization of ZnONPs on the seed coats of bitter gourd plants regulates hormonal balance and reactive oxygen species (ROS) levels. This intricate interplay is pivotal in breaking dormancy. The optimal levels of ROS are necessary to modulate kinase cascade transduction and cellular redox levels essential for robust seed germination and subsequently, improved biomass and growth characteristics. The increased biomass resulting from ZnONPs seed treatment may also be attributed to the activation of alpha amylase, leading to an elevation in sucrose metabolism and, consequently, an increase in overall fresh yield (Wu et al., Citation2020).
Plant secondary metabolites serve as a rich source of pharmacological applications and play a crucial role in aiding plants to endure changing climate conditions (Rahmawati et al., Citation2022). In the present study, we observed that seed priming with ZnONPs led to heightened production of phenolic compounds, flavonoids, and total charantin contents. This increase in secondary metabolites could potentially be attributed to the enhanced sugar metabolism triggered by ZnONPs (Sulaiman and Balachandraan, Citation2012; Aminifard et al., Citation2012). Additionally, the augmentation of bioactive compounds might stem from an intensified shikimate phenylpropanoid metabolism as a result of ZnONPs application (Cui et al., Citation2019; Rahmawati et al., Citation2022). The mechanism underlying ZnONPs’ impact on charantin content elevation in bitter gourds could be linked to the ability of ZnONPs to promote the homeostasis of cytosolic Ca + 2 ions. These ions could function as signalling molecules, stimulating endogenous ROS production and subsequently enhancing the phytochemical profile of bitter gourd plants (Rashmi and Trivedi, Citation2014). Furthermore, epigenetic regulation of cell cycle events by nanoparticles, such as the modulation of Mitogen-Activated Protein Kinase (MAPK), might contribute to the boost in metabolite production. Studies have also suggested that ZnONPs are implicated in upregulating genes involved in the biosynthesis of alkaloids. Additionally, ZnONPs treatment fosters a single-channel-like conductance for Ca2+ ions, promoting the burst of ROS and increased expression of MAPK genes. These collaborative events lead to transcriptomic reprogramming at the genetic level, ultimately resulting in an amplified synthesis of secondary metabolites (Bhat and Bhat, Citation2016).
The improved yield profile serves as a manifestation of the better homeostasis and signalling responses of plants to changing external environments. In the present study, we observed an elevated yield profile in terms of increased fresh fruit yield per plant, greater fruit density, enhanced fruit number, larger fruit diameter, elongated fruit length, higher seed number, and increased seed weights upon cultivating ZnONPs-primed seeds. Additionally, the number of days to flowering significantly reduced, as mentioned in the results. These findings align with recent work reported by Mazhar et al. (Citation2022), where the application of ZnONPs was shown to distinctly enhance the yield profile in rice. Various mechanisms can be inferred to explain these outcomes. Studies have demonstrated that ZnONPs treatment stimulates increased auxin and chlorophyll activities (García-Gómez et al., Citation2017). Zinc plays a pivotal role in the synthesis of the tryptophan amino acid, which is a crucial precursor in auxin biosynthesis in plants. Furthermore, ZnONPs encourage the breakdown of soil organic matter (SOM), releasing minerals into the soil that are effectively absorbed by plant roots (Josko et al., 2014). Improved patterns of nutrient acquisition result in enhanced agronomic performance of the plants. Additionally, ZnONPs boost the activities of enzymes such as glutanyl tRNA reductase and protoporphyrinogen reductase, which play crucial roles in chloroplast synthesis (Wang et al., Citation2018). One potential mechanism contributing to the higher yield trends observed in our current research might involve ZnONPs-mediated enhancement of root growth and increased production of organic acids. Similarly, researchers have suggested that ZnONPs elevate the activities of free nitrogen fixers in the rhizosphere. Zinc also acts as a cofactor for the dehydrogenase enzyme, which plays a role in respiration. Collectively, these findings lend support to our results and elucidate the mechanisms underlying seed priming with ZnONPs (Siddiqui et al., Citation2019).
5. Conclusion
In conclusion, the present study underscores the substantial benefits of employing biostimulants through seed priming to enhance both the yield and biochemical characteristics of bitter gourds. The data regarding improved germination attributes and enhanced growth characteristics strongly advocate for the utilization of ZnONPs as a pre-sowing treatment. Notably, our investigation also revealed elevated concentrations of essential bioactive compounds following seed treatment with ZnONPs. To further deepen our understanding, we recommend conducting additional research involving quantitative phytochemical profiling, ideally using efficient techniques such as GC–TOFMS analysis. This study effectively demonstrates the potential of ZnONPs as valuable candidates in the realm of climate-smart agricultural practices. The insights derived from our research hold particular significance for herbal practitioners and horticultural farmers, as the results substantiate the efficacy of nanopriming as a cost-effective and environmentally friendly approach to augmenting growth, yield, and the biochemical profile of crops. To expand the scope and applicability of these findings, we suggest future research endeavours to explore the effects of ZnONPs on other horticultural crops. This holistic approach will contribute to a more comprehensive understanding of the potential benefits of biostimulants like ZnONPs across diverse agricultural contexts.
Authors’ contributions
Conceptualization, MWM.; HOE and MI.; writing—original draft preparation, EAM; HOE.; MWM.; MI.; FU.; and MM. Writing—review and editing, EAM; HOE.; MWM.; MI.; FU.; and MM. Writing. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to thank Researchers Supporting Project number (RSP2024R118), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability
All data are available from the corresponding author.
Additional information
Funding
References
- Abdul-Baki, A. A., & Anderson, J. D. (1973). Vigor determination in soybean seed by multiple criteria 1. Crop Science, 13(6), 1–14. https://doi.org/10.2135/cropsci1973.0011183X001300060013x
- Adhikari, B., Dhital, P. R., Ranabhat, S., & Poudel, H. (2021). Effect of seed hydro-priming durations on germination and seedling growth of bitter gourd Momordica charantia. PloS One, 16(8), e0255258. https://doi.org/10.1371/journal.pone.0255258
- Aminifard, M. H., Aroiee, H., Azizi, M., Nemati, H., & Jaafar, H. Z. (2012). Effect of humic acid on antioxidant activities and fruit quality of hot pepper Capsicum annuum L. Journal of Herbs, Spices and Medicinal Plants, 18(4), 360–369. https://doi.org/10.1080/10496475.2012.713905
- Atici, Ö., Ağar, G., & Battal, P. E. (2005). Changes in phytohormone contents in chickpea seeds germinating under lead or zinc stress. Biologia Plantarum, 49(2), 215–222. https://doi.org/10.1007/s10535-005-5222-9
- Baig, K. K., Ara, N., Ali, S., Khan, B. P., Wahab, A., & Rabbani, U. (2020). Effect of seed priming on bitter gourd with different sources of phosphorus at various soaking durations. Pure and Applied Biology, 9(1), 80–90. https://doi.org/10.19045/bspab.2020.90010
- Bhat, P., & Bhat, A. (2016). Silver nanoparticles for enhancement of accumulation of capsaicin in suspension culture of Capsicum sp. Journal of Experimental Sciences, 7, 1–6. https://doi.org/10.19071/jes.2016.v7.3001
- Chapman, H. D., & Pratt, P. F. (1962). Methods of analysis for soils, plants and waters. Soil Science, 93(1), 68. https://doi.org/10.1097/00010694-196201000-00015
- Chen, K., & Arora, R. (2013). Priming memory invokes seed stress-tolerance. Environmental and Experimental Botany, 94, 33–45. https://doi.org/10.1016/j.envexpbot.2012.03.005
- Cui, Y., Deng, Y., Zheng, K., Hu, X., Zhu, M., Deng, X., & Xi, R. (2019). An efficient micropropagation protocol for an endangered ornamental tree species Magnolia sirindhorniae Noot. and Chalermglin and assessment of genetic uniformity through DNA markers. Scientific Reports, 9(1), 9634. https://doi.org/10.1038/s41598-019-46050-w
- Dietz, K. J., Mittler, R., & Noctor, G. (2016). Recent progress in understanding the role of reactive oxygen species in plant cell signaling. Plant Physiology, 171(3), 1535–1539. https://doi.org/10.1104/pp.16.00938
- Dimkpa, C. O., McLean, J. E., Latta, D. E., Manangón, E., Britt, D. W., Johnson, W. P., & Anderson, A. J. (2012). CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. Journal of Nanoparticle Research, 149, 1–5.
- Eisvand, H. R., Kamaei, H., & Nazarian, F. (2018). Chlorophyll fluorescence, yield and yield components of bread wheat affected by phosphate bio-fertilizer, zinc and boron under late-season heat stress. Photosynthetica, 564, 1287–1296.
- Faizan, M., Faraz, A., Yusuf, M., Khan, S. T., & Hayat, S. (2018). Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica, 56(2), 678–686. https://doi.org/10.1007/s11099-017-0717-0
- Fang, Y., Wang, L., Xin, Z., Zhao, L., An, X., & Hu, Q. (2008). Effect of foliar application of zinc, selenium, and iron fertilizers on nutrients concentration and yield of rice grain in China. Journal of Agricultural and Food Chemistry, 56(6), 2079–2084. https://doi.org/10.1021/jf800150z
- Farooq, M., Almamari, S. A. D., Rehman, A., Al-Busaidi, W. M., Wahid, A., & Al-Ghamdi, S. S. (2021). Morphological, physiological and biochemical aspects of zinc seed priming-induced drought tolerance in faba bean. Scientia Horticulturae, 281, 109894. https://doi.org/10.1016/j.scienta.2021.109894
- Farooq, M., Basra, S. M., & Ahmad, N. (2007). Improving the performance of transplanted rice by seed priming. Plant Growth Regulation, 51(2), 129–137. https://doi.org/10.1007/s10725-006-9155-x
- Farooq, M., Basra, S. M., Rehman, H. U., & Saleem, B. A. (2008). Seed priming enhances the performance of late sown wheat Triticum aestivum L. by improving chilling tolerance. Journal of Agronomy and Crop Science, 194(1), 55–60. 1941, https://doi.org/10.1111/j.1439-037X.2007.00287.x
- Farooq, M., Basra, S. M., Wahid, A., & Ahmad, N. (2010). Changes in nutrient-homeostasis and reserves metabolism during rice seed priming: consequences for seedling emergence and growth. Agricultural Sciences in China, 9(2), 191–198. https://doi.org/10.1016/S1671-2927(09)60083-3
- Farooq, M., Usman, M., Nadeem, F., Ur Rehman, H., Wahid, A., Basra, S. M., & Siddique, K. H. (2019). Seed priming in field crops: Potential benefits, adoption and challenges. Crop and Pasture Science, 70(9), 731–771. https://doi.org/10.1071/CP18604
- García-Gómez, C., Obrador, A., González, D., Babín, M., Fernández, M. D., & Lojkowski, W. (2017). Comparative effect of ZnO NPs, ZnO bulk and ZnSO4 in the antioxidant defences of two plant species growing in two agricultural soils under greenhouse conditions. The Science of the Total Environment, 589, 11–24. https://doi.org/10.1016/j.scitotenv.2017.02.153
- Hassan, M., Aamer, M., Umer Chattha, M., Haiying, T., Shahzad, B., Barbanti, L., Nawaz, M., Rasheed, A., Afzal, A., Liu, Y., & Guoqin, H. (2020). The critical role of zinc in plants facing the drought stress. Agriculture, 10(9), 396. https://doi.org/10.3390/agriculture10090396
- Horax, R., Hettiarachchy, N., & Islam, S. (2006). Total phenolic contents and phenolic acid constituents in 4 varieties of bitter melons Momordica charantia and antioxidant activities of their extracts. Journal of Food Science, 70(4), C275–C280. https://doi.org/10.1111/j.1365-2621.2005.tb07173.x
- Hou, J., Wu, Y., Li, X., Wei, B., Li, S., & Wang, X. (2018). Toxic effects of different types of zinc oxide nanoparticles on algae, plants, invertebrates, vertebrates and microorganisms. Chemosphere, 193, 852–860. https://doi.org/10.1016/j.chemosphere.2017.11.077
- Jośko, I., Oleszczuk, P., & Futa, B. (2014). The effect of inorganic nanoparticles ZnO, Cr2O3, CuO and Ni and their bulk counterparts on enzyme activities in different soils. Geoderma, 232-234, 528–537. https://doi.org/10.1016/j.geoderma.2014.06.012
- Kabera, J. N., Semana, E., Mussa, A. R., & He, X. (2014). Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. Journal of Pharmaceutical and Pharmacological Sciences, 27, 377–392.
- Kim, Y. K., Park, W. T., Uddin, M. R., Kim, Y. B., Bae, H., Kim, H. H., Park, K. W., & Park, S. U. (2014). Variation of charantin content in different bitter melon cultivars. Asian Journal of Chemistry, 26(1), 309–310. https://doi.org/10.14233/ajchem.2014.15338
- Kosová, K., Vítámvás, P., Urban, M. O., Prášil, I. T., & Renaut, J. (2018). Plant abiotic stress proteomics: the major factors determining alterations in cellular proteome. Frontiers in Plant Science, 9, 122. https://doi.org/10.3389/fpls.2018.00122
- Mazhar, M. W., Ali, Q., Ishtiaq, M., Ghani, A., Maqbool, M., Hussain, T., & Mushtaq, W. (2021). Zinc-aspartate-mediated drought amelioration in maize promises better growth and agronomic parameters than zinc sulfate and l-aspartate. SABRAO Journal of Breeding and Genetics, 532, 1–21.
- Mazhar, WM., Ishtiaq, M., Hussain, I., Parveen, A., Hayat Bhatti, K., Azeem, M., Thind, S., Ajaib, M., Maqbool, M., Sardar, T., Muzammil, K., & Nasir, N. (2022). Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PloS One, 17(3), e0264967. https://doi.org/10.1371/journal.pone.0264967
- Palada, M. C., Ebert, A. W., Yang, R. Y., Chang, L. C., Chang, J., & Wu, D. L. (2017). Progress in research and development of moringa at the World Vegetable Center. Acta Horticulturae, 1158(1158), 425–434. https://doi.org/10.17660/ActaHortic.2017.1158.49
- Pękal, A., & Pyrzynska, K. (2014). Evaluation of aluminium complexation reaction for flavonoid content assay. Food Analytical Methods, 79, 1776–1782.
- Pirzadah, T. B., Malik, B., Maqbool, T., & Rehman, R. U. (2019). Development of nano-bioformulations of nutrients for sustainable agriculture. In Nanobiotechnology in Bioformulations. pp. 381–394. Springer.
- Raei, M., Angaji, S. A., Omidi, M., & Khodayari, M. (2014). Effect of abiotic elicitors on tissue culture
- Rahmawati, M., Mahfud, C., Risuleo, G., & Jadid, N. (2022). Nanotechnology in plant metabolite improvement and in animal welfare. Applied Sciences, 12(2), 838. https://doi.org/10.3390/app12020838
- Rajasree, G., & Pillai, G. R. (2012). Effect of nitrogen nutrition on fruit quality and shelf life of cucurbitaceous vegetable bitter gourd. Journal of Plant Nutrition, 35(8), 1139–1153. https://doi.org/10.1080/01904167.2012.676127
- Rakshit, A., and Singh, H. B. Eds. (2018). Advances in Seed Priming. Springer.
- Rashmi, R., & Trivedi, M. P. (2014). Effect of various growth hormone concentration and combination on callus induction, nature of callus and callogenic response of Nerium odorum. Applied Biochemistry and Biotechnology, 172(5), 2562–2570. https://doi.org/10.1007/s12010-013-0693-1
- Reed, R. B., Ladner, D. A., Higgins, C. P., Westerhoff, P., & Ranville, J. F. (2012). Solubility of nano‐zinc oxide in environmentally and biologically important matrices. Environmental Toxicology and Chemistry, 31(1), 93–99. https://doi.org/10.1002/etc.708
- Rehman, A., Farooq, M., Ahmad, R., & Basra, S. M. A. (2015). Seed priming with zinc improves the germination and early seedling growth of wheat. Seed Science and Technology, 43(2), 262–268. https://doi.org/10.15258/sst.2015.43.2.15
- Rehman, A., Farooq, M., Naveed, M., Nawaz, A., & Shahzad, B. (2018). Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. European Journal of Agronomy, 94, 98–107. https://doi.org/10.1016/j.eja.2018.01.017
- Rehman, A., Farooq, M., Naveed, M., Ozturk, L., & Nawaz, A. (2018). Pseudomonas-aided zinc application improves the productivity and biofortification of bread wheat. Crop and Pasture Science, 69(7), 659–672. https://doi.org/10.1071/CP17441
- Rehman, A., Farooq, M., Ullah, A., Nawaz, A., Ud Din, M. M., & Shahzad, B. (2022). Seed priming with zinc sulfate and zinc chloride affects physio-biochemical traits, grain yield and biofortification of bread wheat (Triticum aestivum). Crop & Pasture Science, 73(5), 449–460. https://doi.org/10.1071/CP21194
- Rehman, A., Khan, I., & Farooq, M. (2023). Secondary metabolites mediated reproductive tolerance under heat stress in plants. Journal of Plant Growth Regulation, 1, 1–19.
- Rizwan, M., Ali, S., Zia Ur Rehman, M., Adrees, M., Arshad, M., Qayyum, M. F., Ali, L., Hussain, A., Chatha, S. A. S., & Imran, M. (2019). Alleviation of cadmium accumulation in maize Zea mays L. by foliar spray of zinc oxide nanoparticles and biochar to contaminated soil. Environmental Pollution (Barking, Essex: 1987), 248, 358–367. https://doi.org/10.1016/j.envpol.2019.02.031
- Sadeghian, S. Y., & Yavari, N. (2004). Effect of water‐deficit stress on germination and early seedling growth in sugar beet. Journal of Agronomy and Crop Science, 190(2), 138–144. https://doi.org/10.1111/j.1439-037X.2004.00087.x
- Saini, R., Rai, P. K., Bara, B. M., Sahu, P., Anjer, T., & Kumar, R. (2017). Effect of different seed priming treatments and its duration on seedling characters of Bitter gourd Momordica charantia L. Journal of Pharmacognosy and Phytochemistry, 65, 848–850.
- Sedghi, M., Hadi, M., & Toluie, S. G. (2013). Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Annales of West University of Timisoara. Series of Biology, 162, 73.
- Sharafi, E., Fotokian, M. H., & Loo, H. (2013). Improvement of hypericin and hyperforin production using zinc and iron nano-oxides as elicitors in cell suspension culture of John’swort Hypericum perforatum L. Journal of Medicinal Plants and by-Products, 2(2),177–184.
- Sher, A., Sarwar, T., Nawaz, A., Ijaz, M., Sattar, A., & Ahmad, S. (2019). Methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings pp. 1–10. Springer.
- Siddiqui, Z. A., Parveen, A., Ahmad, L., & Hashem, A. (2019). Effects of graphene oxide and zinc oxide nanoparticles on growth, chlorophyll, carotenoids, proline contents and diseases of carrot. Scientia Horticulturae, 249, 374–382. https://doi.org/10.1016/j.scienta.2019.01.054
- Sulaiman, C. T., & Balachandran, I. (2012). Total phenolics and total flavonoids in selected Indian medicinal plants. Indian Journal of Pharmaceutical Sciences, 74(3), 258–260. https://doi.org/10.4103/0250-474X.106069
- Thakkar, K. N., Mhatre, S. S., & Parikh, R. Y. (2010). Biological synthesis of metallic nanoparticles. Nanomedicine: nanotechnology, Biology, and Medicine, 6(2), 257–262. https://doi.org/10.1016/j.nano.2009.07.002
- Wang, X. P., Li, Q. Q., Pei, Z. M., & Wang, S. C. (2018). Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biologia Plantarum, 62(4), 801–808. https://doi.org/10.1007/s10535-018-0813-4
- Waqas, M., Korres, N. E., Khan, M. D., Nizami, A. S., Deeba, F., Ali, I., & Hussain, H. (2019). Advances in the concept and methods of seed priming. In Priming and Pretreatment of Seeds and Seedlings. pp. 11–41. Springer.
- Wojnarowicz, J., Chudoba, T., Gierlotka, S., & Lojkowski, W. (2018). Effect of microwave radiation power on the size of aggregates of ZnO NPs prepared using microwave solvothermal synthesis. Nanomaterials, 8(5), 343. https://doi.org/10.3390/nano8050343
- Wojnarowicz, J., Opalinska, A., Chudoba, T., Gierlotka, S., Mukhovskyi, R., Pietrzykowska, E., Sobczak, K., & Lojkowski, W. (2016). Effect of water content in ethylene glycol solvent on the size of ZnO nanoparticles prepared using microwave solvothermal synthesis. Journal of Nanomaterials, 2016, 1–15. https://doi.org/10.1155/2016/2789871
- Wu, F., Fang, Q., Yan, S., Pan, L., Tang, X., & Ye, W. (2020). Effects of zinc oxide nanoparticles on arsenic stress in rice Oryza sativa L.: germination, early growth, and arsenic uptake. Environmental Science and Pollution Research International, 27(21), 26974–26981. https://doi.org/10.1007/s11356-020-08965-0

