 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study aimed to evaluate the effect of botanical pesticides mixed with entomopathogenic Beauveria bassiana on the growth and development of Spodoptera litura. This study adopted an experimental approach using a completely randomized design consisting of three treatments (botanical pesticides, B. bassiana, and a mixture of botanical pesticides and B. bassiana) and two control. The treatment and control had five concentration levels and were repeated four times. The test used the dip feed method. The relation between treatments and S. litura mortality was analyzed using probit analysis. Differences in means between treatment and control were analyzed using ANOVA. The results showed that both single and mixed treatments decreased relative consumption, inhibited relative growth rate and reduced fecundity of S. litura. The activity of the mixture of pesticides at the LC50 level has shown a synergistic effect. Thus, the mixture of botanical pesticides and B. bassiana could be developed as an alternative pesticide for sustainable biological control of S. litura.
REVIEWING EDITOR:
Introduction
Spodotera litura, also known as tobacco cutworm, is a native to Southeast Asia. It spreads to India and becomes an important pest in the Asia Pacific (Ahmad et al., Citation2013). Spodoptera litura is a polyphagous pest of agriculture, causing significant economic impacts on several crops in various countries worldwide. The species has a vast number of host range more than 120 plants including the families Graminae, Malvaceae, Cruciferae, Euphorbiaceae, Araceae, Umbilliferae, Chenopodiaceae, Solanaceae, Leguminasae, Alliaceae, Labitaceae, Capparidaceae, Rosaceae, Compositae, Oleaceae, Apocynaceae, Anacardiaceae, Tilaceae, Moraceae, Meliaceae, Myrtaceae and Fabaceae (Ahmad et al., Citation2013). This species has enormous potential to invade new areas because of its capability to adapt to new climatic and ecological conditions (Arunthirumeni et al., Citation2023).
The S. litura is the primary pest of soybean crops in Indonesia. During the dry season, almost 60% of soybean plantings are attacked by this pest (Marwoto, Citation2008). This also often occurs when soybean crops are planted after rice cultivation. Soybean failure in Indonesia can reach 100% because of S. litura attacks in all soybean stadia (Fateha et al., Citation2020). The cutworm attack on the chili crop may lead to a 40-50% loss (Vijayalakshmi et al., Citation2016). In cabbage crops, this pest can cause losses of 25.8-100% (Sahu et al., Citation2020).
Recent studies have shown that S. litura is resistant to several chemical pesticides, such as organophosphate, carbamate, and pyrethroid (Muthusamy et al., Citation2015). Another study has shown that excessive use of chemicals or chemical insecticides results in intense mortality selection pressure on insect pests, resulting in resistance to certain compounds (Dawkar et al., Citation2013). Insecticides containing synthetic pyrethroids, which kill pests with low toxicity, have been commercially available in Indonesia since the 1970s. Various parties in several countries have reported that cutworms have become resistant to pyrethroids (Sule & Kumar, Citation2019). This is due to the need for proper rotation of insecticide use.
Recently, efforts to seek alternative control of S. litura with botanical pesticides have continued to develop. The application of botanical pesticides made from plant secondary metabolites has been widely reported to suppress the pests. The cutworm larvae fed with ethylacetate extract of Alpinia galanga showed lower body weight changes and affected the relative growth rate of larvae. In addition, A. galanga was shown as toxicity effects on S. litura (Datta et al., Citation2019). Based on statistical tests, in the test of differences in concentration and length of time for the application of ar turmerone from Curcuma longa extract (Zingiberaceae), the results showed that turmerone from Curcuma longa extract can be a solid natural pest repellent, even in a low concentration of 10 liters per 20 grams of corn seeds (Tavares et al., Citation2013). Likewise, another study showed effective the treatment of Dioscorea hispida extract against the insect pest C. curvignathus termites that attack wood. Dioscorea hispida is an antifeedant or feeding inhibitor for C. curvignathus termites up to 99.2% (Azman et al., Citation2016). In addition, hydrogels made from D. hispida can act as a promising antibacterial to inhibit bacterial activity (Azman et al., Citation2016).
The application of botanical pesticides to control S. litura can be done with a single source or mixture. Using botanical pesticide mixtures with entomopathogenic has a synergistic impact that can reduce the amount of material used to achieve toxic levels and minimize the possibility of negative impacts on non-target organisms. Based on literature studies, research on the properties of biopesticides with several secondary metabolite plants, with a mixture of entomopathogenic fungi against S. litura has yet to be widely reported. Potential pesticides as a biocontrol in the field are relatively short and are influenced by environmental factors. For this reason, research about pesticides must continue through many innovations. In this study, an exploration was conducted to determine the potential of a mixture of botanical pesticides and entomopathogenic fungi to control agricultural insect pests. Various morphological character parameters, larval antifeedant effect and imago fecundity were assessed to support the toxicity and effectiveness of biopesticides against S. litura. This study aimed to evaluate the effect of botanical pesticides mixed with entomopathogenic B. bassiana on the growth and development of S. litura.
Methods
Insect culture
The research was conducted at Field laboratory in Wagir District, Malang, East Java, from September 2022 to March 2023. Spodoptera litura eggs were obtained from Research Institute for Sweetener and Fiber Crops in Karangploso, Malang, East Java. The Institute has reared the species for several decades. Before rearing S. litura larvae as test insects, acclimatization was carried out for 24 hours to adjust the newly hatched larvae in new external environment. The S. litura larvae were put and maintained in plastic boxes (16.2 cm × 16.2 cm × 7.5 cm) and fed with castor bean leaves. Food for test larvae using Ricinus communis L. leaves from R. communis that were 3 to 4 months old were used as. After the larvae entered the sixth instar or before the pupal phase, the larvae were transferred into a plastic jar 11 × 11 × 19.5 cm3, containing sifted sand seven cm as a medium for pupal formation. The five-day-old pupae were transferred to a plastic jar until they hatched into the imago. The temperature and humidity were maintained at 29° to 31 °C and 60 to 62.5% throughout the experiment.
Imago S. litura hatching from pupae were fed with 10% honey solution by applying the solution to the surface of a screen cover 25.5 cm x 28.5 cm until the entire surface wet with honey solution. Imago will fly to the top surface of the jar to obtain food nutrients. After seven days, S. litura eggs appear attached to the jar’s top side surface or to the inner screen cover. Furthermore, the imago was moved to another jar so that it was not mixed with eggs that later entered the larval phase that hatched. After the larvae hatch, the larvae are fed with Ricinus communis leaves again. Insect maintenance is carried out periodically to maintain the availability of larvae for testing.
Preparation of botanical pesticide and test solution
The botanical pesticide extract solution was prepared by mixing D. hispida tubers, traditional plant and other supporting materials. The materials used in making botanical pesticide include D. hispida tubers, Zingiber officinale, traditional plant consisting of Curcuma longa Linn., Alpinia galanga, Curcuma zanthorrhiza, Boesenbergia rotunda (L.), some fruits such as, Carica papaya L., Ananas comosus, Aegle marmelos, molasses, shrimp paste, coconut water, rice washing water, EM4, tofu waste and cow urine.
The process of making the botanical pesticide was done by peeling the fruit and zingiberacea tubers, then blending all the ingredients until smooth and mixing with coconut water, rice water waste, EM4, tofu waste, and cow urine. The whole materials were stirred until fully dissolved. The mixture was fermented anaerobic for two weeks at a room temperature of 25 °C.
Preparation of test solution
Testing solution entomopathogenic using Beauveria bassiana was obtained by commercially available B. bassiana. The B. bassiana used for testing is GMN Beauveria, which is produced by Turrima Trubus Prima Co. Ltd. In addition to tests using organic materials, tests were also carried out using insecticides as a comparison. The insecticide used was SAPPORO 52EC insecticide, Santani Agro Mandiri Co. Ltd.
The test consisted of a botanical pesticide, B. bassiana solution, a mixture of botanical pesticide, and B. bassiana, insecticide, and control. The botanical pesticide test solution was prepared by taking the botanical pesticide according to the predetermined concentration using a pipette into a 100 ml measuring cup and then gradually adding aquades while stirring and putting it in a container until the volume reached 100 ml. Furthermore, the preparation of the B. bassiana solution was carried out by first weighing the B. bassiana powder according to the predetermined concentration, then gradually adding aquades while stirring until the B. bassiana dissolved and put into a container until the volume reached 100 ml. The next step was determining pesticide treatment. Pesticide solution was taken as much as 0.1 ml and put into a measuring cup, then added aquades until the volume reached 100 ml. Determination of the pesticide concentration for testing is based on the maximum dose of pesticide specified for application.
Furthermore, the preparation of a mixture of botanical pesticide solutions and B. bassina is carried out by mixing the LC50 botanical pesticide solution that has been obtained first with B. bassiana according to a predetermined concentration. The mixture of botanical pesticide and B.bassiana is 1:1. Aqudes as a diluent solvent was used in the test solution.
Testing Methods
Single and mixed toxicity tests of botanical pesticide
Single and mixed toxicity tests consisted of preliminary tests and further tests using the feed leaf residue method. Preliminary tests were carried out at several concentrations to determine the concentration level that could cause mortality of test insect larvae. Further tests were carried out using five levels and a control. The concentration level that became the basis for further testing was based on the preliminary test results. Preliminary tests were conducted at several concentrations to determine the two concentration levels that could cause 5%-95% mortality of test larvae. In addition, further tests were conducted using five concentration levels and a control. As the results of the preliminary tests were obtained, the concentrations were determined for further plant-based pesticide testing using a concentration of 10%, 25%, 50%, 75%, and 100%. The concentration level that became the basis for further testing was based on the preliminary test results. In the follow-up test of a single botanical pesticide concentration, concentration levels are 10%, 25%, 50%, 75% and 100%. Single testing of B. bassiana was done using concentrations of 0.1 g, 0.25 g, 0.5 g, 0.75 g and 1 g. Furthermore, single testing of a mixture of botanical pesticides and entomopathogenic B. bassiana based on the results of a single botanical pesticide test obtained the LC50 value of botanical pesticide toxicity against test insects. This LC50 toxicity value will be the basis for testing the mixture of plant-based pesticides and B. bassiana with 34% concentration.
The larvae were put in the jars or containers. It consisted of 20 larvae per jar, and the treatment was replicated six times (in the jars). So that the total jars used in one treatment were 30 jars. The test was carried out by cutting the leaves of R. communis, which were previously weighed first, to determine the initial weight of the leaves. Then, it dipped into each predetermined concentration for 20 seconds to ensure the mixture has evenly moistened both sides of the surface, then dried on newsprint. The air-dried R. communis leaves were put into a Petri dish with a diameter of 9 cm and a height of 1.5 cm that had been covered with tissue paper, then ten instar II larvae were inserted. Testing of botanical pesticide treatments, entomopathogenic Beauveria bassiana and their mixture was carried out with four replicates. The control treatment was fed with Ricinus communis L. leaves that were only dipped in solvent according to the preparation. After 48 hours of treatment and control exposure, the feed was replaced with untreated R. communis.
After 48 hours of treatment, the remaining feed from each treatment was wrapped in aluminum foil and dried in an oven at 90 °C for 48 hours, then weighed again to determine the ratio of feed weight after and before drying as the value of the proportion of dry weight to wet weight. Observations were made on the mortality of test larvae from the first day after application, from the second instar larvae until the surviving larvae developed to the sixth instar phase. Observations were also made by recording the developmental time of instars II-VI, differences in feed consumption, pupa weight and imago fecundity or the ability of imago to lay eggs.
Observations of differences in feed consumption were made by recording the values of dry weight and wet weight of leaves. The difference in pupal weight was observed by weighing the weight of S. litura pupae with an analytical balance. The conversion of food digested by V instar larvae was calculated by the method according to Datta et al. (Citation2019) as follows:
Where
I = the weight of food (leaf) consumed (g),
B = the larval weight gain (g)
T = the feeding duration (day)
ΔB = the body weight change of larva (g) and
BI = the initial weight of larva (g).
The activity characteristics of the combination interaction of the mixture were analyzed using the combination index (CI) based on different co-acting models at the LC50 and LC95 levels with the following formula: (Chou, Citation2006)
CI = combination index
LCx1(cm) = lethal concentration × combination extract on the first single extract
LCx2(cm) = lethal concentration × combination extract on the second single extract
LCx1 = lethal concentration × first single extract
LCx2 = lethal concentration × second single extract,
The combination index results obtained can determine the nature of the mixture interaction referring to Chou, Citation2006 criteria ().
Table 1. Category of combination extract interaction.
Test of the effect of botanical pesticide, B. bassiana and its mixture on the fecundity of S. litura
S. litura larvae at each concentration that successfully survived in the next phase were reared until they formed pupae and became imago. Imago that successfully formed in each test and control group were paired for the female imago fecundity test. One pair of imago was mated from imago between treatments, but not from the same treatment, except in the control treatment, with the result that in one jar, there was only one pair of imago. Some things underlie the pairing between treatments, including that, in each test group, not all pupae succeeded in becoming imago. In addition, the distinguishing character of wing color on the imago cannot be seen clearly because the shape and color of the wings are reduced, this makes it difficult to distinguish the sex of the imago.
Data analysis
Data on the relationship between concentration and mortality of test larvae were analyzed using probit analysis of the IBM SPSS package Statistics version 25© program. Data relative consumption rate, relative growth rate and fecundity analyzed by ANOVA (p < 0.05) after checking for normality, means were separated with Tukey’s multiple range test at the 5% significant level using IBM SPSS package statistics version 25©. Data on the developmental time of test larvae and pupal weights were presented as mean values and standard deviations. Morphological observations during the larval phase include body size difference morphology, cuticle damage, larval movement, color changes, changes in larval body texture, pupa shape, imago wing shape and imago wing color.
Result
Single toxicity of botanical pesticides, Beauveria bassiana and mixture of both on the development of S. litura
The S. litura larvae treated with a single plant-based pesticide, B. bassiana and a mixture of both showed increasing mortality in line with the concentration given to each treatment (). Mortality of S. litura after the application of botanical pesticides (BP), B. bassiana (BB) and mixture of both pesticides (BP + BB) with the feed dipping method correlated with the amount of each treatment as shown in . The percentage mortality of S. litura along with the change of observation time until 15 days after treatment is shown in . Larvae of S. litura treated with botanical pesticides, B. bassiana and mixture of Botanical pesticides + B. bassiana at the concentrations tested showed increasing result in mortality at 2nd day until 15th day and relatively constant at 10th day.
Figure 1. Mortality of S. litura (%) at various levels of concentration treatments as follows (a) Plant-based pesticides; (b) B. bassiana; (c) Plant-based + B. bassiana; (d) Avermectin.
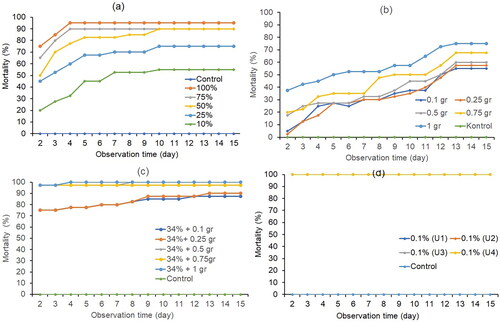
Treatment of botanical pesticides showed that at a concentration of 100%, larval mortality increased from 75% on the second day to 95% on the fourth day. At a concentration of 75%, larval mortality also increased from 65% on the second day to 90% on the fourth day. At a concentration of 50%, mortality also increased from 50% on the second day to 90% on the tenth day. At a concentration of 25%, the second-day mortality rate was less than 50% (). Single application of B. bassiana showed lower mortality (low effectiveness) than botanical pesticides, because larva mortality was less than 50% in all treatment. That in a concentration of 1 g, showed that larval mortality increased from 37.5% on the second day to 70% on the thirteenth day. At a concentration of 0.75 gr, larval mortality also increased from 20% on the second day to 65% on the thirteenth day (). The combination application showed that at a concentration of 34% PB and 1 gr BB the larval mortality rate reached 97.5% on the second day, increasing to 100% on the fourth day. At a concentration of 34% PB and 0.75 gr BW, larval mortality on the second day was 97.5% and did not increase until the last day. The lowest concentration, 34% PB and 0.1 gr BW, showed that larval mortality increased from 75% on the second day to 87.5% on the 12th day (). Treatment using Avermectin (positive control) showed 100% mortality at all concentrations (). Meanwhile, the probit analysis of LC50 and LC95 showed that mixture pesticide produced smaller values. The results of small value indicate a higher level of toxicity along with the increase in observation time and the development of mortality of test insects (). shows the effect of the application of botanical pesticides (PN), Beauveria bassiana (BB), and a mixture of both (PN + BB) on S. litura mortality, probit regression values, relative consumption rate, relative growth rate, length of the larval growth phase, pupa weight and imago fecundity. Statistical results showed the effect of the application on S. litura indicate changes in several physiological characters in each S. litura development, which was also reinforced by evidence of changes in S. litura morphology from larval development to pupal and imago phases.
Table 2. Probit regression of the toxicity of plant-based pest, B. bassiana and its mixture based on the development al stage of S. litura.
Based on the analysis using SPSS 25, LC50 values were obtained from day 4 to day 11 after treatment. The LC50 value used as an estimate of the concentration that kill half the number of S. litura larvae. The LC50 and LC95 values of the mixture of pesticides in all observations were lower than the LC50 and LC95 values in the single test of botanical pesticides and the single test of B. bassiana. Meanwhile, the single test of botanical pesticides showed a value close to the LC50 and LC95 of the mixture of botanical pesticides and B. bassiana. The test results showed that the mixture of botanical pesticides and B. bassiana (BP + BB) was effective in killing S. litura larvae. LC50 and LC95 in each treatment decreased from day to day, indicating a higher level of toxicity, can also kill more S. litura pests than the previous day ().
Effect of single plant-based pesticide, B. bassiana and mixture of both in relative consumption rate (RCR) and relative growth rate (RGR)
The treatment of a single botanical pesticide, a single B. bassiana, and a mixture of both resulted in the inhibition of larval development according to the high concentration given (). S. litura larvae in the mixture of pesticides had completed the larval development stage 1-4 days longer than the control treatment, while that in the single treatment of botanical pesticides and B. bassiana took 1-2 days longer than the control. This can be evidence that the treatment using a mixture of pesticides can effectively inhibit larval development compared with the single treatment.
Table 3. Effect of plant-based pesticide, B. bassiana and its mixture on consumption rate, and growth rate.
presents the average relative consumption of S. litura larvae in treating a single botanical pesticide, a single B. bassiana, and a mixture of both. The value of the 5% Tukey test on the average relative consumption of S. litura larvae in the single and mixed botanical pesticide treatments was significantly lower than in the control treatment, except for the single treatment of B. bassiana at a concentration of 0.1 g. Presumably, due to the low compound content in the single treatment of low concentration, B. bassiana does not interfere with the process of stopping eating directly. The treatment that causes the lowest relative consumption is the botanical pesticide at a concentration of 100%, which can cause feeding inhibition 1.8%, which shows the highest feeding inhibition activity. The following highest food inhibition results were in the mixed treatment of botanical pesticides + B. bassiana, which showed more effectiveness than the single B. bassiana treatments ().
Figure 2. Comparing RCR and RGR indices of S. litura after feeding on various concentrations of plant-based pesticide, B. bassiana and its mixture.

The 5% Tukey test showed that the value of the relative growth rate (RGR) of the control treatment was higher and significantly different from the mixed treatment of botanical pesticides and B. bassiana, the single treatment of botanical pesticides, and the single treatment of B. bassiana (). The lowest value of the relative growth rate was in the single treatment of B. bassiana concentration of 1 g. The RGR value in the sole treatment of B. bassiana concentration of 1 g and the mixture of botanical pesticides and B. bassiana concentrations of 34% + 0.5 g, 34% + 1 g, which was very low, shows that the larvae have begun to dry out, and getting thinner. In the single treatment of B. bassiana, low concentrations 0.1 and 0.25 showed high RGR values in line with the high average value of relative consumption. The increased consumption of larvae in the treatments was in order for larvae to fulfill their nutritional needs due to the use of energy to detoxify toxic compounds that enter the body.
Changes in larval morphology
Morphological changes were also seen in the single treatment of plant-based pesticides, single B. bassiana, and a mixture of both (plant-based pesticides + B. bassiana) at the larval, pre-pupal, and imago stages, as shown in . Morphological observations during the larval phase include body size difference morphology, cuticle damage, larval movement, color changes, changes in larval body texture, pupa shape, imago wing shape and imago wing color.
Figure 3. Effect of plant-based pesticide, B. bassiana and their mixture on morphological changes of S. litura. (a) Morphological changes in the mixed treatment of plant-based pesticide + B.bassiana. (b) morphological changes in B.bassiana treatment. (c) morphological changes in plant-based pesticide treatment.

Morphological changes were also seen in the single treatment of botanical pesticides, single B. bassiana, and a mixture of both (plant-based pesticides + B. bassiana) at the larval, pre-pupal, and imago stages as shown in . There is a morphological change between the mixed treatment of botanical pesticides and B. bassiana and the single treatment of botanical pesticides and the B. bassiana treatment, which shows striking morphological changes. Larval body size in all single and mixed treatments was smaller.
The S. litura larvae showed visible disturbances with severe cuticle damage. The larvae treated with a mixture of pesticides demonstrated slowed movement, and after death, the larval body became hard and stiff. The posterior abdominal part appears broken and looks smaller than the thorax and dorsal parts (). shows B. bassiana larvae infection characterized by mycelial growth on dead larvae. Morphological changes in larvae that are seen due to botanical pesticide treatment show the characteristics of the body becoming increasingly soft and watery, as seen in .
Furthermore, larvae shrink along the mesothorax to the posterior abdominal and experience color changes, where the pest’s body becomes dark. In some cases, it affected the pre-pupal stage and often led to mortality or abnormal pupal formation, seen in all single and mixed treatments. Abnormal pre-pupal stage changes are characterized by cuticle rupture above the protonum of the head capsule along the dorsal, meso and metathoracic midline and result in not being able to continue in the complete pupal process and lead to death during the pre-pupal stage (). Other abnormality seen in the failure of the pre-pupal stage are a wrinkled and dry body shape (), anterior and posterior parts begin to form but fail, which causes an abnormal body shape.
During the pupal phase, the pupae appear to have shrunk (). Even in the botanical pesticide treatment, the pre-pupal stage has shrunk (). The ventral part of the pupa does not appear to be fully formed, with the abdominal segment not formed (). The treatment of Beauveria bassiana and botanical pesticides showed the failure of pupa formation, larvae that had been covered with soil during the pupa phase process, the larvae then died in the soil () or abnormal pupa body shape (posterior not formed) (). Developmental disorders also occur, with the imago failing to emerge from the pupa and only making it halfway out (). Developmental changes can also be seen in the reduced size and color of the imago wings (3c).
Male and female images can be determined by looking at the spots on the wing scales. Male imago shows fewer wing spots than female imago. In the treated imago, the wing color was reduced, making it quite difficult to determine the sex. The growth and development period of S. litura larvae is highly dependent on adequate nutrients such as carbohydrates, protein, and fat. However, these nutrients are needed due to the synergistic effect of active compounds contained in botanical pesticides, B. bassiana, and the mixture used in this study. In that case, it will cause disruption of growth and development, such as larval mortality, the inability of larvae to develop in each instar, larvae not successfully entering the pupal phase, pupal mortality, low pupal weight, pupae not successfully forming imago and low fecundity of imago.
The results show that botanical pesticides, B. bassiana, and the mixture have the same potential for biological control of S. litura larvae by affecting growth and development. Some imago only makes it out of the pupa partially. Only the antennae, caput, and thorax were successfully released. B. bassiana-treated imago showed hatching activity, characterized by the appearance of golden fibers on the genitalia of the imago. However, the appearance of the golden fibers was not accompanied by the fecundity of the imago or the eggs did not come out (). Observations showed that there were a number of S. litura imago that successfully hatched from the treatment, showing slow-walking characters and even some individuals were unable to walk. Eggs produced by imago from single and mixed treatments could not survive or did not mature. The decreased feeding activity of S. litura after testing impacts the reduction of energy sources for growth and development, which can inhibit insect physiological processes such as molting, reproduction and the ability to produce eggs.
Effect of single plant-based pesticide, B. bassiana and mixture of both on imago viability and fecundity
Compared to the control, a single treatment of botanical pesticides and a mixture of pesticides caused impaired viability of S. litura imago. Imago from the treatment group had lower viability compared to the control. Imago in the treatments can survive 6-8 days, while in the control treatment, the imago can survive 8-11 days (). The results of the study on the fecundity of S. litura imago were shown in . The fecundity ability of S. litura imago in all treatments can be seen from the number of eggs laid by female S. litura imago. The total number of eggs female S. litura imago produced in the control treatment was significantly higher and significantly different from the treatment data of all concentrations of single botanical pesticides, single B. bassiana and a mixture of pesticides. The parameter of the length of life of female and male imago in the control treatment proved to be significantly longer than in all single botanical pesticide treatments, B. bassiana, and mixture of pesticides. The mixed treatment of botanical pesticides and B. bassiana showed a shorter length of life of female and male imago than the B. bassiana treatment, but it was not significantly different from the single botanical pesticide treatment.
Table 4. Effect of several treatments, plant-based pesticide, B. bassiana and their mixture on S. litura fecundity.
Activity characteristics of botanical pesticide mixtures and B. bassiana
The combination index (CI) of the mixture of pesticides at 4 DAT LC50 observations was moderately synergistic, 7-11 DAT LC50 observations were synergistic, and 13 DAT LC50 observations were strongly synergistic. Observation of 4 DAT LC95 was synergistic, and at 7-11 DAT, observations were strongly synergistic. At observation 13 DAT, result showed powerful synergistic properties (). The characteristics of the interaction of the pesticide and B. bassiana mixture at LC50 and LC95 changes synergistically in line with the concentration applied and the exposure time. According to the previous explanation, changes in the activity characteristics of the mixture are influenced by the amount of concentration applied, the mode of action of an active compound in the mixture and the time of exposure.
Table 5. Activity characteristics of plant-based pesticide, B. bassiana, and their mixture against S. litura larvae.
Discussion
The application of single and mixed pesticides effectively caused the gradual mortality of S. litura along with the increase in concentration level and observation time. The chronic effect of toxic materials contained in the single and mixture of pesticides caused the death of S. litura larvae after the treatment. Based on the percent mortality value, applying a mixture of pesticides (BP + BB) demonstrated a higher toxicity of 1.3 times that of the single botanical pesticide and 3 times that of the single B. bassiana. It shows that botanical pesticides and B. bassiana are more effective when used in combined form and have a synergistic effect for S. litura. A mixture of extracts may produce various interactions, including synergistic, antagonistic, non-synergistic, and additive effects.The antagonistic effect occurs when the mixed material treatment produces a lower effect than the single treatment. The synergistic effect occurs when the mixed material treatment leads to a higher effect than the calculation of the effect of a single material (Cui et al., Citation2022). When the botanical pesticide was combined with B. bassiana, it showed a synergistic effect. In addition to the significant mortality of S. litura, this synergistic effect also increased larval growth and feeding activity inhibition. This situation may lead to larval morphological and fecundity changes.
Extrinsic and intrinsic factors may influence the growth and development of S. litura larvae. Intrinsic factors are the metabolism within the larval body, while extrinsic factors are the nutrients consumed by the larvae. The active compounds in a single botanical pesticide, a single B. bassiana, and a mixture enter the larva’s gut. The active compounds are digested and absorbed in the larval midgut until they disrupt the body’s metabolic processes.
The commercial Beauveria bassiana is a fungus often used to control insects. A study by Ardika demonstrated that B. bassiana from the same company effectively controlled Oryctes rhinoceros (Ardika, Citation2023). Research conducted by Sianturi et al. (Citation2014) showed that commercial Beauveria bassiana was effective against H. antonii on the 10th day with a mortality rate of 100%. Another study reported effective B. bassiana isolates as a biological control agent against S. frugiperda. The isolates caused significant cumulative mortality rates ranging from 71.3% to 93.3% at 14 days post-treatment and reduced larval feeding efficacy from 69.4 to 77.8% at 48 h post-treatment (Idrees et al., Citation2022). The other study demonstrated evidence of B. bassiana killing S. litura by infecting larval tissues. The mycelium appears on the larvae’s body after consuming food treated with B. bassiana (Kartohardjono, Citation2011). B. bassiana conidia coat the food leaves and can stick to the skin of the larvae. When the larvae eat the leaves, the B. bassiana conidia develop to form hyphae, then produce chitinase, protease, lipolytic and amylase enzymes. When these enzymes enter the larval tissue, they can hydrolyze proteins in the integument, causing damage to the cuticle layer. When the cuticle is damaged, the hyphae of B. bassiana may penetrate and develop inside the larva’s body.
The application of B. bassiana may have less impact to non-target species. The effects of B. bassiana on non-target arthropods were studied in rangeland and alfalfa agroecosystems in Canada, and assessed after field application. That study showed that B. bassiana had a low effect on spiders, carabids, coccinellids, and tenebrionid beetles (Goettel et al., Citation2021). The application of B. bassiana can affect non-target species when it is used at doses exceeding the recommended limits (Hanif et al., Citation2020).
Plant-based pesticides are thought to contain active secondary metabolite compounds such as tannins, dioscorin, saponins, phenols, alkaloids, and terpenoids. A study reported that D. hispida and A. marmelos have the toxic active compound dioscorin and can reduce the growth of the S. litura pest population through antifeedant activity (Darmanto et al., Citation2019). Dioscorin’s active compound is neurotoxic and convulsant, which causes paralysis of the central nervous system in animals (Ismawati et al., Citation2021). The average S. litura imago life span in the mixture of pesticides is 6 to 7 days. The viability female and male imago in the mixture of pesticides is low. It may reduce the time to find a mate and the time for females to lay eggs while in the field (Duarte et al., Citation2020).
D. hispida has a cyanogenic glycoside compound that can cause death in insects more quickly according to the concentration applied because it has high toxicity and disrupts the nervous system when it enters the insect’s body (Yuliana & Rustam, Citation2023). This cyanogenic glycoside compound forms Hydrogen Cyanide or Cyanide Acid through a hydrolysis process, entering the insect’s body through stomach poison. This cyanide acid will affect the work of insect hemolymph. Cyanide acid may cause hemolymph to be unable to form antibodies in free quantities in suppressing antigens, so the hemosid cells contained hemolymph will be damaged. These cells play a role in transporting metabolite products, disturbing osmotic pressure and pH regulation (Azman et al., Citation2016).
The previous study reported that D. hispida and A. marmelos have active compounds of dioscorin which are toxic and can reduce the growth of S. litura pest populations through antifeedant activity (Darmanto et al., Citation2019). Dioscorin’s active compound is neurotoxic and convulsant, which causes paralysis of the central nervous system in animals (Ismawati et al., Citation2021). Other studies using D. hispida extract can provide more than 50% mortality values when applied to leafhopper pests. A dose of 100 grams/liter of D. hispida can provide 87% pest mortality for 65 hours after treatment; further, for 69 hours, it can provide mortality of 89.40% (Muhidin et al., Citation2020). Feed containing botanical pesticide enters and undermine the digestion tract of S. litura as a gut poison. The active compounds dioscorin and tannin contained in D. hispida and A. marmelos had repellant and antifeedant effects, which may lead to larval death. The stomach toxins contained in the active compounds dioscorin and tannin are absorbed by the walls of the digestive tract to the middle digestive tract (mesenteron), then translocated to the target site, such as the insect’s nervous system (Darmanto et al., Citation2019). This result of this study also demonstrated similar result. The RCR (relative consumption rate) and RGR (relative growth rate) values at the highest concentration of botanical pesticide treatment and the highest concentration of mixture pesticides showed significantly different results from the control treatment. The value of (RCR) relative consumption rate at a concentration of 100% botanical pesticide showed the lowest consumption rate (short-term effect) compared to the control, which decreased feeding efficiency then affected the long-term effect, namely the value of relative growth rate.
A few species of Zingiberaceae have been reported to exhibit antifeedant activity against test insects. Zingiber officinale for botanical pesticide is reported to contain phenolic compounds that act as antioxidants and enter the larvae in a contact toxic manner through the anal opening or mouth of the larvae. Some of these phenolic compounds are gingerol, kaempferol, zingiberol, paradol and shogaol (Singh et al., Citation2008). Shogaol and gingerol compounds in Z. officinale works as a neurotoxin, damaging plasma membrane permeability resulting in lysis. Meanwhile, kaempferol compounds work as a stomach poison by disrupting mitochondrial activity. If the mitochondrial activity is disrupted, the electron transport and ATP formation processes are reduced. The larvae then do not have enough energy to eat, causing low larval consumption and eventually death (Su et al., Citation2018).
Curcuma longa is also included in the botanical pesticide content of the treatment. Previous research reports on Curcuma longa showed that curcumin as an active ingredient contained in Curcuma longa gave a synergistic effect, reaching 2.651 when combined with the pesticide Avermectin, with a mixture ratio of 10%. Previous research also reported that the effectiveness of curcumin in killing S. litura when applied singly only showed a slight effect on mortality, this is suspected that curcumin can be an active ingredient that is auxiliary in nature to be combined with other active compounds. Evidence of the synergistic effect of curcumin with avermectin is reported to increase growth inhibition, destroy larval midgut morphology, and increase inhibition of cell proliferation observed in Sf9. The mixture of curcumin and avermectin was able to induce autophagy and cell apoptosis processes in vitro and in vivo (Cui et al., Citation2022).
Research on Alpinia galanga recently reported by Abdullah et al. (Citation2015) showed antifeedant activity against subterranean termite pests. The main place of digestive enzyme synthesis and secretion is in the midgut epithelium (Huang et al., Citation2015). The secondary metabolite compounds of A. galanga may pass through the larval peritrophic membrane and cause damage to the insect midgut epithelium, disrupting enzyme secretion and nutrient absorption (Kagon, Citation1986). This is similar to research conducted by Datta et al. (Citation2019), A. galanga extract purified 1′-acetoxy chavicol acetate, and galanin caused a significant decrease in the RGR and RCR values of S. litura test insects when compared to the control and showed an inhibitory effect on nutritional parameters. 1′-acetoxychavicol was reported to be more effective than taro. This was similar to the results of the RGR values, which were significantly different from the control treatment. This very low RGR value causes cell damage because the compound has been lysed into the cell and causes a decrease in nutrient absorption (Jansen & Groot, Citation2004). It is possible that the metabolites of A. galanga sequestrants have passed through the insect’s peritrophic membrane and damaged the insect’s midgut epithelium causing impaired enzyme secretion and nutrient absorption (Datta et al., Citation2019).
In treating a mixture of 34% + 1 g botanical pesticide and B. bassiana, there was an increase in consumption rate when compared to the single treatment of 100% botanical pesticide concentration which had the lowest consumption rate value among other treatments. The same event also occurred at the lowest concentration of B. bassiana, which showed that the consumption rate value was not significantly different from the control. The B. bassiana treatment at a concentration of 0.1 has the highest feed consumption value compared to all other treatments. This is likely because low concentrations have not been able to play an important role in larval digestive disorders. In addition, there was an increase in the consumption rate of surviving S. litura larvae. When toxic compounds enter the body, larvae will increase their ability to digest food. B. bassiana treatment is probably affecting the structure of the midgut microbial community of S. litura larvae. Microbes in the midgut detoxify secondary metabolite compounds and pathogens that have been introduced (Grobler, Citation2019). Generally, a single treatment of botanical pesticide, B. bassiana and a mixture of both disrupted larval growth by reducing feed consumption.
About the oviposition activity of S. litura imago, it is stopped, causing the total number of eggs produced to be low and, of course, affecting the shorter life span of the imago. The treatment of botanical pesticide and B. bassiana singularly or in mixture significantly affected the imago phase of S. litura. Previous studies have also reported that toxins from B. bassiana impair the average balance of physiological systems. Eggs produced from B. bassiana-treated larvae showed a significant reduction in hatchability (Kaur et al., Citation2011). According to previous studies, Aegle marmelos was reported to show maximum activity in preventing S. litura oviposition (Arivoli & Tennyson, Citation2013). Under treatment, female S. litura imago have sensory receptors sensitive to the host plant’s biochemical composition (Chen et al., Citation1996). Upon sensory reception, phytophagous insects tend to reduce oviposition (Showler, Citation2001).
The interaction of the mixture of botanical pesticide and B. bassiana at LC50 and LC95 changes in synergistic properties according to the concentration given and the time after application. Changes in the mixture’s effectiveness are influenced by several factors, such as the amount of concentration used, exposure time and how the active compound works on the target. The synergistic activity of the mixture of botanical pesticide and B. bassiana can be concluded to have a joint effect of the active compounds contained in the mixture on the target part of the pest S. litura.
Conclusion
The results showed that a mixture of of botanical pesticide and B. bassiana affected S. litura larval mortality escalation. The treatment decreased relative consumption, inhibited relative growth rate and reduced fecundity of S. litura. The activity of the mixture pesticides at the LC50 level has shown a synergistic effect. Thus, the mixture of botanical pesticides and B. bassiana could be developed as an alternative pesticide for sustainable biological control of S. litura.
Author contributions
The conception and design (Amin Setyo Leksono), analysis and interpretation of the data (Tasafima Tesari); the drafting of the paper (Tasafima Tesari), revising it critically for intellectual content (Irfan Mustafa); and the final approval of the version to be published (Amin Setyo Leksono). All authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Excel (804.7 KB)Acknowledgements
The authors thank Rector and Head of Research and Community Empowerment Institute, Brawijaya University.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article [and/or] its supplementary materials.
Additional information
Funding
Notes on contributors
Tasafima Tesari
Tasafima Tesari is a student at the Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, Indonesia. She obtained her master degree in Entomology from Brawijaya University. Email: [email protected]
Amin Setyo Leksono
Amin Setyo Leksono is a professor at the Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, Indonesia. His specialty is Entomology and Insect Ecology. He obtained his Ph.D. in ecology from Hiroshima University, Japan. He is serving as an editorial member and reviewer of several international journals. He has also authored many research articles/books on Animal Ecology and related fields. Email: [email protected]
Irfan Mustafa
Irfan Mustafa is an assistant professor at the Department of Biology, Faculty of Mathematics and Natural Sciences, Brawijaya University, Malang, Indonesia. His specialty is microbiology. He graduated from Kumamoto University in Japan. He is serving as an editorial member and reviewer of several national journals. He has also authored many research articles related to microbiology. Email: [email protected]
References
- Jansen, B. J. M., & Groot, A. D. (2004). Occurrence, biological activity and synthesis of dri- mane sesquiterpenoids. Natural Product Reports, 21(4), 1–14. https://doi.org/10.1039/b311170a
- Abdullah, F., Subramanian, P., Ibrahim, H., Abdul Malek, S. N., Lee, G. S., & Hong, S. L. (2015). Chemical composition, antifeedant, repellent, and toxicity activities of the rhizomes of galangal, Alpinia galanga against Asian subterranean termites, Coptotermes gestroi and Coptotermes curvignathus (Isoptera: Rhinotermitidae). Journal of Insect Science, 15(1), 7–7. https://doi.org/10.1093/jisesa/ieu175
- Ahmad, M., Gaffar, A., & Rafig, M. (2013). Host plants of leaf worm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in Pakistan. Asian Journal of Agriculture and Biology, 1(1), 23–28.
- Ardika, M. (2023). Potensi produk komersial berbahan aktif beauveria bassiana sebagai agensia pengendalian Oryctes rhinoceros. Non-Published Report.
- Arivoli, S., & Tennyson, S. (2013). Screening of plant extracts for oviposition activity against Spodoptera litura (Fab). (Lepidoptera: Noctuidae). 20 ∼ International Journal of Fauna and Biological Studies, 1(1), 20–24.
- Arunthirumeni, M., Vinitha, G., & Shivakumar, M. S. (2023). Antifeedant and larvicidal activity of bioactive compounds isolated from entomopathogenic fungi Penicillium sp. for the control of agricultural and medically important insect pest (Spodoptera litura and Culex quinquefasciatus). Parasitology International, 92(April 2022), 102688. https://doi.org/10.1016/j.parint.2022.102688
- Azman, I., Mutalib, S. A., Yusoff, S. F. M., Fazry, S., Noordin, A., Kumaran, M., & Mat Lazim, A. (2016). Novel Dioscorea hispida starch-based hydrogels and their beneficial use as disinfectants. Journal of Bioactive and Compatible Polymers, 31(1), 42–59. https://doi.org/10.1177/0883911515597704
- Bunga Yuliana, and Rusli Rustam. 2023. Test of effectiveness Gadung tuber extract (Dioscorea hispida Dennst.) against cacao pod sucker pest (Helopeltis Spp.) in the laboratory. International Journal of Science and Research Archive, 9(1): 571–576. https://doi.org/10.30574/ijsra.2023.9.1.0460
- Chen, C. C., Dong, Y. J., Cheng, L. L., & Hou, R. F. (1996). Deterrent effect of neem seed kernel extract on oviposition of the oriental fruit fly (Diptera: Tephritidae) in guava. Journal of Economic Entomology, 89(2), 462–466. https://doi.org/10.1093/jee/89.2.462
- Chou, T.-C. (2004). Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacological Reviews, 58(3), 621–681.
- Cui, G., Yuan, H., He, W., Deng, Y., Sun, R., & Zhong, G. (2022). Synergistic effects of botanical curcumin-induced programmed cell death on the management of Spodoptera litura Fabricius with avermectin. Ecotoxicology and Environmental Safety, 229, 113097. https://doi.org/10.1016/j.ecoenv.2021.113097
- Darmanto, I. W., Supriyatdi, D., & Sudirman, A. (2019). Pengendalian Ulat grayak (Spodoptera litura F.) dengan ekstrak Ubi Gadung dan Ekstrak Buah Maja. Jurnal Agro Industri Perkebunan, 7(1), 23. https://doi.org/10.25181/jaip.v7i1.1052
- Darmanto, I. W., Supriyatdi, D., & Sudirman, A. (2019). Pengendalian Ulat grayak (Spodoptera litura F.) dengan Ekstrak Ubi Gadung dan Ekstrak Buah Maja (Armyworm [Spodoptera litura F.] Management using Dioscorea Tuber and Aegle Fruit Extract). Jurnal Agro Industri Perkebunan, 7(1), 23–30.
- Datta, R., Kaur, A., Saraf, I., Singh, I. P., & Kaur, S. (2019). Effect of crude extracts and purified compounds of alpinia galanga on nutritional physiology of a polyphagous lepidopteran pest, Spodoptera litura (Fabricius). Ecotoxicology and Environmental Safety, 168(October 2018): 324–329. https://doi.org/10.1016/j.ecoenv.2018.10.065
- Dawkar, V. V., Chikate, Y. R., Lomate, P. R., Dholakia, B. B., Gupta, V. S., & Giri, A. P. (2013). Molecular insights into resistance mechanisms of lepidopteran insect pests against toxicants. Journal of Proteome Research, 12(11), 4727–4737. https://doi.org/10.1021/pr400642p
- Duarte, J. P., Redaelli, L. R., Silva, C. E., & Jahnke, S. M. (2020). Effect of Azadirachta indica (Sapindales: Meliaceae) oil on the immune system of Spodoptera frugiperda (Lepidoptera: Noctuidae) immatures. Journal of Insect Science, 20(3), 1–6. https://doi.org/10.1093/jisesa/ieaa048
- Fateha, R. N., Grasela, M., Ichwan, M. N., Purwanti, E. W., & Kurniasari, I. (2020). Larvicidal and antifeedant activities of clove leaf oil against Spodoptera litura (F.) on soybean. Jurnal Hama Dan Penyakit Tumbuhan Tropika, 21(1), 20–25. https://doi.org/10.23960/j.hptt.12120-25
- Goettel, M. S., Douglas Inglis, G., Duke, G. M., Lord, J. C., & Jaronski, S. T. (2021). Measurement of internal Beauveria bassiana to ascertain non-target impacts on arthropods in field environments. Biocontrol Science and Technology, 31(8), 834–849. https://doi.org/10.1080/09583157.2021.1895072
- Grobler, J. M. (2019). The effect of Ricinus communis on larval behaviour and midgut microbe communities of Spodoptera frugiperda (Lepidoptera: Noctuidae [Doctoral dissertation].
- Hanif, K. I., Herlinda, S., Irsan, C., Pujiastuti, Y., Prabawati, G., Hasbi, Karenina, T. (2020). The impact of bioinsecticide overdoses of Beauveria bassiana on species diversity and abundance of not targeted arthropods in South Sumatra (Indonesia) freshwater swamp paddy. Biodiversitas Journal of Biological Diversity, 21(5), 2124–2136. https://doi.org/10.13057/biodiv/d210541
- Huang, J. H., Jing, X., & Douglas, A. E. (2015). The multi-tasking gut epithelium of insects. Insect Biochemistry and Molecular Biology, 67, 15–20. https://doi.org/10.1016/j.ibmb.2015.05.004
- Idrees, A., Afzal, A., Qadir, Z. A., & Li, J. (2022). Bioassays of Beauveria bassiana isolates against the fall armyworm, Spodoptera frugiperda. Journal of Fungi, 8(7), 717. https://doi.org/10.3390/jof8070717
- Ismawati, R., Nurhidayanti, F., Dhewi, T. A., & Yuningsih, C. (2021). The potential use of anti-nutritional compounds of Gadung tubers as biopesticides. Jurnal Pena Sains, 8(2), 58–63. https://doi.org/10.21107/jps.v8i2.9518
- Kagon, M. (1986). Natural chemicals in plant resistance to insects. Iowa State Journal of Research, 60, 501–527.
- Kartohardjono, A. (2011). Penggunaan musuh alami sebagai komponen pengendalian hama padi berbasis ekologi. Pengembangan Inovasi Pertanian, 4(1), 29–46. (Indonesian language).
- Kaur, S., Preet Kaur, H., Kaur, K., & Kaur, A. (2011). Effect of different concentrations of Beauveria bassiana on development and reproductive potential of Spodoptera litura (Fabricius). Journal of Biopesticides, 4(2), 161–168.
- Marwoto, S. (2008). Strategi dan komponen teknologi pengendalian ulat grayak (Spodoptera litura Fabricius) pada tanaman kedelai. Jurnal Litbang Pertanian, 27(4), 131–136.
- Muhidin, M., Muchtar, R., & Hasnelly, H. (2020). Pengaruh insektisida nabati umbi gadung terhadap wereng batang cokelat (Nillavarpata lugens Stall) pada tanaman padi. Jurnal Ilmiah Respati, 11(1), 62–68. https://doi.org/10.52643/jir.v11i1.856
- Muthusamy, B., Arumugam, E., Dhamodaran, K., Thangarasu, M., Kaliyamoorthy, K., & Kuppusamy, E. (2015). Bioefficacy of Caesalpinia bonducella extracts against tobacco cutworm, Helicoverpa armigera (Hub.)(Lepidoptera: Noctuidae). Journal of Coastal Life Medicine, 3(5), 382–388.
- Sahu, B., Pachori, R., Navya, R. N., & Patidar, S. (2020). Extent of damage by Spodoptera litura on cabbage. Journal of Entomology and Zoology Studies, 8(3), 1153–1156.
- Showler, A. T. (2001). Spodoptera exigua oviposition and larval feeding preferences for pigweed, Amaranthus hybridus, over squaring cotton, Gossypium hirsutum, and a comparison of free amino acids in each host plant. Journal of Chemical Ecology, 27(10), 2013–2028. https://doi.org/10.1023/a:1012238803311
- Sianturi, N. B., Pangestiningsih Y, & Dan Lubis, L. (2014). Uji efektifitas jamur entomopatogen Beauveria bassiana (Bals.) dan Metarrhizium anisopliae (Metch) terhadap Chilo sacchariphagus Boj. (Lepidoptera: Pyralidae) di Laboratorium. Jurnal Agroteknologi, 2(4), 1607–1613.
- Singh, G., Kapoor, I. P. S., Singh, P., de Heluani, C. S., de Lampasona, M. P., & Catalan, C. A. N. (2008). Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association, 46(10), 3295–3302. https://doi.org/10.1016/j.fct.2008.07.017
- Su, Q., Zhou, Z., Zhang, J., Shi, C., Zhang, G., Jin, Z., Wang, W., & Li, C. (2018). Effect of plant secondary metabolites on common cutworm, Spodoptera litura (Lepidoptera: Noctuidae.)Entomological Research, 48(1), 18–26. https://doi.org/10.1111/1748-5967.12238
- Sule, R., & Kumar, D. (2019). Insecticide resistance studies of Cypermenthrin 25EC and Chlorpyriphos 20EC against Spodoptera litura fabricius, 1775 (Lepidoptera: Noctuidae). Indian Journal of Agricultural Research, 53(of), 453–457. https://doi.org/10.18805/IJARe.A-5260
- Tavares, W. d S., de Sousa Freitas, S., Grazziotti, G. H., Parente, L. M. L., Lião, L. M., & Zanuncio, J. C. (2013). Ar-turmerone from Curcuma longa (Zingiberaceae) rhizomes and effects on Sitophilus zeamais (Coleoptera: Curculionidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae). Industrial Crops and Products, 46, 158–164. https://doi.org/10.1016/j.indcrop.2013.01.023
- Vijayalakshmi, P., Vijayalakshmi, T., & Naidu, L. N. (2016). Evaluation of certain insecticide molecules against chillipod borer, Spodoptera litura in Andhra Pradesh. The Journal of Research ANGRAU, 44(1&2), 26–30.

