?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Drought severely affects maize growth and productivity, making the identification of tolerant genotypes essential, especially in local maize. Therefore, a greenhouse pot experiment was conducted in Sundarbazar, Lamjung, from February to April using 30 local maize accessions under irrigated and drought conditions in a completely randomized block design to study nine vegetative traits and screen drought tolerance for upcoming breeding programs. Accession NGRC05570 showed relative tolerance to root length and number, NGRC05586 to shoot length, NGRC05589 to root-to-shoot ratio, NGRC05591 to moisture content in roots, NGRC05578 to moisture in shoot and relative water content (RWC), NGRC05561 to root volume and NGRC05573 to normalized difference vegetation index (NDVI). Accession NGRC05571, although lacking singular dominance in traits, exhibited an overall tolerance for NDVI, root-to-shoot ratio, root number, root length and RWC. Corelation analysis between shoot moisture content and shoot length showed a significant negative correlation under drought (−0.38) but a significant positive correlation under irrigation (0.38). Similarly, a significant negative correlation between the root-to-shoot ratio and shoot moisture content was observed under drought (−0.62) but not under irrigation. The possibility of accession NGRC05571, collected from Surkhet, being used in upcoming breeding studies has been seen, but a study on its ability to tolerate reproductive drought would be more insightful.
Reviewing Editor:
Introduction
By 2050, the human population on Earth is expected to reach 9.6 billion. For the growing population to have enough food, grain production must increase from 2.1 billion tons to 3 billion tons (Food and Agricultural Organization of the United Nations, Citation2015). Climate change poses about a serious threat to agricultural production and yield stability in many parts of the world (Ray et al., Citation2015; Summary of Policymakers, Intergovernmental Panel on Climate Change, Citation2013). Unpredictable climate changes and altered rainfall patterns have led to recurrent episodes of drought stress worldwide (Lobell et al., Citation2011). The consequences of drought stress are significant, leading to the annual loss of 12 million hectares of agricultural land (0.4% of the global total) or a loss of 20 million tons of grain (Azadi et al., Citation2018; Hall & Leng, Citation2019). Additionally, it results in a reduction of 0.9% in gross primary productivity annually (Du et al., Citation2018) by interfering with plant growth, physiology and reproduction (Gheysari et al., Citation2017). It disrupts morphophysiological processes, leading to low grain yield (Maheswari et al., Citation2016).
Maize plays a crucial role as the primary staple food for over 900 million people worldwide and ranks third in terms of calorie contribution, following rice and wheat (Adebayo & Menkir, Citation2015). The global maize consumption pattern is versatile, with 61% used for feed, 17% for food and 22% for industrial purposes (ICAR-IIMR, Citation2021). Webber et al. (Citation2018) reported that climate change would cause intensive yield loss in maize. In Nepal, maize holds the second position as an important cereal crop in terms of area and production (Kandel, Citation2021). As of the year 2020/21 A.D., the area of land under maize cultivation in Nepal is estimated to be 979,776 ha, with a productivity of 3.06 t/ha (MOALD, 2020/2021). Drought reduced maize cultivation by 13%, 6% and 8% in Nepal’s western, central and eastern regions over the last two decades (Hamal et al., Citation2020). A 1.5 °C global warming scenario from 2021 to 2055, according to studies, would result in a 41% reduction in maize yield and an increased risk of drought in the mid-latitudes (Yin et al., Citation2021). A projected 22% decrease in maize production by 2050 has been reported (Barbosa et al., Citation2021).
Consequently, addressing the response to drought risks remains a prominent area of research as 80% of globally cultivated land relies on rain-fed agriculture (Messina et al., Citation2021). Developing drought-tolerant varieties is a cost-effective approach and reliable strategy to stabilize crop yield and farmers incomes (Tani et al., Citation2019). Drought tolerance is a complex trait with quantitative characteristics involving various shoot and root morphological features (Yadav & Sharma, Citation2016). Crop improvement heavily relies on genetic variability (Kaushik et al., Citation2011). Maize, as a crop, exhibits significant genetic variation, offering immense possibilities for enhancing its genetics (Tandzi et al., Citation2015). More than 650 maize landraces were conserved in the National Gene Bank of Nepal (B. K. Joshi, personal communication, 2022, January 23), but our maize hybridization program is totally dependent on foreign germplasm (Habiyaremye et al., Citation2017). Although local cultivars yield less and have excessive heights than hybrid and composite kinds, they offer advantages like early blooming (anthesis, silking), a shorter harvest period and climate resilience (Windra Sukma, Citation2018). The availability of such heritable drought-tolerant features in the parent is required for maize breeders to produce elite hybrids with drought tolerance (Magar et al., Citation2019). As a result, preserving these local varieties, screening them under controlled moisture deficiency and incorporating them into breeding populations is vital to creating improved maize varieties and hybrids for drought tolerance and, ultimately, food security (Mustamu et al., Citation2023). Potential use of valuable local genetic resources can be achieved by identification, characterization and thorough understanding of genotypes, serving as the foundation for the development of drought-tolerant cultivars (Naveed et al., Citation2012). The objective of this study is to identify locally adapted drought-tolerant varieties for use in contemporary breeding programs by analyzing and comparing various vegetative parameters under drought stress and under normal conditions.
Materials and methods
Experimental site
A greenhouse pot experiment was conducted at the Institute of Agriculture and Animal Science (IAAS), Lamjung, located at 650 masl with latitude 28°7′ 41.93′′ N and longitude 84° 24’ 51.23′′ E, during the summer season (February-April) of 2023.
Experimental design and treatments
The greenhouse pot experiment was conducted in a two-factor factorial completely randomized design (CRD). Seeds of 30 local maize accessions were collected from the Nepal Agriculture Genetic Resources Center, Khumaltar (NAGRC) based on local climate and soil conditions, as shown in . Two water conditions: irrigated (i) and drought (d) were maintained for the maize accessions in two replications. As such 120 pots in total (60, each of irrigated and drought condition) were arranged under homogenous condition in the greenhouse.
Table 1. List of local maize accessions collected from NAGRC, Khumaltar.
Cultural practices
Plastic pots of nine liter size, 25 cm top diameter, 25 cm height and 0.45 kg weight were used. Soil was left to air dry for two days. 6.34 kg of the air-dried soil was transferred to each pot after mixing with farmyard manure in a ratio of 2:1. Two seeds were placed in each pot at a finger distance apart about 3–4 cm below the soil and later thinned out at 13 days after sowing (DAS). 1.48 gm Urea, 2.43 gm Di-ammonium Phosphate and 1.245 gm Muriate of Potash (MoP) were added to each pot as basal dose at the rate of 120:60:40 kg NPK/ha on the basis of population. Hand weeding was performed whenever necessary. Earthing up was done 25 DAS.
Soil was oven dried at 104 °C for 24 hr and weighed. To determine the weight of soil at field capacity (FC), water was continuously added to the pot until water dripped out of the hole at the lower end. Thereafter, the mouth of the pot was covered with plastic and kept as such for one to two days to prevent loss of water by evaporation. Thereafter, the weight was taken. The experiment was performed at 65% of FC.
Weight of air-dried soil= 6.39 kg
Weight of oven dried soil= 5.24 kg
Water in air-dried soil= 6.39–5.24 = 1.1 kg
Soil weight at FC= 8.05 kg
Amount of water at FC= 8.05–5.24 = 2.81 kg
Since we will work at 65% of FC, the amount of water at this level= 65% of 2.81 = 1.82 kg
Amount of water to be added at 1st irrigation= 1.82–1.1 = 0.72 kg
On every third day, individual pots of irrigation treatment were weighed to calculate the amount of water required to counter the loss until harvesting. A blank pot was assigned to eliminate the error of the plant weight during the calculation. A similar process was carried out for pots of drought treatment until the fourth leaf stage (21 DAS), and then, water was completely checked. Harvesting was done at 48 DAS.
Data collection and analysis
The shoot length and root length of the pulled plant was measured using a scale in cm. The ratio of measured root fresh weight to shoot fresh weight is computed as the root: shoot ratio. Primary roots greater than 5 cm in length were counted as the number of roots. The fresh weight of the root (RFW) was measured in grams. Roots were oven-dried at 70 ± 5 °C for 48 hr and then weighed (RDW) (Badr et al., Citation2020). Thereafter, the root moisture content was computed. The same procedure was followed by replacing roots with shoots to measure shoot fresh weight (SFW), shoot dry weight (SDW) and shoot moisture content. For relative water content, 50 punched pieces of leaf lamina were collected from different maize leaves at 1/3, 1/2 and 2/3 of the leaf tip collectively using a punching machine. They were immediately placed inside aluminum foil to prevent moisture loss, and weight was taken (LFW) in grams. These same pieces were then placed in a petri plate and distilled water was poured over them such that they submerged and remained undisturbed for 24 hr. Weight was taken the other day (LTW). Thereafter, they were oven dried at 80 °C for 24 hr and weighed (LDW) (Badr et al., Citation2020). Thereafter, the relative water content was computed. For root volume, water was added to a measuring cylinder with the fitted calibration. Roots were dipped into the cylinder. Displacement of water was noted from the original level. Difference between the levels yielded root volume in cm3. Normalized Difference Vegetation Index (NDVI) was measured using a GreenSeeker Handheld Crop Sensor. Values of each trait under stress are divided by the value under irrigation for relative value of tolerance (Liu et al., Citation2015).
(Badr et al., Citation2020)
(Badr et al., Citation2020)
(Badr et al., Citation2020)
Data entry was done in MS-Excel and analysis was done using R studio version 1.4.1106. ANOVA analysis was performed and mean separation was carried out using Duncan’s Multiple Range Test (DMRT).
Results
Analysis of variance for nine quantitative traits at 48 DAS is shown in .
Table 2. ANOVA for nine quantitative traits.
Mean performance comparison of the study parameters
Shoot length
A significant difference was observed between the shoot length in 30 different maize accessions as shown in . The interaction between accessions and watering conditions was found to be significant as shown in . Under drought conditions, the most significant shoot length was observed in the NGRC05589, which was 88.85 cm. This trend was statistically consistent with the performance of NGRC05563 (88.55), NGRC05561 (84), NGRC05566 (83.75), NGRC05569 (83.4), NGRC05562 (82.6), NGRC05586 (82.5), NGRC05570 (81.5) and NGRC05582 (80.4). Conversely, under well-watered conditions, the highest shoot elongation was exhibited by the NGRC05572 cultivar, registering a value of 145.7 cm. This outcome demonstrated statistical similarity with NGRC05568 (145.1), NGRC05575 (138.475), NGRC05571 (137.9), NGRC05563 (135.65), NGRC05565 (132.75), NGRC05561 (132.5), NGRC05566 (129.1), NGRC05564 (126.75), NGRC05569 (125.35) and NGRC05574 (125.05). This finding is in line with Ali et al. (Citation2016), Khan et al. (Citation2016) and Abrokwah et al. (Citation2017), who reported reduced shoot growth under drought stress.
Figure 1. Interaction of 30 maize accessions with error bars representing standard error for the trait ‘shoot length’ under irrigated (SL_I) and drought (SL_D) conditions. Interaction is significant at p ≥ .001.
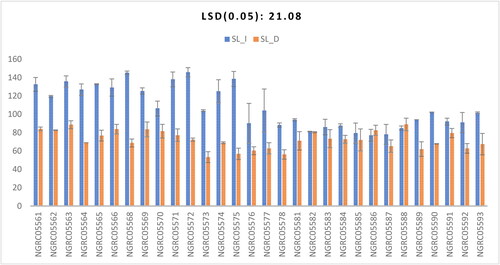
Table 3. Mean separation table for nine quantitative traits in 30 maize accessions.
Root length
Noticeable variations were identified among the different accessions concerning the root length as shown in . The interaction between accessions and watering conditions also exhibited statistical significance, as shown in . Except for NGRC05586, NGRC05590, NGRC05591, NGRC05592 and NGRC05593, most of the maize accessions exhibited greater root lengths under drought conditions. Notably, NGRC05570 displayed the most extensive root length under drought conditions, measuring 76.1 cm and was statistically similar to NGRC05562 (74.5), NGRC05565 (70.5), NGRC05566 (67.4), NGRC05574 (65.65), NGRC05561 (65.3) and NGRC05564 (58.5). As longer roots are considered the primary basis of drought tolerance in maize plants (Liu et al., Citation2015; Zhu et al., Citation2010), these accessions exhibit tolerance in decreasing order of root length. Conversely, under irrigated conditions, the most considerable root length was observed in NGRC05593, reaching 75 cm. Greater root length under drought stress is in agreement with the findings of Shahzad et al (Citation2023).
Figure 2. Interaction of 30 maize accessions with error bars representing standard error for the trait ‘root length’ under irrigated (RL_I) and drought (RL_D) conditions. Interaction is significant at p ≥ .001.
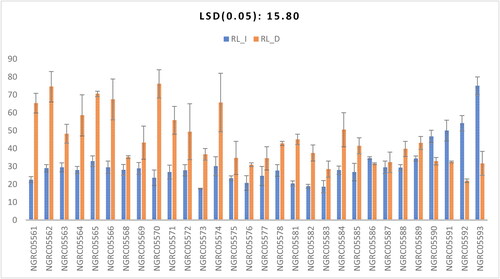
Root:shoot ratio
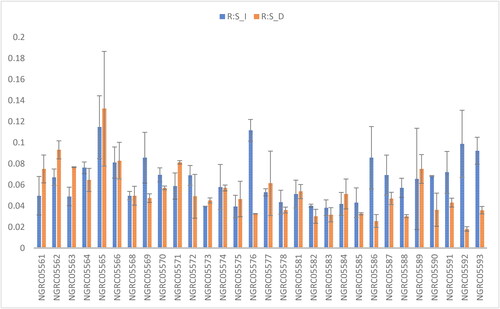
Significant variances were noted among the accessions concerning the root-to-shoot ratio. as shown in . The highest root-to-shoot ratio was recorded in NGRCO5565 (0.123), whereas the lowest ratio was observed in NGRCO5576 (0.032). A substantial disparity in the ratio was evident between the two watering conditions: irrigated (0.062) and drought (0.053). Under drought stress, increased root fresh weight was observed, in agreement with Pokhrel et al. (Citation2019). There was no significant interaction between accessions and watering conditions, as shown in .
Number of roots
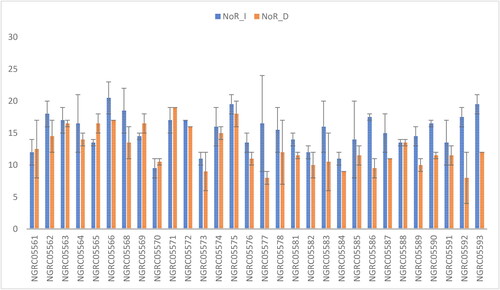
Statistically significant differences were observed in the number of roots among maize accessions, as shown in . Under different watering conditions: irrigated (15.35) and drought (12.63), such differences were observed. Pokhrel et al. (Citation2019) reported decreased root number under stress conditions. Similarly, noticeable distinctions were observed among the 30 maize accessions. The highest root number was recorded in NGRC05566 and NGRC05575 (18.75), with statistical similarity to NGRC05571 (18), NGRC05563 (16.75), NGRC05572 (16.5), NGRC05562 (16.25), NGRC05568 (16), NGRC05574 (15.5), NGRC05593 (15.75), NGRC05564 (15.25), NGRC05565 (15), NGRC05590 (14), NGRC05578 (13.75), NGRC05586 (13.5), NGRC05588 (13.5), NGRC05583 (13.25) and NGRC05587 (13). Conversely, the lowest root number was observed in NGRC05570, NGRC05573 and NGRC05584 (10). The interaction between accessions and watering conditions was insignificant, as shown in .
Root moisture content
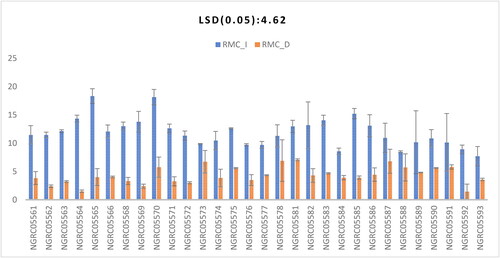
Significant differences in root moisture content were found among the 30 maize accessions studied, as shown in . The interaction between different maize types and watering conditions was also found to be significant, as shown in . Root moisture content was observed to be lower for all 30 accessions under drought condition than in irrigated condition. In the irrigated condition, NGRC05565 (18.31) showed the greatest value statistically at par with NGRC05570 (18.15), NGRC05585 (15.23), NGRC05564 (14.33), NGRC05583 (14.03), NGRC05569 (13.78), NGRC05582 (13.17), NGRC05523 (13.09), NGRC05568 (13.03) and NGRC05581 (12.95). However, under drought stress, NGRC05581 (7.06) showed the greatest root moisture content. Similar higher root moisture content under normal conditions was observed by Qayyum et al. (Citation2012).
Shoot moisture content
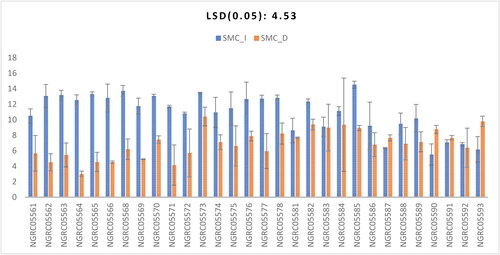
Significant differences in shoot moisture content were found among the 30 maize accessions studied, as shown in . The interaction between accessions and watering conditions was found to be significant, as shown in . The highest shoot moisture content under irrigated conditions was observed in NGRC05585 (14.52), having statistical equivalence with NGRC05568 (13.71), NGRC05573 (13.5), NGRC05565 (13.32), NGRC05563 (13.18), NGRC05562 (13.06), NGRC05570 (13.04), NGRC05580 (12.81), NGRC05566 (12.8), NGRC05579 (12.71), NGRC05578 (12.66), NGRC05564 (12.52), NGRC05582 (12.34), NGRC05569 (11.75), NGRC05571 (10.81), NGRC05575 (11.47), NGRC05584 (11.12), NGRC05574 (10.94), NGRC05572 (10.81), NGRC05561 (10.5), NGRC05589 (10.16), NGRC05588 (9.48), NGRC05586 (9.2) and NGRC05583 (9.1). Similarly, the greatest shoot moisture content under drought conditions was recorded in NGRC05573 (10.41), statistically on par with NGRC05593 (9.79), NGRC05582 (9.38), NGRC05584 (9.36), NGRC05583 (8.97), NGRC05585 (8.93) and the previously mentioned accessions under irrigated conditions. A similar greater shoot moisture content under irrigated conditions was observed by Pokhrel et al. (Citation2019).
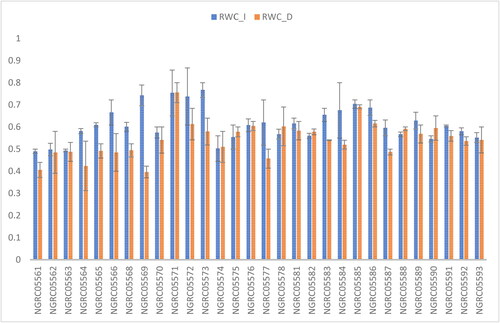
Relative water content
The primary effect of drought on a plant is its impact on the plant’s water budget (Farooq et al., Citation2012). A statistically significant variation was observed in terms of relative moisture content within the collection of 30 maize accessions as shown in . The highest relative water content was recorded for NGRC05571 (0.754), followed by NGRC05585 (0.697), NGRC05572 (0.675), NGRC05573 (0.673) and NGRC05586 (0.651), whereas the lowest relative water content was identified for NGRC05561 (0.448). Significant differences in relative water content were evident among various watering conditions: irrigated (61.11%) and drought-stressed (54.38%) conditions. Water stress decreased the relative water content in maize leaves, similar to the findings of Aslam et al. (Citation2015). However, the interaction between diverse maize accessions and watering conditions lacked statistical significance in relation to this specific trait, as shown in .
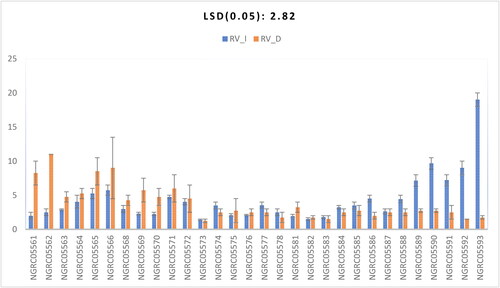
Root volume
We observed a significant difference in root volume among the 30 maize accessions, as shown in . The manner in which accessions responded to watering conditions showed a significant impact, as shown in . The most significant root volume under irrigated conditions was observed in NGRC05593 (19). Conversely, under drought conditions, NGRC05562 displayed the highest root volume of 11 cm3, exhibiting statistical similarity with NGRC05566 (9), NGRC05565 (8.5) and NGRC05561 (8.25) under drought conditions, as well as with NGRC05590 (9.65) and NGRC05592 (9) under irrigated conditions.
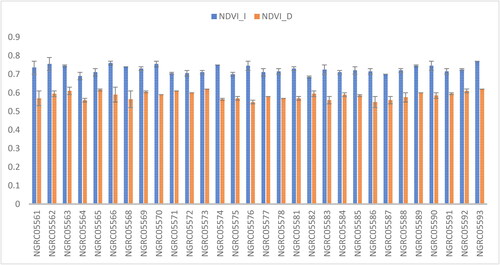
NDVI
We observed significant variations in the NDVI across the 30 different maize accessions studied, as shown in . The maize variety NGRC05593 (0.695) displayed the highest NDVI, statistically similar to NGRC05563 (0.678). Additionally, NGRC05566 (0.675), NGRC05562 (0.675), NGRC05570 (0.673), NGRC05589 (0.673), NGRC05569 (0.668), NGRC05592 (0.668), NGRC05573 (0.665), NGRC05590 (0.665), NGRC05565 (0.663), NGRC05571 (0.658), NGRC05574 (0.658) and NGRC05591 (0.655) also exhibited noteworthy NDVI values. Substantial differences were observed between the watering conditions: irrigated (0.725) and drought (0.585). Drought stress has been found to affect greenness in plants (Khayatnezhad and Gholamin Citation2012). Nevertheless, no meaningful interaction between watering conditions and maize types was seen, as shown in .
Relative value of tolerance
The relative values of tolerance, as shown in , vary across nine quantitative traits among 30 maize accessions. Greater the value for a trait, more tolerant the variety is under drought for that trait. Genotype NGRC05570 stands out for having the highest relative tolerance value for root length (3.383) and high tolerance to number of roots (1.125) and RWC (0.938). This is consistent with its substantial root trait performance, suggesting its strong drought tolerance potential. Genotype NGRC05586 has the highest relative tolerance value for shoot length (1.072) and high RWC (0.898), indicating efficient water conservation, contributing to its potential drought tolerance. Genotype NGRC05589 displayed a peak relative value of tolerance for root: shoot ratio (2.133), indicating an enhanced allocation of resources to the root system, high tolerance to NDVI (0.805) and for the number of roots (1.125), further highlighting its robust root development. Genotype NGRC05591 exhibited the highest relative tolerance value for root moisture content (0.789), relatively high tolerance for shoot moisture content (1.086) and NDVI (0.833). This combination suggests effective water storage in roots and shoots, indicating potential drought adaptation. Genotype NGRC05578 has notable relative tolerance value for relative water content (1.057). Genotype NGRC05561 stands out with the most significant value for root volume (4.633). Its extensive relative tolerance to root length (2.924), root: shoot ratio (1.858) and number of roots (1.136) could contribute to enhanced water uptake and storage. Genotype NGRC05573 showed the greatest tolerance to NDVI (0.873) and a high root: shoot ratio (1.139). Genotype NGRC05571, although has none of the greatest tolerance values, exhibits high tolerance for NDVI (0.865), root: shoot ratio (1.441), number of roots (1.133), root length (2.078) and relative water content (1.030).
Table 4. Relative value of tolerance (RVT) for nine quantitative traits in 30 maize accessions.
Correlation between the traits
Correlation identifies the mutual relationships among the traits under study, as shown in . Under drought conditions, shoot length was found to be significantly correlated with root length (0.44), shoot moisture content (−0.38) and root volume (0.52). However, it was only positively correlated with shoot moisture content (0.35) and the number of roots (0.34) under irrigated conditions. Similarly, root length was correlated with root: shoot ratio (0.76; 0.48), shoot moisture content (−0.52; −0.64), root volume (0.79; 0.94) and the number of roots (0.47; 0.38) under both watering conditions. However, it correlated with root moisture content (0.38) under irrigated conditions only. Root: Shoot ratio correlated with root length (0.76; 0.48) and root volume (0.8; 0.45) under both conditions but was correlated with the number of roots (0.52) and shoot moisture content (−0.62) under drought only. The number of roots correlated with root length (0.47; 0.38) and root volume (0.59; 0.4) under both watering conditions but was significantly correlated with shoot length (0.37) under irrigated conditions and with root: shoot ratio (0.52) and shoot moisture content (−0.64) under drought. Root moisture content correlated with shoot moisture content (0.3; 0.45) and root volume (−0.39; −0.38) under both conditions but with root length (−0.38) under irrigated conditions. Shoot moisture content correlated negatively with root volume (−0.74; −0.58), shoot length (−0.38; 0.38), root length (−0.52; −0.64), root moisture content (0.3; 0.45) and root volume (−0.74; −0.58) under both watering conditions, while it correlated with root: shoot ratio (−0.62) and the number of roots (−0.64) under drought. Relative water content significantly correlated with root volume (−0.38) under drought only. Root volume had a positive correlation with NDVI (0.39) under irrigated conditions only.
Table 5. Correlation among plant growth attributes of 30 maize accessions under different growing conditions: drought condition (above diagonal) and irrigated condition (below diagonal).
Discussion
Under drought stress, a reduction in shoot length is primarily attributed to constrained cell elongation, enlargement and division due to reduced water absorption (Anjum et al., Citation2017; Avramova et al., Citation2016). This stress triggers the movement of assimilates from the shoot to the root (Aslam et al., Citation2015). Roots are crucial drought sensors, with traits such as root length, density, volume and number being disrupted under drought (Nejad et al. Citation2010). Researches involving polyethylene glycol, which limits water absorption, thus mimicking a drought condition, affected the histological makeup of plants, including the size of the cortex, stele and xylem in soybean genotypes. Drought stress may also induce damage to the root membrane, as demonstrated by the presence of lipid peroxides (Rosawanti et al., Citation2015). Aslam et al. (Citation2015) found that a maize plant experiencing mild drought tends to grow its root longer in search of extra water in the soil. However, when drought becomes severe, the plant shortens its roots. Longer roots are a key factor in maizés drought tolerance (Zhu et al., Citation2010), with a higher root-to-shoot weight ratio being important (Liu et al., Citation2015). Maize seedlings adjust to low water potentials by increasing expansin activity at the molecular level, causing the walls in the apical region of the root to extend more (Wu & Cosgrove, Citation2000). However, Beebe et al. (Citation2014) concluded that a deeper root is not enough if not combined properly with other traits. Dar et al. (Citation2018) focused on deep root mass for selection in drought tolerance. Deeper, thicker roots with greater volume and extensive branching are better at extracting water from soil (Aslam et al., Citation2015; Gowda et al., Citation2011). Maize’s drought tolerance relies on root traits such as length, density and biomass (Kavar et al., Citation2007). Leaf rolling, firing, canopy temperature, stomata closure and root traits are essential criteria for selecting drought-tolerant maize (Aslam et al., Citation2015).
An increment in the histological size of root epidermis, cortex and stele by 28.67%, 11.3% and 20.85% in maize genotypes under drought conditions imposed by 50% PEG was found by Mustamu et al. (Citation2023) in comparison to control with local maize accessions. Larger root tissue size signifies a good adaptation to drought stress (Mustamu et al., Citation2023). Contrastingly, in an experiment conducted by Grzesiak et al. (Citation1999) with hybrid maize genotypes, it was found that plants exposed to drought showed reduced seminal root diameter, stele diameter and cortical thickness compared to the control treatment, which indicated their sensitivity to drought stress. Root fresh weight increases under drought as plants search for water (Pokhrel et al., Citation2019). Increased root tissue size is associated with higher water absorption, potentially boosting drought resistance. Higher cortical thickness protects roots from extreme environmental situations, including drought, by allowing for better absorption of water and nutrients. This is helpful for the plant as it helps assure its survival (Mustamu et al., Citation2023). This allocation strategy optimizes water uptake through the root system, which is influenced by various root architecture traits (Comas et al., Citation2013). Shoot mass is more sensitive to drought than root mass (Adhikari et al., Citation2019; Widuri et al., Citation2018) because resources are prioritized for water uptake and transpiration reduction (Chimungu et al., Citation2014). Drought affects leaf number (Zhang et al., Citation2018), greenness (Khayatnezhad & Gholamin, Citation2012), photosynthesis rate and efficiency (Jha, Citation2019; Wang et al., Citation2018), thereby affecting plant survival. Reduced water potential in cells due to drought leads to decreased cell division and size, hampering plant growth (Nonami, Citation1998). Leaves lose turgor, curl and fold under drought conditions (Du Plessis, Citation2003), reducing light interception and photosynthesis. Increased leaf temperature under drought inhibits enzymatic activity, further reducing photosynthesis (Chaves et al., Citation2002). Relative water content is an integral drought tolerance indicator, with closed stomata reducing CO2 accumulation under drought conditions (Gindaba et al., Citation2004).
Proline and soluble sugars comprising glucose, sucrose, maltose and trehalose play significant roles in osmoregulation under drought stress and are thus well associated with drought tolerance (Ibrahim & Abdellatif, Citation2016; Zhang et al., Citation2020). In an experiment conducted by Makbul et al. (Citation2011), a significant decline in cumulative chlorophyll content under drought conditions was found. Research suggests that a reduction in chlorophyll content in leaves is efficient under drought conditions, as it leads to conservation of nitrogen and energy (which would otherwise be invested in the formation of pigment-protein), which can now be utilized to enhance the efficiency of incident radiation into biomass (also known as radiation interception efficiency), thus contributing to adaptation against drought stress. Also, a reduced chlorophyll level could potentially have significant effects at the canopy level, as it leads to a decrease in light absorption and thus increases albedo at the top of the canopy, which may consequently lower leaf temperature in the upper canopy (Slattery et al., Citation2017), eventually lessening the transpiration rate. It can be speculated that the prominence of promising accessions under drought conditions in our experiment could have been well associated with these explanations, with increased content of osmo-regulates and reduced levels of chlorophyll.
The importance of traits when water availability changes is shown by differences in their relationship to one another under various water conditions (Comas et al., Citation2013). In times of drought, we see longer roots have a high shoot length, suggesting that longer roots are better at searching the soil for water (Boyer, Citation1996; Ludlow & Muchow, Citation1990). However, there is a negative link between shoot length and shoot moisture content, indicating that longer shoots might actually have less water, possibly because they are losing more water through transpiration. The positive relationship between shoot length and root volume under drought suggests that larger shoots often come with larger roots, whicht can be pretty helpful for sucking up water (Sarker et al., Citation2005). It is important for roots to expand in order to support the growth of shoots, as shown by the positive correlation between root length and the root: shoot ratio (Addington et al., Citation2006; Mencuccini, Citation2003). Longer roots are advantageous in both dry and wet conditions because there is a positive relationship between root length and number. This approach successfully increases the total root surface area, making it the best configuration for water absorption. When water is abundant, those longer roots are friendly with root moisture content, implying that they are effective at storing water. The strong positive relationship between the root: shoot ratio and root length indicates that root growth impacts shoot growth (Novák & Lipiec, Citation2012). Similarly, the root: shoot ratio gets along well with the root volume, showing that having bigger roots is good. However, the negative relationship between shoot moisture content and root volume revealed that larger root systems might reduce shoot moisture content because the roots are drinking up more water. In addition, shoot moisture content correlates negatively with root length under drought conditions, suggesting that longer roots might mean less moisture in the shoot, possibly because the water is heading down to the roots for storage (Lynch et al., Citation2005). There is a balance seen in between shoot moisture content and root moisture content. Relative water levels decrease with large root systems under drought; it appears that they might be drinking up more water. In well-watered conditions, larger root volume is a sign of healthier plants, as indicated by the positive connection to NDVI. When water is scarce, shoot moisture content becomes super important, suggesting that genotypes with lower shoot moisture content use water efficiently. The significance of roots maintaining their hydration under stress is demonstrated by the positive correlations between root moisture content and the number of roots.
For assessing drought-tolerant accessions, lower reductions in shoot length, longer root lengths (Ahsan et al., Citation2013; Khan et al., Citation2014; Sarker & Oba, Citation2018), higher root-to-shoot ratios (Khan et al., Citation2016; Liu et al., Citation2015), more roots and greater volume (Aslam et al., Citation2015), balanced moisture content, relatively higher water content (Sarker & Oba, Citation2018) and lower reductions in greenness (Ahsan et al., Citation2013; Khayatnezhad & Gholamin, Citation2012) seem to indicate the ability of maize to thrive under water-deficient conditions. Considering the relative tolerance values, accessions NGRC05570, NGRC05571, NGRC05589, NGRC05561, NGRC05591, NGRC05578 and NGRC05586 stand out as strong competitors for drought tolerance. Particularly, NGRC05571 exhibits a higher relative tolerance value in all root-associated traits, relative water content and NDVI under drought stress, establishing it as the drought-tolerant genotype. However, it’s important to emphasize that selecting drought-tolerant genotypes should consider various factors like biochemical responses, anatomical changes, reproductive traits, environmental conditions, extensive field trials and overall plant performance. While NGRC05571 shows promise, more rigorous testing under real-world conditions is essential. Moreover, further research attempts must focus on its ability to endure reproductive drought, which would be beneficial to promote its application in breeding programs for drought resistance.
Conclusion
The disruption of the physiological, biochemical and molecular mechanisms of the maize plant under drought is the most damaging abiotic stress factor and a significant barrier to its productivity. For genetic improvements in this regard, it is essential to identify genotypes with the capacity to flourish under drought stress. The plant’s tolerance to a reduction in shoot length, root: shoot ratios, root number and volume, balanced moisture content, relative water content, greenness and longer root lengths showed that it could efficiently absorb, retain and manage water in a drought-stricken environment. Among the examined accessions, NGRC05561, NGRC05570, NGRCO5571, NGRC05578, NGROCO5586, NGRC05589 and NGRC05591 showed significant potential for drought tolerance. These genotypes showed significant relative tolerance values, especially in root-related traits, relative water content and greenness, indicating their ability to survive under water stress. While irrigated conditions showed positive links between shoot length and shoot moisture content, drought conditions showed positive correlations between shoot length, root length and root volume. Key characteristics such as root volume and the root:shoot ratio are consistently correlated with root length. Strong connections were observed between the root:shoot ratio, particularly under drought. Notably, shoot moisture content had a detrimental effect on several traits, which were more pronounced under drought. Furthermore, root volume was positively correlated with NDVI under irrigation, whereas root volume was uniquely correlated with relative water content under drought conditions. However, a thorough analysis of their agronomic performance must be performed before recommending them as the best options for cultivation in drought-prone areas.
Author contributions
Anubhav Tripathi: Designed & conducted experiment, data collection & analysis and manuscript writing
Rashmi Poudel: Designed & conducted experiment and data analysis
Reema Gurung: Designed & conducted experiment
Unisha Ghimire: Designed & conducted experiment
Mamata Pandey: Conducted the experiment & data collection
Bishnu Prasad Kandel: Data analysis and manuscript writing
Bal Krishna Joshi: Provided research materials and manuscript writing.
Acknowledgments
The authors express their gratitude to the Nepal Agriculture Genetic Resources Center, Khumaltar (NAGRC), for generously providing the maize seeds for this research. In addition, heartfelt thanks are extended to the Institute of Agriculture and Animal Science, Lamjung campus, Tribhuvan University, Nepal, for their invaluable research support and the use of their facilities throughout the course of the experiment.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data will be made available upon request via the corresponding email address.
Additional information
Funding
References
- Abrokwah, O. A., Antwi-Boasiako, A., & Effah, Z. (2017). Effects of drought stress on maize genotypes (Zea mays L.) using some plant parameters. Journal of Science Research and Allied Sciences, 6(3), 1–17.
- Addington, R. N., Donovan, L. A., Mitchell, R. J., Vose, J. M., Pecot, S. D., Jack, S. B., Hacke, U. G., Sperry, J. S., & Oren, R. (2006). Adjustments in hydraulic architecture of Pinus palustris maintain similar stomatal conductance in xeric and mesic habitats. Plant, Cell & Environment, 29(4), 535–545. https://doi.org/10.1111/j.1365-3040.2005.01430.x
- Adebayo, M. A., & Menkir, A. (2015). Combining ability of adapted and exotic drought-tolerant maize inbred lines under full irrigation and rainfed conditions in Nigeria. Journal of Crop Improvement, 29(1), 117–130. https://doi.org/10.1080/15427528.2014.980484
- Adhikari, B., Sa, K. J., & Lee, J. K. (2019). Drought tolerance screening of maize inbred lines at an early growth stage. Plant Breeding and Biotechnology, 7(4), 326–339. https://doi.org/10.9787/PBB.2019.7.4.326
- Ahsan, M., Farooq, A., Khaliq, I., Ali, Q., Aslam, M., & Kashif, M. (2013). Inheritance of various yield-contributing traits in maize (Zea mays L.) at low moisture condition. African Journal of Agricultural Research, 8, 413–420. https://doi.org/10.5897/AJAR13.004
- Ali, Q., Ahsan, M., Malook, S., Kanwal, N., Ali, F., Ali, A., Ahmed, W., Ishfaq, M., & Saleem, M. (2016). Screening for drought tolerance: Comparison of maize hybrids under water deficit condition. Advances in Life Sciences, 3, 51–58.
- Anjum, S. A., Ashraf, U., Tanveer, M., Khan, I., Hussain, S., Shahzad, B., Zohaib, A., Abbas, F., Saleem, M. F., Ali, I., & Wang, L. C. (2017). Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Frontiers in Plant Science, 8, 69. https://doi.org/10.3389/fpls.2017.00069
- Aslam, M., Maqbool, M. A., & Cengiz, R. (2015). Effects of drought on maize (pp. 5–17). Springer. https://doi.org/10.1007/978-3-319-25442-5_2
- Avramova, V., Nagel, K. A., Abdelgawad, H., Bustos, D., Duplessis, M., Fiorani, F., & Beemster, G. T. S. (2016). Screening for drought tolerance of maize hybrids by multi-scale analysis of root and shoot traits at the seedling stage. Journal of Experimental Botany, 67(8), 2453–2466. https://doi.org/10.1093/jxb/erw055
- Azadi, H., Keramati, P., Taheri, F., Rafiaani, P., Teklemariam, D., Gebrehiwot, K., Hosseininia, G., van Passel, S., Lebailly, P., & Witlox, F. (2018). Agricultural land conversion: Reviewing drought impacts and coping strategies. International Journal of Disaster Risk Reduction, 31, 184–195. https://doi.org/10.1016/j.ijdrr.2018.05.003
- Badr, A., El-Shazly, H. H., Tarawneh, R. A., & Börner, A. (2020). Screening for drought tolerance in maize (Zea mays L.) germplasm using germination and seedling traits under simulated drought conditions. Plants, 9(5), 565. https://doi.org/10.3390/plants9050565
- Barbosa, P. A. M., Fritsche-Neto, R., Andrade, M. C., Petroli, C. D., Burgueño, J., Galli, G., Willcox, M. C., Sonder, K., Vidal-Martínez, V. A., Sifuentes-Ibarra, E., & Molnar, T. L. (2021). Introgression of maize diversity for drought tolerance: Subtropical maize landraces as a source of new positive variants. Frontiers in Plant Science, 12, 691211. https://doi.org/10.3389/fpls.2021.691211
- Beebe, S. E., Rao, I. M., Cajiao, C., & Grajales, M. (2014). Selection for drought tolerance in common beans. Crop Science, 48(2), 582–592. https://doi.org/10.2135/cropsci2007.07.0404
- Boyer, J. S. (1996). Advances in drought tolerance in plants. Advances in Agronomy, 56, 187–218.
- Chaves, M. M., Pereira, J. S., Maroco, J., Rodrigues, M. L., Ricardo, C. P. P., Osório, M. L., Carvalho, I., Faria, T., & Pinheiro, C. (2002). How plants cope with water stress in the field. Annals of Botany, 89(7), 907–916. https://doi.org/10.1093/aob/mcf105
- Chimungu, J. G., Brown, K. M., & Lynch, J. P. (2014). Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiology, 166(4), 1943–1955. https://doi.org/10.1104/pp.114.249037
- Comas, L. H., Becker, S. R., Cruz, V. M. v., Byrne, P. F., & Dierig, D. A. (2013). Root traits contributing to plant productivity under drought. Frontiers in Plant Science, 4(NOV), 442. https://doi.org/10.3389/fpls.2013.00442
- Dar, I. A., Sofi, P. A., Dar, Z. A., Luddin, K., & Lone, A. A. (2018). Screening of maize genotypes for drought tolerance related trait variability. International Journal of Current Microbiology and Applied Sciences, 7(04), 668–682. https://doi.org/10.20546/ijcmas.2018.704.076
- Du, L., Mikle, N., Zou, Z., Huang, Y., Shi, Z., Jiang, L., McCarthy, H. R., Liang, J., & Luo, Y. (2018). Global patterns of extreme drought-induced loss in land primary production: Identifying ecological extremes from rain-use efficiency. The Science of the Total Environment, 628-629, 611–620. https://doi.org/10.1016/j.scitotenv.2018.02.114
- Du Plessis, J. (2003). Maize production. Department of Agriculture, Directorate Agricultural Information Services.
- Food and Agricultural Organization of the United Nations. (2015). Food and Agricultural Organization of the United Nations (FAO), FAO statistical database. http://faostat3.fao.org
- Farooq, M., Hussain, M., Wahid, A., & Siddique, K. H. (2012). Drought stress in plants: an overview. In: Plant responses to drought stress. From morphological to molecular features; Aroca R., Springer, Berlin, Heidelberg, 1–33. https://doi.org/10.1007/978-3-642-32653-0_1
- Gheysari, M., Sadeghi, S.-H., Loescher, H. W., Amiri, S., Zareian, M. J., Majidi, M. M., Asgarinia, P., & Payero, J. O. (2017). Comparison of deficit irrigation management strategies on root, plant growth and biomass productivity of silage maize. Agricultural Water Management, 182(C), 126–138. https://doi.org/10.1016/j.agwat.2016.12.014
- Gindaba, J., Rozanov, A., & Negash, L. (2004). Response of seedlings of two Eucalyptus and three deciduous tree species from Ethiopia to severe water stress. Forest Ecology and Management, 201(1), 119–129. https://doi.org/10.1016/j.foreco.2004.07.009
- Gowda, V. R. P., Henry, A., Yamauchi, A., Shashidhar, H. E., & Serraj, R. (2011). Root biology and genetic improvement for drought avoidance in rice. Field Crops Research, 122(1), 1–13. https://doi.org/10.1016/j.fcr.2011.03.001
- Grzesiak, S., Hura, T., Grzesiak, M. T., & Pieńkowski, S. (1999). The impact of limited soil moisture and waterlogging stress conditions on morphological and anatomical root traits in maize (Zea mays L.) hybrids of different drought tolerance. Acta Physiologiae Plantarum, 21(3), 305–315. https://doi.org/10.1007/s11738-999-0046-4
- Habiyaremye, C., Barth, V., Highet, K., Coffey, T., & Murphy, K. M. (2017). Phenotypic responses of twenty diverse proso millet (Panicum miliaceum L.) accessions to irrigation. Sustainability, 9(3), 389. https://doi.org/10.3390/su9030389
- Hall, J. W., & Leng, G. (2019). Can we calculate drought risk and do we need to?. Wiley Interdisciplinary Reviews: Water, 6, e1349.
- Hamal, K., Sharma, S., Khadka, N., Haile, G. G., Joshi, B. B., Xu, T., & Dawadi, B. (2020). Assessment of drought impacts on crop yields across Nepal during 1987–2017. Meteorological Applications, 27(5), e1950. https://doi.org/10.1002/met.1950
- Ibrahim, H. A., & Abdellatif, Y. M. R. (2016). Effect of maltose and trehalose on growth, yield and some biochemical components of wheat plant under water stress. Annals of Agricultural Sciences, 61(2), 267–274. https://doi.org/10.1016/j.aoas.2016.05.002
- ICAR-IIMR. (2021). World maize scenario. Indian Council of Agricultural Research.
- Intergovernmental Panel on Climate Change. (2013). Summary for policymakers. In T. F. Stocker, D. Qin, G. -K. Plattner, M. Tignor, S. K. Allen, J. Doschung, A. Nauels, Y. Xia, V. Bex, & P. M. Midgley (Eds.), Climate change 2013: The physical science basis. Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change (pp. 3–29). Cambridge University Press. https://doi.org/10.1017/CBO9781107415324.004
- Jha, Y. (2019). Regulation of water status, chlorophyll content, sugar, and photosynthesis in maize under salinity by mineral mobilizing bacteria. Photosynthesis: Production and Environmental Stress. https://doi.org/10.1002/9781119501800.ch5
- Kandel, B. P. (2021). Status, prospect, and problems of hybrid maize (Zea mays L.) in Nepal: A brief review. Genetic Resources and Crop Evolution, 68(1), 1–10. https://doi.org/10.1007/s10722-020-01032-0
- Kaushik, S. K., Tomar, D. S., & Dixit, A. K. (2011). Genetics of fruit yield and its contributing characters in tomato (Solanum lycopersicum). Journal of Agricultural Biotechnology and Sustainable Development, 3(10), 209.
- Kavar, T., Maras, M., Kidrič, M., Šuštar-Vozlič, J., & Meglič, V. (2007). Identification of genes involved in the response of leaves of Phaseolus vulgaris to drought stress. Molecular Breeding, 21(2), 159–172. https://doi.org/10.1007/s11032-007-9116-8
- Khan, N. H., Ahsan, M., Naveed, M., Sadaqat, H. A., & Javed, I. (2016). Genetics of drought tolerance at seedling and maturity stages in Zea mays L. Spanish Journal of Agricultural Research, 14(3), e0705. https://doi.org/10.5424/sjar/2016143-8505
- Khan, N. H., Ahsan, M., Saleem, M., & Ali, A. (2014). Genetic association among various morpho-physiological traits of Zea mays under drought condition. Life Science Journal, 11, 112–122.
- Khayatnezhad, M., & Gholamin, R. (2012). The effect of drought stress on leaf chlorophyll content and stress resistance in maize cultivars (Zea mays). African Journal of Microbiology Research, 6, 2844–2848. https://doi.org/10.5897/AJMR11.964
- Liu, M., Li, M., Liu, K., & Sui, N. (2015). Effects of drought stress on seed germination and seedling growth of different maize varieties. Journal of Agricultural Science, 7(5), 231–240. https://doi.org/10.5539/jas.v7n5p231
- Lobell, D., Bänziger, M., Magorokosho, C., & Bindiganavile, V. (2011). Nonlinear heat effects on African maize as evidenced by historical yield trials. Nature Climate Change, 1(1), 42–45. https://doi.org/10.1038/nclimate1043
- Ludlow, M. M., & Muchow, R. C. (1990). A critical evaluation of traits for improving crop yields in water-limited environments. Advances in Agronomy, 43, 107–153.
- Lynch, J. P., Ho, M. D., & Phosphorus, L. (2005). Rhizoeconomics: Carbon costs of phosphorus acquisition. Plant and Soil, 269(1-2), 45–56. https://doi.org/10.1007/s11104-004-1096-4
- Magar, M. M., Parajuli, A., Sah, B. P., Shrestha, J., Sakh, B. M., Koirala, K. B., & Dhital, S. P. (2019). Effect of PEG induced drought stress on germination and seedling traits of maize (Zea mays L.) lines. Türk Tarım ve Doğa Bilimleri Dergisi, 6(2), 196–205. https://doi.org/10.30910/turkjans.556607
- Maheswari, M., Tekula, V. L., Yellisetty, V., Sarkar, B., Yadav, S. K., Singh, J., G, S. B., Kumar, A., Amirineni, S., Narayana, J., & Maddi, V. (2016). Functional mechanisms of drought tolerance in maize through phenotyping and genotyping under well-watered and water-stressed conditions. European Journal of Agronomy, 79, 43–57. https://doi.org/10.1016/j.eja.2016.05.008
- Makbul, S., Saruhan Güler, N., Durmuş, N., & Güven, S. (2011). Changes in anatomical and physiological parameters of soybean under drought stress. Turkish Journal of Botany, 35(4), 369–377. https://doi.org/10.3906/bot-1002-7
- Mencuccini, M. (2003). The ecological significance of long‐distance water transport: Short‐term regulation, long‐term acclimation and the hydraulic costs of stature across plant life forms. Plant, Cell & Environment, 26(1), 163–182. https://doi.org/10.1046/j.1365-3040.2003.00991.x
- Messina, C., McDonald, D., Poffenbarger, H., Clark, R., Salinas, A., Fang, Y., Gho, C., Tang, T., Graham, G., Hammer, G. L., & Cooper, M. (2021). Reproductive resilience but not root architecture underpins yield improvement under drought in maize. Journal of Experimental Botany, 72(14), 5235–5245. https://doi.org/10.1093/jxb/erab231
- MOALD. (2020/21). Statistical information on nepalese agriculture 2077-78 [Online] https://moald.gov.np/wp-content/uploads/2022/07/STATISTICAL- INFORMATION-ON-NEPALESE-AGRICULTURE-2077-78.pdf
- Mustamu, N. E., Tampubolon, K., Alridiwirsah Basyuni, M., Al-Taey, D. K A, Jawad Kadhim Al Janabi, H., & Mehdizadeh, M. (2023). Drought stress induced by polyethylene glycol (PEG) in local maize at the early seedling stage. Heliyon, 9(9), e20209. https://doi.org/10.1016/j.heliyon.2023.e20209
- Naveed, A., Khan, A. A., & Rauf, S. (2012). The potential of breeding okra (Abelmoschus esculentus L.) for water stress tolerance. In M. Ashraf, M. Öztürk, M. Ahmad, & A. Aksoy (Eds.), Crop production for agricultural improvement. Springer. https://doi.org/10.1007/978-94-007-4116-4_8
- Nejad, S. K., Bakhshande, A., Nasab, S. B., & Payande, K. (2010). Effect of drought stress on corn root growth. Reports in Opinion, 2(2), 1–7.
- Nonami, H. (1998). Plant water relations and control of cell elongation at low water potentials. Journal of Plant Research, 111(3), 373–382. https://doi.org/10.1007/BF02507801
- Novák, V., & Lipiec, J. (2012). Water extraction by roots under environmental stresses. In George J. Halasi-Kun (Ed.), Pollution and water resources, Columbia University seminar proceedings: Impact of anthropogenic activity and climate changes on the environment of Central Europe and USA (Vol XLI).
- Pokhrel, S., Paudel, S., Shrestha, N., Kandel, B. P., Aryal, K., & Pokhrel, K. R. (2019). Screening at an early seedling stage for the identification of drought-tolerant genotypes in maize. Journal of Genetics, Genomics & Plant Breeding, 3(3), 45–52.
- Qayyum, A., Ahmad, S., Liaqat, S., Malik, W., Noor, E., Saeed, H. M., & Memoona Hanif, M. (2012). Screening for drought tolerance in maize (Zea mays L.) hybrids at an early seedling stage. African Journal of Agricultural Research, 7(24), 3594–3604.
- Ray, D. K., Gerber, J. S., MacDonald, G. K., & West, P. C. (2015). Climate variation explains a third of global crop yield variability. Nature Communications, 6(1), 5989. https://doi.org/10.1038/ncomms6989
- Rosawanti, P., Ghulamahdi, M., Khumaida, N., Agroteknologi, J., Pertanian, F., Muhammadiyah, U., Rta, J., Km, M., & Tengah, K. (2015). Respon_Anatomi_dan_Fisiologi_Akar_Kedelai_terhadap. Jurnal Agronomi Indonesia (Indonesian Journal of Agronomy) 43(3), 186–192. https://doi.org/10.24831/jai.v43i3.11243
- Sarker, A., Erskine, W., & Singh, M. (2005). Variation in root and shoot traits and their relationship in drought tolerance in lentil. Genetic Resources and Crop Evolution, 52(1), 89–97. https://doi.org/10.1007/s10722-005-0289-x
- Sarker, U., & Oba, S. (2018). Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biology, 18(1), 258. https://doi.org/10.1186/s12870-018-1484-1
- Shahzad, A., Gul, H., Ahsan, M., Wang, D., & Fahad, S. (2023). Comparative genetic evaluation of maize inbred lines at seedling and maturity stages under drought stress. Journal of Plant Growth Regulation, 42(2), 989–1005. https://doi.org/10.1007/s00344-022-10608-2
- Slattery, R. A., Vanloocke, A., Bernacchi, C. J., Zhu, X. G., & Ort, D. R. (2017). Photosynthesis, light use efficiency, and yield of reduced-chlorophyll soybean mutants in field conditions. Frontiers in Plant Science, 8(April), 549. https://doi.org/10.3389/fpls.2017.00549
- Tandzi, L. N., Ngonkeu, E. M., Youmbi, E., Nartey, E., Yeboah, M., Gracen, V., Ngeve, J., & Mafouasson, H. A. (2015). Agronomic performance of maize hybrids under acid and control soil conditions. International Journal of Agronomy and Agricultural Research (IJAAR), 6(4), 275–291.
- Tani, E., Chronopoulou, E. G., Labrou, N. E., Sarri, E., Goufa, M., Vaharidi, X., Tornesaki, A., Psychogiou, M., Bebeli, P. J., & Abraham, E. M. (2019). Growth, physiological, biochemical, and transcriptional responses to drought stress in seedlings of Medicago sativa L., Medicago arborea L. and their hybrid (Alborea). Agronomy, 9(1), 38. https://doi.org/10.3390/agronomy9010038
- Wang, Z., Li, G., Sun, H., Ma, L., Guo, Y., Zhao, Z., Gao, H., & Mei, L. (2018). Effects of drought stress on photosynthesis and photosynthetic electron transport chain in young apple tree leaves. Biology Open, 7(11). bio035279. https://doi.org/10.1242/bio.035279
- Webber, H., Ewert, F., Olesen, J. E., Müller, C., Fronzek, S., Ruane, A. C., Bourgault, M., Martre, P., Ababaei, B., Bindi, M., Ferrise, R., Finger, R., Fodor, N., Gabaldón-Leal, C., Gaiser, T., Jabloun, M., Kersebaum, K.-C., Lizaso, J. I., Lorite, I. J., … Wallach, D. (2018). Diverging importance of drought stress for maize and winter wheat in Europe. Nature Communications, 9(1), 4249. https://doi.org/10.1038/s41467-018-06525-2
- Widuri, L. I., Lakitan, B., Sodikin, E., Hasmeda, M., Meihana, M., Kartika, K., & Siaga, E. (2018). Shoot and root growth in common bean (Phaseolus vulgaris L.) exposed to gradual drought stress. AGRIVITA Journal of Agricultural Science, 40(3), 442–452. https://doi.org/10.17503/agrivita.v40i0.1716
- Windra Sukma, K. P. (2018). Growth and Production of Local, Hybrid and Composite Corn in Pamekasan Madura. Journal of Agrosains: Creative and Innovative Work, 4(2), 34. https://doi.org/10.31102/agrosains.2017.4.2.34-38
- Wu, Y., & Cosgrove, D. J. (2000). Adaptation of roots to low water potentials by changes in cell wall extensibility and cell wall proteins. Journal of Experimental Botany, 51(350), 1543–1553. https://doi.org/10.1093/jexbot/51.350.1543
- Yadav, S., & Sharma, K. D. (2016). Molecular and morphophysiological analysis of drought stress in plants. In E. C. Rigobelo (Ed.), Plant growth (pp. 149–173). InTechOpen. https://doi.org/10.5772/65246
- Yin, Y., Gao, Y., Lin, D., Wang, L., Ma, W., & Wang, J. (2021). Mapping the global-scale maize drought risk under climate change based on the GEPIC-vulnerability-risk model. International Journal of Disaster Risk Science, 12(3), 428–442. https://doi.org/10.1007/s13753-021-00349-3
- Zhang, Q., Liu, H., Wu, X., & Wang, W. (2020). Identification of drought-tolerant mechanisms in a drought-tolerant maize mutant based on physiological, biochemical and transcriptomic analyses. BMC Plant Biology, 20(1), 315. https://doi.org/10.1186/s12870-020-02526-w
- Zhang, X., Lei, L., Lai, J., Zhao, H., & Song, W. (2018). Effects of drought stress and water recovery on physiological responses and gene expression in maize seedlings. BMC Plant Biology, 18(1), 68. https://doi.org/10.1186/s12870-018-1281-x
- Zhu, J., Brown, K. M., & Lynch, J. P. (2010). Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant, Cell & Environment, 33(5), 740–749. https://doi.org/10.1111/j.1365-3040.2009.02099.x