?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Phytophthora meadii causes fruit rot disease (FRD) in arecanut palm trees in the Western Ghats of Southern India. The control of this disease is challenging due to intrinsic factors like continuous rainfall, skilled climbers being required and the emergence of virulent biotypes. Farmers have been relying on the fungicides for the FRD control, however efficacy of these fungicides is largely dependent on application strategy, schedule and timings. The main aim of this investigation was to evaluate commonly used and newly developed 12 oomycete-specific fungicidal products with different application strategies in 2018 and 2019 at Malnad regions. Fungicides viz., Bordeaux mixture, Mandipropamid, Metalaxyl + Mancozeb and Fosetyl-Al were the most effective in reducing FRD and efficiently controlling (70–80%) the disease with a statistically significant difference compared to untreated control (p ≤ 0.05). However, the efficacy of the evaluated fungicides greatly varied over the years. In terms of application strategies, the pre-monsoon application proved to be more effective in controlling FRD under field conditions. Further, this study suggests that a calendar-based approach to fungicidal application is recommended for effective management of FRD.
Reviewing Editor:
1. Introduction
In India, the area and production of arecanut (Areca catechu L.) have increased significantly over the past 20 years. This is attributed to the establishment of new arecanut plantations in non-traditional areas with changing production models (Balanagouda et al., Citation2021b; DASD, Citation2022). India is the world’s leading producer of arecanut, with a total acreage of 793,705 ha and a production of 1,559,753 tonnes/ha during the 2021–22 year (DASD, Citation2022; Mitra & Devi, Citation2018). However, newly established plantations in non-traditional areas face greater challenges in controlling arecanut pests and diseases, particularly the emergence of new diseases and the reoccurrence of old ones (Balanagouda et al., Citation2021a).
Notably, the occurrence of fruit rot disease (FRD) in newer arecanut plantations is one such example (Jose et al., Citation2008). The occurrence of FRD has increased recently in India, mainly attributed to the expansion of newer arecanut plantations to the Maidan region, providing suitable climatic conditions for FRD development and growing of highly susceptible varieties in newly established plantations (Balanagouda et al., Citation2022a). Recently, arecanut plantations have been grown with varied intensive cropping systems, which provided a favorable situation for the emergence of numerous fungal diseases due to high-density planting. Therefore, there is an urgent need to develop FRD control measures in newly established plantations of non-traditional areas to ensure the maximum system productivity and economic viability of the growers (Balanagouda et al., Citation2023a).
Till now, many Phytophthora species have been reported and described on arecanut as a causal agent (Chowdappa et al., Citation2002; Das & Cheeran, Citation1986; Sastry & Hedge, Citation1985), however, an oomycete organism Phytophthora meadii is a major species which predominantly occurs on arecanut-growing regions (Balanagouda et al., Citation2022b). FRD mainly affects the fruit/nuts, causing initially brownish water-soaked lesions near the perianth region of the nuts, dropping off the infected nuts and leading to fruit rot in severe infections. This entity can infect buds and crown regions leading to bud/crown rots, which can cause severe infections in the fruit (Saraswathy, Citation1994; Citation2004). FRD being a fatal disease to arecanut palms, posed a serious threat to arecanut production which resulted in severe yield reductions of about 50–90%, 72–350 kg nuts/acre and almost two lakh rupees losses/ha (Coleman, Citation1910; Jose et al., Citation2008; Koti Reddy & Anandaraj, Citation1980; Saraswathy, Citation1994; Sastry & Hedge, Citation1985).
Due to lack of resistant varieties, and difficulty in reducing the pathogen inoculum through crop management approaches under field conditions, the application of fungicides has been the only option for the control of FRD. In line with this, previous research has mainly focused on the application and evaluation of multiple fungicides to safeguard the arecanut plantations from FRD infection (Chowdappa et al., Citation2000; Naik et al., Citation2019; Narayanaswamy et al., Citation2017). However, previous studies have majorly tested the efficacy of Bordeaux mixture and other copper-based fungicides and recommended the application of Bordeaux mixture (1%) before the onset of monsoon rains at 30–45 days intervals for effective control of FRD (Ravikumar et al., Citation2019). Over some time, the indiscriminate use of Bordeaux mixture and copper-based fungicides led to the deposition of copper residues on the nut surface (Anandaraj, Citation1985; Mathew et al., Citation2015; Rao, Citation1962).
Furthermore, to avoid the problem of copper deposition, some studies have employed biocontrol agents (BCA) for the eco-friendly management of FRD under field conditions (Naik et al., Citation2019). Subsequently, the fungicide-amended urea briquettes (FAUBs) were attempted against FRD in arecanut plantations located in the Konkan region of Maharashtra and disease-prone areas of Karnataka, India (Balanagouda et al., Citation2023a; Pande et al., Citation2016). Attempts have been made to use integrated approaches in the field, such as old copper-based fungicides, BCA formulations and soil application of FAUBs. However, due to the limited availability of registered fungicides and a lack of newer fungicides with multi-actions against pathogens, there are challenges in effectively controlling fungal diseases.
This posed a serious concern regarding FRD control in India and the difficulty of implementing a fungicidal management program without repetitive application of the same registered fungicides. This eventually led to the development of fungicidal resistance among the pathogens responsible for FRD (Heungens et al., Citation2016; Pérez-Sierra et al., Citation2011; Turner et al., Citation2008; Vercauteren et al., Citation2010; Wagner et al., Citation2008). Therefore, it is of utmost importance to evaluate newer fungicidal molecules with different modes of action and explore application strategies, which can aid in better managing FRD with oomycete-specific fungicides. Hence, the present investigation aimed to improve the available FRD control approaches in India by the usage of novel fungicides. Further, the efficacy of systemic and contact oomycete-specific fungicides was tested and time-based application strategies were evaluated for FRD control under field conditions.
2. Materials and methods
2.1. Experimental plots
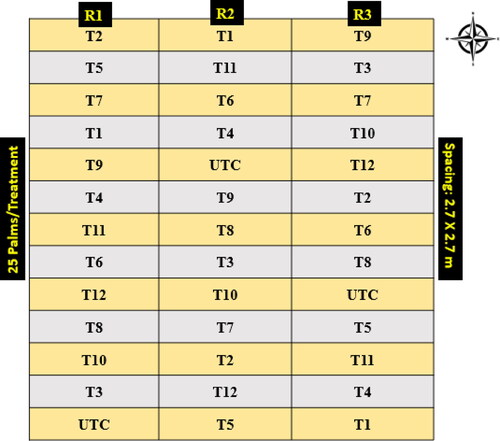
Field experiments were carried out in a commercial arecanut plantation located in the Malnad region, Karnataka, India belongs to Central Western Ghats of agro-ecological region (13.41600°N latitude, and 75.13480°E longitude) with mean altitude of 603 m above the MSL. This location is highly prone to FRD infection and the hot-spot area comes under the jurisdiction of the University of Agricultural and Horticultural Sciences (UAHS), Shivamogga, Karnataka, India. The arecanut plantation was planted in 2010 with a susceptible variety (A. catechu cv. Mangala) following the spacing of 2.7 m between the rows and 2.7 m distance between the trees. The experiment was conducted in 2018 and 2019 during the monsoon season and was designed in a completely randomized block (RCBD) with three replications and 25 trees were maintained per treatment. During this investigation, a total of 13 treatments were maintained including one reference control and one untreated control (UTC) ().
2.2. Fungicidal products
All the oomycete-specific fungicidal products applied in the trial were procured as commercially formulated products (). These products were applied in the experiments based on recommendations given by the manufacturers (Crop Care Chemical Suppliers, Mangalore, Karnataka, India). The rates of non-tested or no-registered fungicides in India were tested using similar doses to those used on the other plantations or fruit or vegetable crops. But, the Bordeaux mixture (1%) was freshly prepared on the day of spraying by following a standard protocol (Narayanaswamy et al., Citation2017; Ravikumar et al., Citation2019). None of the other fungicides were applied or evaluated in the arecanut plantation during the experimental period.
Table 1. Oomycete-specific fungicidal products for the control of fruit rot disease (FRD) of arecanut during 2018 and 2019 in the Malnad region, Karnataka, India.
2.3. Fungicidal products application
The oomycete-specific fungicidal products were sprayed to arecanut bunches during 2018 and 2019 by using the rocket sprayer® (model RHSD-0131 with heavy-duty, Maico Pipe Industries, Gujarat, India) was equipped with an adjacent cone jet tip (Narayanaswamy et al., Citation2017). The sprayer has a flexible nozzle, that is, ABS plastic nozzles (Maico Pipe Industries, Gujarat, India) and the operational pressure of the nozzle is about 10–15 bar; the performance of the sprayer could reach up to 25–30 ft. height with 75–80 gallons on one battery charge. The volume of the fungicidal solution was set up to approximately 5 L palm−1, which is a commercially used spraying solution in major arecanut-growing regions of Karnataka, India. In each experiment, an untreated control (UTC) or check was included which consisted of tap water instead of fungicidal solutions. The palms were treated with each fungicide with adhesive or wetting agent (Indetran, commercially available at the local market was used) and phytosanitation was kept common for all the treatments.
2.4. Fungicide application strategies
Different fungicidal application programs were tested in 2018 and 2019 (, footnote) using newer oomycete-specific combi-products and commonly registered copper-based fungicides against FRD in Karnataka, India. All the strategies were initiated at immature and marble nut stages. In all the conditions, the spray strategies did not involve any alternating of fungicides, and the number of spraying schedules as well as intervals was mostly dependent on seasonal strategy.
In each trial, UTC sprays with water were performed at each interval of fungicidal application to the arecanut bunches. We compared the calendar-based fungicide application strategies viz., the first spray on the 30th of May, the second on the 15th of July, and the third on the 30th of August during monsoon season at 30–45 days intervals. The efficacy of oomycete-specific fungicides at different spray programs was evaluated and compared in response to the moment of FRD occurrence.
2.5. Disease assessment
During each trial, various disease-related factors were observed and measured, including the incidence and severity of FRD and the cumulative fallen nut rate (CFNR). To determine the FRD incidence, the ratio of infected palms to healthy palms was calculated. Meanwhile, the severity of FRD was determined by assessing the percentage of the arecanut bunch surface that displayed symptoms of FRD, using a standard 0–6 scale () as established by Sastry and Hedge (Citation1987). CFNR was calculated by averaging the number of fallen nuts caused by FRD infection per palm weekly (it was expressed in numbers). To evaluate FRD incidence, FRD severity and CFNR, 50 palms per treatment including an untreated control were randomly selected and analyzed
Table 2. The following rating scale was utilized to estimate the percent disease severity of FRD on arecanut.
Further, we calculated the effectiveness of the fungicidal products by modifying Abbott’s formula (Abbott, Citation1925). The formula used is Efficacy = 100 × (1 − (ST/SUTC)). ST represents the average FRD severity in a particular treatment, while SUTC indicates the average FDR severity in UTC.
2.6. Statistical analysis
Before analysis, we initially assessed the normality of the data by checking the Kolmogorov–Smirnov test (Vannini et al., Citation2010), and the two-year dataset was pre-processed and the percentage data was arcsine-transformed. Each trial of the experimental dataset was analyzed separately using JMP (version 16.0.0, SAS Institute Inc., Cary, NC, USA). To estimate the marginal means of the treatment, we used the ‘emmeans’ function from the emmeans R package (Length et al., Citation2018). Mean comparisons were conducted among treatments using Tukey–Kramer’s test with a significance level of 5%.
3. Results
3.1. FRD severity, incidence and CFNR in different treatments
During the experiments conducted in 2018 and 2019, it was observed that the treatment with UTC had the highest rates of FRD severity, incidence and CFNR. The average values for FRD severity, incidence and CFNR were 26.14%, 27.59% and 117.64 in 2018, respectively. These values increased to 31.61%, 32.59% and 142.24, respectively, in 2019.
A total of 12 multi-site, single/combi fungicides which mainly target oomycetes including UTC were evaluated in 2018 and 2019 under field conditions. Among the different treatments during 2018, significantly lower FRD severity, incidence and CFNR were observed in bunch spraying of Bordeaux mixture (5.10%, 7.63% & 22.95, respectively) followed by Mandipropamid (5.32%, 8.75% & 23.96, respectively), a combi-product of Metalaxyl + Mancozeb (8.13%, 11.69% & 36.61, respectively) and Fosetyl-Al (8.71%, 12.62% & 39.20, respectively). Thrice-bunch spraying of these fungicides significantly reduced the FRD severity, incidence and CFNR under field conditions (). Therefore, these treatments proved to be significantly better than all other treatments, including untreated control (UTC). However, UTC had the highest recorded severity and incidence of FRD, and CFNR with 26.14%, 27.59% and 117.64, respectively.
Figure 2. Fruit rot disease (FRD) severity (green colored) and reduction in severity or control efficiency (%, yellow colored) of different contact and systemic oomycete-specific fungicides in 2018 and 2019. The error bars indicate the standard error of the mean.
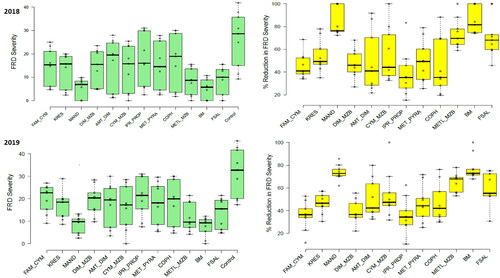
Figure 3. Fruit rot disease (FRD) incidence (green colored) and the corresponding reduction in incidence or control efficiency (%, yellow colored) of different contact and systemic oomycete-specific fungicides in 2018 and 2019. The error bars indicate the standard error of the mean.
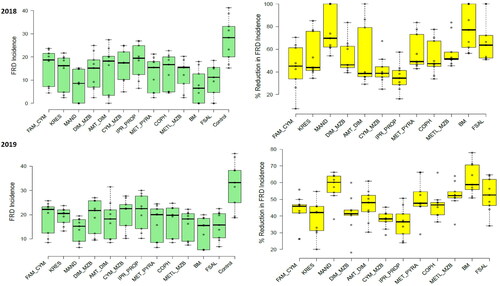
Figure 4. CFNR (green colored) and the corresponding reduction in incidence or control efficiency (%, yellow colored) of different oomycete-specific fungicides in 2018 and 2019. The error bars indicate the standard error of the mean.
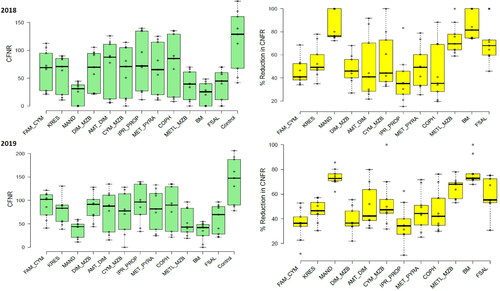
In 2019, the bunch spraying of Bordeaux mixture (7.57%, 12.98% & 34.08, respectively), followed by Mandipropamid (8.67%, 13.89% & 39.01, respectively), a combi-product of Metalaxyl + Mancozeb (11.76%, 18.53% & 52.93, respectively) and Fosetyl-Al (13.33%, 15.54% & 60, respectively) had the most substantial effect on reducing FRD severity, incidence and CFNR. Thrice-bunch spraying of these fungicides significantly reduced FRD under field conditions (), proving to be significantly better than all other treatments, including the untreated control. UTC had the highest recorded severity and incidence of FRD, and CFNR with 31.61%, 32.59% and 142.24, respectively. The results in 2019 showed a higher FRD severity, incidence and CFNR than in 2018 under field conditions.
3.2. Control efficiency of FRD in different treatments
Based on the results of the experiments conducted over two years, the Bordeaux mixture and Mandipropamid demonstrated the highest efficacy rates, exceeding 70%. A combination of Metalaxyl + Mancozeb, as well as Fosetyl-Al, showed moderate efficacy rates in the range of 50–60%. The remaining tested products showed less than 50% efficacy in controlling FRD, as shown in .
In 2018, there were significant reductions in the severity of FRD for all the products compared to the corresponding UTC, with a p value of less than 0.05. All fungicides had efficacy indices above 50% as shown in . The most effective products in order of efficacy were the Bordeaux mixture, Mandipropamid, Metalaxyl + Mancozeb and Fosetyl-Al, all with efficacies ranging from 60% to 80%. The highest efficacies were achieved with Bordeaux mixture (80.49%) and Mandipropamid (79.63%). Combi-product Metalaxyl + Mancozeb and Fosetyl-Al had control efficiencies of 68.88% and 66.68%, respectively. The other tested fungicides had lower efficacies ranging from 30% to 50% and showed substantial differences among them. Under field conditions, the Iprovalicarb + Propineb and Ametoctradin + Dimethomorph combi-products; copper hydroxide demonstrated the lowest efficacies at 32.78%, 40.31% and 36.12%, respectively. However, the other fungicides displayed moderate efficacies against FRD.
In 2019, a similar trend was observed, significant reductions in FRD severity between all the products and the corresponding UTC were shown at p < 0.05. All the tested fungicides exhibited efficacy indices greater than 50% but lesser control efficiency was noticed in 2019 than in 2018 (). The best products this season, in the order of efficacy, were the Bordeaux mixture, Mandipropamid, Metalaxyl + Mancozeb and Fosetyl-Al; all of them showed efficacies ranging from 57% to 76%. However, the highest efficacies were with the Bordeaux mixture (76.04%), and Mandipropamid (72.58%). While combi-product Metalaxyl + Mancozeb and Fosetyl-Al had a control efficiency of 62.79% and 57.82%, respectively. The other tested fungicides represented the lower efficacies ranging from 30% to 46% with substantial differences among them. The lowest efficacies were demonstrated by a combi-products Iprovalicarb + Propineb (34.56%) and Famoxadone + Cymoxanil (36.88%), whereas the rest of the fungicides showed moderate efficacies against FRD under field conditions.
The results from studies conducted in 2018 and 2019 on FRD severity, incidence and CFNR, as well as the control efficacy of various oomycete-specific fungicides evaluated under field conditions, confirmed that the Bordeaux mixture, Mandipropamid, Metalaxyl-Mancozeb and Fosetyl-Al were the most effective in reducing FRD and efficiently controlling the disease with a statistically significant difference compared to the other tested fungicides and UTC.
3.3. Efficacies of fungicides against FRD applied at different strategies
The application strategies that were evaluated showed moderate to high control of the disease. In 2018, the strategies had higher efficacies, with an average of over 70%, compared to 2019, where they ranged from 45% to 55%. All treatments proved effective in significantly reducing FRD severity when compared to the UTC (p < 0.01) ().
Table 3. FRD severity, control efficiency and other corresponding statistics of 12 fungicides evaluated at pre-monsoon time for control of FRD in 2018 and 2019.
Table 4. FRD severity, control efficiency and associated parameters for 12 fungicides tested during the Mid-monsoon period for control of FRD in 2018 and 2019.
Table 5. FRD severity, control efficiency and associated parameters for 12 fungicides were evaluated during fag-end monsoon for control of FRD in 2018 and 2019.
The FRD severity experiments where fungicides were applied during the pre-monsoon period (30th May), indicated that the mean FRDseverity at the UTC plots was higher (28.88%). Bordeaux mixture resulted in the lowest value of pooled FRDseverity of 6.34%, and the latter values represented 78.04% control efficiency (CE) relative to the corresponding UTC. There was no significant difference observed between the Bordeaux mixture and Mandipropamid (7.00% FRDseverity and 75.76% CE) but Metalaxyl-Mancozeb and Fosetyl-Al showed moderate FRDseverity (9.95% and 11.02%) and control efficiencies (65.54% and 61.84%). The rest of the tested fungicides showed intermediate to lower control efficiencies and FRDseverity levels corresponding to the UTC ().
In the field experiment where fungicides were sprayed in the mid-monsoon, that is, 15th July (30–45 days after the first spray), the average FRD severity in the non-treated control (UTC) was 30.10%. The Bordeaux mixture and Mandipropamid had the lowest FRDseverity values with no significant difference: 10.20% and 10.90% (control efficiency of 66.11% and 63.78%, respectively) (p < 0.05). The Bordeaux mixture and Mandipropamid were the most efficient fungicides to control FRD, with the lowest FRDseverity. No significant differences in FRDseverity between the combi-products viz., Copper hydroxide, Ametoctradin + Dimethomorph, Kresoxymethyl and Dimethomorph + Mancozeb but these fungicides had significant differences with Metalaxyl + Mancozeb and Fosetyl-Al ().
In the FRDseverity experiments, fungicides were applied at fag-end of the monsoon season on 30th August. The mean FRD severity at the UTC plots was found to be higher at 130.00%. However, the Bordeaux mixture resulted in the lowest pooled FRDseverity value of 28.50%. This resulted in a control efficiency of 78.07% relative to the corresponding UTC. There was no significant difference observed between the effectiveness of the Bordeaux mixture and Mandipropamid, which had a FRDseverity of 31.50% and a control efficiency of 75.76%. On the other hand, Metalaxyl + Mancozeb and Fosetyl-Al showed moderate FRD severity at 44.80% and 49.60%, respectively. Their corresponding control efficiencies were 65.53% and 61.84%. The remaining tested fungicides showed lower control efficiencies and FRD severity levels compared to the UTC ().
There was a noticeable difference in the severity of FRD among all treatments and UTC. The pre-monsoon application proved to be more effective in controlling FRD under field conditions. While a fixed timing of application showed higher efficiency, there was a significant difference (p ≤ 0.05) found between fungicide application timing and treatment control efficiency. This study suggests that a calendar-based approach to fungicidal application is recommended for the effective management of FRD ().
Table 6. Variations in FRD severity and control efficiency between the time of fungicidal application for control of FRD in 2018 and 2019.
4. Discussion
To effectively control the arecanut FRD using fungicides, it is necessary to use the most effective fungicides and improve application strategies. This is particularly important in areas where highly susceptible cultivars to FRD are planted. In the southern states of India, previous research has shown that the Bordeaux mixture has good efficacy, while some copper-based products have medium efficacy. It is recommended to apply one treatment before the onset of monsoon and two additional treatments at intervals of 30–45 days (Chowdappa et al., Citation2000; Naik et al., Citation2019; Narayanaswamy et al., Citation2017; Ravikumar et al., Citation2019). In India, various fungicides such as Copper hydroxide, Copper oxychloride, Metalaxyl + Mancozeb, and Potassium Phosphonate were once recommended for controlling FRD disease in arecanut plantations (Hegde, Citation2015; Lokesh et al., Citation2014; Pande et al., Citation2016). However, due to their inefficacy and other inherent factors, they are no longer considered effective. Recently, new systemic fungicidal products have been introduced in the Indian market to combat FRD disease in arecanut plantations (Balanagouda et al., Citation2023b). We aimed to assess the effectiveness of these new products in controlling FRD disease and develop an optimized strategy for their application timing.
P. meadii is a ubiquitous and hemi biotrophic organism, quite difficult to isolate and grow on synthetic culture media (Balanagouda et al., Citation2022a, Citation2022b). Consequently, the testing of the effectiveness of fungicides is better conducted under natural field conditions that are conducive to pathogen development. This is a serious constraint for several fungicides and strategies to be tested under such conditions. However, our trials were conducted in an area where FRD occurs naturally (Balanagouda et al., Citation2023b; Coleman, Citation1910; Ravikumar et al., Citation2019). During the experimental period, FRD levels in the UTC (45–55%) were similar to those reported by Narayanaswamy et al. (Citation2017). The results of the fungicide trials confirmed that different fungicides behave differently in terms of their effectiveness in controlling FRD.
Our study aimed to evaluate the efficacy of 12 fungicides that are specific to oomycetes. These fungicides have contact and penetrant products that operate through different modes of action. After conducting three spray applications, we found that the products showed high variability in their efficacy, ranging from 30% to 80%. Over the course of two years, the Bordeaux mixture and Mandipropamid demonstrated the highest efficacy rates, exceeding 70%. The combination of Metalaxyl + Mancozeb and Fosetyl-Al showed moderate efficacy rates in the range of 50-60%. The differences in efficacy values could be attributed to the various modes of action of the active ingredients used, with higher FRD control observed in the latter case. We suggest that the superior efficacy performance of Mandipropamid is due to its unique mode of action as a membrane disruptor, as well as its additional penetrant action, as described by Shashidhara (Citation2007) and Shah (Citation2009).
In the past, copper-based fungicides such as Copper hydroxide and Copper oxychloride were commonly used to control FRD in field conditions. However, recent studies have shown that these fungicides have lower efficacy rates of less than 30% when applied seasonally. Multiple studies in arecanut-growing regions, including those conducted by Narayanaswamy et al. (Citation2017) and Naik et al. (Citation2019) have found that these fungicides are not suitable for effectively managing FRD in field conditions. On the other hand, the use of a Bordeaux mixture (prepared manually and freshly) provides better control of FRD. Farmers rely on this fungicide because of its higher efficacy (75–80%), which is mainly due to its ability to penetrate the cuticle of tender nuts and arecanut bunches, allowing soluble copper to enter the plant’s system (Anandaraj & Saraswathy, Citation1986; Balanagouda et al., Citation2023b; Chowdappa et al., Citation2002).
In general, the efficacies of fungicides were lower in years with low levels of disease (e.g. in 2018) in both the fungicide application strategy trials, as compared to the rest of the experiments. This issue could be related to less precise measurements of both disease incidence and severity at low disease levels, which resulted in a higher variability and non-significant mean treatment comparisons and hence, in a disputed disease control efficacy (Ojiambo et al., Citation2010). The fungicide products used in the study were classified into three main categories based on their overall efficacy during the trials. The first category showed higher efficacies (65–80%) and included Bordeaux mixture and Mandipropamid. The second group had moderate efficacies (50–65%) and included a combination of Metalaxyl + Mancozeb and Fosetyl-Al. Finally, the third group showed the lowest efficacies (<50%) and included the remaining tested fungicides in the trials.
Regarding the evaluation of the different application strategies, all the treatments reduced the disease levels compared to the UTC with significant differences between strategies in terms of disease incidence reduction. Treatments with a fixed application timing showed higher efficacies as compared to the blind application of the fungicides. This is consistent with recommendations from various authors who suggest applying fungicides before the monsoon season followed by two more sprays at 30–45 days intervals for the best results in reducing disease (Balanagouda et al., Citation2023a; Naik et al., Citation2019; Narayanaswamy et al., Citation2017; Ravikumar et al., Citation2019). The pre-monsoon application was found to be particularly effective in controlling disease in field conditions. While a fixed timing of application was more efficient, there was a significant difference (p ≤ 0.05) in control efficiency between different fungicide application timings. This study suggests that a calendar-based approach for fungicidal application is recommended for the effective management of the disease. However, there were significant variations in the number of seasonal applications among the different strategies, which is an important consideration in terms of economic and environmental impact.
To sum up, our research has shown that using fungicides before the monsoon season is essential in controlling FRD in field conditions. Instead of blindly applying fungicides, growers and researchers should follow a calendar-based approach. Our results have emphasized the importance of timing the application of fungicides to effectively control FRD. Our study has confirmed the effectiveness of oomycete-specific fungicides on FRD disease levels and CFNR when used in different application strategies. This research is a crucial first step in developing a sustainable fungicide program based on different strategies for managing FRD disease with fungicides. Further studies are necessary to validate the best combinations of products and their application at specific times when disease infections may occur. This information will be useful for growers, stakeholders, policymakers and the scientific community in selecting and applying fungicides at the right time to control FRD.
Author contributions
Conceptualization, BP and SS; Methodology, BP and SS; Investigation, BP; Writing-original draft preparation, BP, RTPP and SHT; Writing-review and editing, RC, HOE, FAA, SS, RTPP, SHT, NCV and P. All authors agree with the content of this publication and consent to submit this article to the journal. The authors declare that they obtained content from the responsible authorities of the university and institute for the article.
Ethical approval
The authors declare that (i) the manuscripts have not been submitted to another journal simultaneously, (ii) the submitted work contains original data not published earlier, (iii) this article follows the experimental guidelines of the country, (iv) no human beings or animal are not involved during the study.
Acknowledgments
This study was part of the Ph.D. thesis of the first author and we are thankful to the Keladi Shivappa Nayaka University of Agricultural and Horticultural Sciences, Shivamogga, Karnataka for providing the required financial assistance and collaborative institute ICAR-Central Plantation Crops Research Institute, Kasaragod, Kerala, India for technical support, consultative facility. The authors would like to thank the Researchers Supporting Project Number (RSP2024R118), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Balanagouda Patil], upon reasonable request.
Additional information
Funding
References
- Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18(2), 1–11. https://doi.org/10.1093/jee/18.2.265a
- Anandaraj, M. (1985). Effect of adhesives on the retention of Bordeaux mixture on arecanut bunches. Annals in Tropical Research, 7, 62–65.
- Anandaraj, M., & Saraswathy, N. (1986). Effect of systemic fungicides on fruit rot of arecanut. Indian Phytopathology, 39, 607–609.
- Balanagouda, P., Hegde, V., Sridhara, S., Narayanaswamy, H., Naik, M. K., Patil, K. K. R., Rajashekara, H., & Mishra, A. K. (2022a). Exploring the impact of climatic variables on the arecanut fruit rot epidemic by understanding the disease dynamics in relation to space and time. Journal of Fungi, 8(7), 745. https://doi.org/10.3390/jof8070745
- Balanagouda, P., Hegde, V., Sridhara, S., Pandian, R. T. P., Thube, S. H., Gangaraj, K. P., Gangurde, S. S., & Jha, P. K. (2022b). Multigene phylogeny and haplotype analysis reveals predominance of oomycetous fungus, Phytophthora meadii (McRae) associated with fruit rot disease of arecanut in India. Saudi Journal of Biological Sciences, 29(8), 103341. https://doi.org/10.1016/j.sjbs.2022.103341
- Balanagouda, P., Narayanaswamy, H., Hegde, V., Sridhara, S., Pandian, R. T. P., & Thube, S. H. (2023a). Development and evaluation of fungicide-amended urea briquettes (FAUB’s) to combat fruit rot disease of arecanut: A farmers-friendly approach. Crop Protection, 165, 106155. https://doi.org/10.1016/j.cropro.2022.106155
- Balanagouda, P., Sridhara, S., Narayanaswamy, H., Hegde, V., Mishra, A. K., & Pruthviraj. (2023b). Efficacy of new generation oomycete-specific fungicides on life stages of Phytophthora meadii and field evaluation through bunch spraying system. Crop Protection, 168, 106232. https://doi.org/10.1016/j.cropro.2023.106232
- Balanagouda, P., Sridhara, S., Shil, S., Vinayaka, H., Naik, M. K., Narayanaswamy, H., & Siva, K. B. (2021a). Assessment of the spatial distribution and risk associated with fruit rot disease in Areca catechu L. Journal of Fungi (Basel, Switzerland), 7(10), 797. https://doi.org/10.3390/jof7100797
- Balanagouda, P., Vinayaka, H., Maheswarappa, H. P., & Narayanaswamy, H. (2021b). Phytophthora diseases of arecanut in India: Prior findings, present status, and future prospects. Indian Phytopathology, 74(3), 561–572. https://doi.org/10.1007/s42360-021-00382-8
- Chowdappa, P., Saraswathy, N., Vinayagopal, K., & Somala, M. (2000). Control of fruit rot o arecanut through polythene covering. Indian Phytopathology, 53, 321.
- Chowdappa, P., Saraswathy, N., Vinayagopal, K., & Somala, M. (2002). Natural occurrence of Phytophthora heveae. Indian Phytopathology, 55, 366.
- Coleman, L. C. (1910). Diseases of the areca palm. In Koleroga, mycological series. Bulletin no. 2 (pp. 92). Department of Agriculture, Mysore State.
- Das, T. P. M., & Cheeran, A. (1986). Infectivity of Phytophthora spp. on cash crops in Kerala. Agricultural Research Journal of Kerala, 24, 7–13.
- Directorate of Arecanut and Spices Development. (2022). Area, production and productivity statistics of arecanut. http://www.dasd.gov.in/statistics (accessed on 15 December 2022).
- FRAC. (2022). FRAC Code List 2021: Fungicides Sorted by Mode of Action. http://www.phi-base.org/images/fracCodeList.pdf (accessed on 31 July).
- Hegde, G. M. (2015). Bio-efficacy of potassium phosphonate against nut rot disease of arecanut (Areca catechu L.) in the northern Karnataka of India. Sri Lanka Journal of Food and Agriculture, 1(2), 9–14. https://doi.org/10.4038/sljfa.v1i2.14
- Heungens, K., De Dobbelaere, I., & Maes, M. (2016). Fungicide control of Phytophthora ramorum on Rhododendron. In Frankel, S. J., Shea, P. J., & Haverty, M. I. (Eds.). Proceedings of the sudden oak death second science symposium: the state of our knowledge (pp. 241–257). US Department of Agriculture, Forest Service, Pacific Southwest Research Station.
- Jose, C. T., Balasimha, D., & Kannan, C. (2008). Yield loss due to fruit rot (Mahali) disease of arecanut in Karnataka. Indian Journal of Arecanut, Spices and Medicinal Plants, 10, 45–51.
- Koti Reddy, M., & Anandaraj, M. (1980). Koleroga of arecanut. In Proc: Workshop on phytophthora diseases of tropical cultivated plants (pp. 71–79). Central Plantation Crops Research Institute.
- Lenth, R., Singmann, H., Love, J., Buerkner, P., & Herve, M. (2018). Emmeans: estimated marginal means, aka least-squares means. R Package Version 1:3.
- Lokesh, M. S., Patil, S. V., Palakshappa, M. G., & Gurumurthy, S. B. (2014). Role of systemic fungicide metalaxyl mancozeb in management of Koleroga (Phytophthora meadii Mc Rae) of arecanut (Areca catechu L.) in Central Western Ghats of Karnataka. Asian Journal of Biological Sciences, 9, 131–133.
- Mathew, P., Austin, R. D., Varghese, S. S., & Manoj Kumar, A. D. (2015). Effect of copper-based fungicide (Bordeaux mixture) spray on the total copper content of areca nut: Implications in increasing prevalence of oral submucous fibrosis. Journal of International Society of Preventive & Community Dentistry, 5(4), 283–289. https://doi.org/10.4103/2231-0762.161755
- Mitra, S. K., & Devi, H. (2018). Arecanut in India – Present situation and future prospects. Acta Horticulturae, 1205(1205), 789–794. https://doi.org/10.17660/ActaHortic.2018.1205.99
- Naik, B. G., Maheshwarappa, H. P., Nagamma, G., & Latha, S. (2019). Management of fruit rot disease of arecanut (Areca catechu L.) caused by (Phytophthora meadii Mc Rae.). International Journal of Current Microbiology and Applied Sciences, 8(04), 837–847. https://doi.org/10.20546/ijcmas.2019.804.094
- Narayanaswamy, H., Raju, J., & Jayalakshmi, K. (2017). Management of fruit rot disease of arecanut incited by Phytophthora meadii. International Journal of Current Microbiology and Applied Sciences, 6(7), 2824–2828. https://doi.org/10.20546/ijcmas.2017.607.393
- Ojiambo, P., Paul, P. A., & Holmes, G. J. (2010). A quantitative review of fungicide efficacy for managing downy Mildew in Cucurbits. Phytopathology, 100(10), 1066–1076. https://doi.org/10.1094/PHYTO-12-09-0348
- Pande, V. S., Dademal, A. A., Kasture, M. C., & Bhagwat, R. G. (2016). Management of Koleroga of arecanut caused by application of fungicide amended fertilizer briquettes Phytophthora meadii. Journal of Indian Society of Coastal Agricultural Research, 34, 88–90.
- Pérez-Sierra, A., Álvarez, L. A., Vercauteren, A., Heungens, K., & Abad-Campos, P. (2011). Genetic diversity, sensitivity to phenyl amide fungicides, and aggressiveness of Phytophthora ramorum on Camellia, Rhododendron, and Viburnum plants in Spain. Plant Pathology, 60(6), 1069–1076. https://doi.org/10.1111/j.1365-3059.2011.02485.x
- Rao, K. S. N. (1962). Estimation of copper on the finites sprayed with Bordeaux mixture. Agricultural Research, 2, 293.
- Ravikumar, M., Pradeep Kumar, B. A., & Niranjana, K. S. (2019). Standardization of concentration of Bordeaux mixture for management of fruit rot disease (P. meadii) of arecanut. Green Farming, 10, 115–117.
- Saraswathy, N. (2004). Diseases and disorders. In Rajagopal, V., & Balasimha, D. (Eds.). Arecanut (pp. 134–169). Central Plantation Crops Research Institute.
- Saraswathy, N. (1994). Studies on Phytophthora spp. on arecanut and arecanut based cropping systems [Ph.D. Thesis]. Mangalore University.
- Sastry, M. N. L., & Hedge, R. K. (1985). Taxonomic identity of arecanut Phytophthora isolates from the gardens of Sirsi, Uttara Kannada. In Shama Bhat, K., & Radhakrishnan Nair, C. P. (Eds.). Arecanut research and development (pp. 92–94). Central Plantation Crops Research Institute.
- Sastry, M. N. L., & Hedge, R. K. (1987). Phytophthora associated with arecanut (Areca catechu L) in Uttara Kannada, Karnataka. Current Science, 56, 367–368.
- Shah, T. A. (2009). Cause and management of Phytophthora fruit rot of tomato (Lycopersicon esculentum Mill.) in Kashmir (pp. 22–76) [Ph.D. Thesis]. Division of Plant Pathology. Sher-e-Kashmir University of Agricultural Sciences & Technology, Shalimar Campus.
- Shashidhara, S. (2007). Studies on foot rot of black pepper caused by Phytophthora capsici Leonian, emend, Alizedeh and Tsao (pp. 63) [M.Sc. Thesis]. Department of Plant Pathology, University of Agricultural Sciences.
- Turner, J., Jennings, P., Werres, S., & Wagner, S. (2008). Report response of isolates of Phytophthora ramorum exposed to fungicides commonly used to control Phytophthora diseases in nurseries. Deliverable Report 5, Forest Research, Central Sciences Laboratory.
- Vannini, A., Natili, G., Anselmi, N., Montaghi, A., & Vettraino, A. M. (2010). Distribution and gradient analysis of Ink disease in chestnut forests. Forest Pathology, 40(2), 73–86. https://doi.org/10.1111/j.1439-0329.2009.00609.x
- Vercauteren, A., De Dobbelaere, I., Grünwald, N. J., Bonants, P., Van Bockstaele, E., Maes, M., & Heungens, K. (2010). Clonal expansion of the Belgian Phytophthora ramorum populations based on new microsatellite markers. Molecular Ecology, 19(1), 92–107. https://doi.org/10.1111/j.1365-294X.2009.04443.x
- Wagner, S., Kaminski, K., & Werres, S. (2008). Evaluation of fungicides for control of Phytophthora ramorum. In Frankel, S. J., Kliejunas, J. T., & Palmieri, K. M. (Eds.). Proceedings of the sudden oak death second science symposium: The state of our knowledge (pp. 481–482). US Department of Agriculture, Forest Service, Pacific Southwest Research Station.