?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Pepper is an important source of income for Ethiopian smallholder farmers. However, Fusarium wilt has been hindering pepper production and productivity. This study characterized the morphological variability of Fusarium oxysporum f. sp. capsici isolates collected from potential pepper production areas of West Gojjam, Ethiopia. A total of 196 symptomatic plant samples were collected and screened. Based on the macroscopic and microscopic characteristics, 67 F. oxysporum f. sp. capsici isolates were characterized. The distribution of F. oxysporum varied among districts, of which the largest number of the Fusarium isolates was recorded in Womberma (36%) and the lowest was in Burie Zuria (15%). The frequency of F. oxysporum occurrence varied in different plant parts including, roots (56.2%), stem (31.7%), and leaf (12.1%). Colony color varied from white (43.28%) to pink (10.45%). The radial growth of the colony varied from 2.5 – 4.5 cm on the 7th day of culture, of which, 6 isolates had >3 cm, 37 isolates had 3-4 cm, and 24 isolates had >4 cm radial growth. Two types of shapes were recorded with almost similar proportions (50.75% filamentous and 49.25% round). From the total 67 identified isolates, 74.6% had adherent mycelial and 26.4% of them had fluffy growth pattern. Microscopically, all the isolates produced micro- and macro-conidia. The microconidia had oval (67.16%), kidney (29.85%), and globose (2.99%) shaped morphology. The macroconidia were with curved (25) and straight (42) shaped morphology, with three to five septa. The isolates had an entire (73%) and filiform margin (27%). Further, pathogenicity and molecular characterization needs to be conducted.
PUBLIC INTEREST STATEMENT
Hot pepper is one of the most important vegetable cum spice crops valued for its aroma, taste, flavor, pungency and to make food tasty. Especially for most Ethiopians, most foods without the presence of hot pepper as a spice are tasteless. However, the production and productivity of this important crop has become reduced due to different biotic and abiotic factors. Among the biotic factors, fungal disease caused by Fusarium oxysporum f. sp. capsici. contributes for the major yield reduction of hot pepper. Hence, isolation and characterization based on morphological and cultural characters of this disease is a benchmark for enhancing production and productivity of hot pepper through screening resistant pepper variety from pathogenic isolates and breeding of resistant pure lines.
REVIEWING EDITOR:
Introduction
Hot pepper (Capsicum annuum L.) is one of the most important vegetable spices in the world, which is among the oldest domesticated crops in the Western Hemisphere (Kim et al., Citation2014). It belongs to the family Solanaceae and genus Capsicum with a chromosome number of 2n = 24 (Dias et al., Citation2013). Hot pepper is a very important multipurpose crop, which is consumed as a vegetable, spice, or condiment in its fresh, dried, or processed forms (Belay & Tsehaye, Citation2020). It is also serve as an industrial raw material for the manufacturing of oleoresin (Kibiru et al., Citation2021). Besides its agricultural importance, it has immense applications in medicine (Alehegn et al., Citation2022). Therefore, hot pepper production is a vital agribusiness worldwide, where the market stimulates domestic farming and increased employment and source of income generation from agriculture (Dessie et al., Citation2019). Ethiopia is gifted with suitable agro-ecological zones for horticultural crop production including pepper (Belay & Tsehaye, Citation2020). Nationally, among all crops, vegetables cover 1.08% of the farming area, of which 69.31% dedicated to hot pepper production (CSA, Citation2006). In Ethiopia, pepper is mainly produced by smallholder farmers in Amhara, Southern Nations Nationalities and Peoples Regional States (SNNPR), and Oromia regions (Abebe et al., Citation2019).
The total area coverage, production and productivity of hot pepper in the country was estimated to be 174,463.6 ha, 3,131,154 quintals and 17.95 qt/ha respectively, in 2019/2020 main cropping season (CSA, Citation2020). In Amhara region area coverage, production and productivity, was 71,857.92 ha, 1,362,342.45 quintals and 18.96 qt/ha from 962,199 smallholder farmers for the same year. Thus, the contribution of Amhara region for the country pepper production was 43.5% and 68% of its production in the region being supplied to market (Gobie, Citation2019). In addition in West Gojjam Zone, the place where our field experiment was conducted, average estimated hot pepper production was 700,646.71 quintals and yield of 19.99 qt/ha from 35,050.07 ha of land for the year 2019/2020 main cropping season (CSA, Citation2020), Hence, this zone contributed 51.4% for the regional pepper production.
Regardless of its importance, the production practice of the crop has faced different challenges nationally and regionally (Alehegn et al., Citation2022). The current average national level productivity of hot pepper (17.95 qt/ha) showed a significant yield reduction compared to the national average yield of 21.96 qt/ha for the year 2005/2006 main cropping season (CSA, Citation2006). It is even lower to that of the national in Amhara Region pepper growing areas (Abebe & Abera, Citation2019). The major production constraints of the crop are the use of local varieties, unscientific agricultural practices, and increasing prevalence as well as intensity of fungal, bacterial, and viral diseases (Fekadu & Dandena, Citation2006; Sharma et al., Citation2021). Faisal & Muhammad (Citation2011), stated that the productivity of hot pepper is declining slowly each year because of different pests and pathogens which result heavy yield losses. Among biotic factors, diseases are one of the most vital limiting factors in red pepper production and it causes losses of 25-90% (Mengist et al., Citation2019). Wilt disease which causes enormous yield losses is presently a main constraint of hot pepper production in the Amhara Region each year (Abebe & Abera, Citation2019). Fungal disease, Fusarium oxysporum is a serious disease attacking pepper plants and causing yield loss (Assefa et al., Citation2015). Fusarium wilt is the leading fungal disease in Southern Nations, Nationalities, and People’s Regional State (SNNPR) of Ethiopia (Mekonen & Chala, Citation2014). FOC is the most important causative agent of severe vascular wilts and root rot diseases in hot pepper and ultimately brings heavy economic losses (Nelson, Citation1992). According to Teshome (Citation2012), in his study at Bako areas of Ethiopia, Fusarium wilt showed a significant yield loss that ranged between 68 to 71%. FOC is one of the most frequently occurring microorganisms isolated from pepper wilting disease (Abebe & Abera, Citation2019). Alehegn et al. (Citation2022), also confirmed that FOC was the predominant wilt complex disease causal pathogens associated with diseased hot pepper stems and roots in the studied areas. It is a soil-borne pathogen and is cosmopolitan which spreads in all types of soil throughout the world (Burgess & Summerell, Citation1992). However, its effect is more aggravated and attacks hot peppers during the rainy season (Ferniah et al., Citation2014). The most common symptoms of this disease are damping-off (Rivera-Jiménez et al., Citation2018), partial wilting, vascular discoloration, stunting growth, yellowing of older leaves, complete wilting, and death of plants (Velarde-Félix et al., Citation2018). Nowadays, this disease is becoming more destructive and farmers are forced to quit and shift their cultivation to other crops species (Endriyas et al., Citation2020).
Preliminary identification of Fusarium species is mainly based on cultural and morphologically unique characteristics such as shape and size of macro and microconidia, the number of septation in macroconidia, presence or absence of chlamydospores as well as colony appearance, pigmentation, and growth rate on Potato Dextrose Agar (PDA) medium (Campbell & Johnson, Citation2013; Leslie & Summerell, Citation2008).
In Ethiopia, several scholars assessed and identified pepper wilt diseases (Abebe & Abera, Citation2019; Assefa et al., Citation2015; Mekonen & Chala, Citation2014; Mengist et al., Citation2019), characterized Fusarium species (Getnet, Citation2019), isolated and characterized wilt-causing pathogens of local growing pepper (Oljira & Berta, Citation2020), and studied the cultural and morphological variability of FOC isolates (Endriyas et al., Citation2020). The above mentioned scholars except for Endriyas et al. (Citation2020) concentrated on the general identification of the causal agents of pepper wilt in different parts of Ethiopia and confirmed that FOC was the most destructive fungal pathogen of pepper wilt but failed to describe its detailed characteristics. In addition, most assessments and identification of pepper diseases were focusing on some geographical locations including Central Rift valley, Southern Ethiopia, West Shewa Zone, Central Gondar, and Gurage Zone. Gebre kiristos and his colleagues described the morphological variability of FOC in the central rift valley of Ethiopia (Gabrekiristos et al., Citation2020). However, to the best of our knowledge, in the Amhara region, detailed characterization of FOC has not been performed so far. Therefore, the objective of this study was to characterize the morphological variability of FOC isolates collected from the main pepper producing areas of Amhara region, West Gojjam, Ethiopia.
Materials and methods
Description of the study area
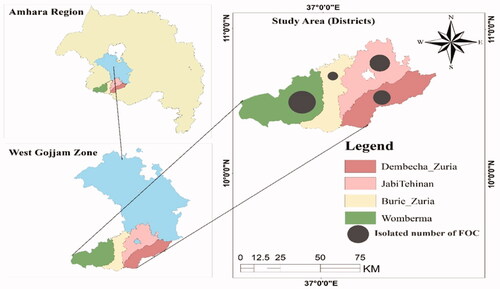
The study was conducted in major pepper-growing area of West Gojjam Zone, Amhara Regional State, Ethiopia. The districts included JabiTehinan, Burie Zuria, Dembecha Zuria, and Womberma (). These areas are known for their high pepper production potential and incidence of Fusarium wilt. The study areas are situated in the Northwest direction of Addis Ababa at 385 km and 171 km South direction from the capital city of Amhara Region, Bahir Dar (Motbainor et al., Citation2016). The sampling areas are located between the geographical coordinates of 10°24’49” to 10°41’53” north latitude and 36°51’20” to 37°20’53” east longitude. Data related to disease incidence and severity were collected from four districts during 2021 main cropping season. The altitudinal range, mean minimum and maximum temperatures, total seasonal annual rainfall, and mean relative humidity of the studied districts are presented in . May–October (six growing months) meteorological data were obtained from the National Meteorology Agency, Bahir Dar Branch, Ethiopia.
Table 1. Altitudinal range, mean minimum and maximum temperatures, total annual rainfall and mean relative humidity of studied areas in West Gojjam Zone, Ethiopia during 2021 main cropping seasons.
Sampling techniques and sample collection
The sampled districts (Womberma, Burie Zuria, Dembecha Zuria, and Jabi Tehnnan) were purposively selected based on the extend of pepper production and expansion potential (). Out of four districts, sixteen major hot pepper producing farmer associations were purposively selected based on hot pepper production, productivity, and incidence of diseases potential to represent the major hot pepper growing areas of the district. Random sampling technique was used to select hot pepper producer farmers. Hot pepper cultivated fields were sampled randomly at intervals of 5-10 km from initial field by following all accessible roads and distances between each farmer’s fields depended on the topography and the relative importance of hot pepper cultivation within each district. A total of 96 farmer’s field were assessed for sample collection and inspected for disease incidence and severity. In each farmer’s field, disease data were recorded using a 1 m × 1 m area quadrant at five points by moving in an X-fashion transect from the initial point. Five observations were made per field, and mean incidence and severity values were used to analyze the data.
Figure 2. Procedures followed for sample collection and isolation of FOC from Pepper in Amhara region.
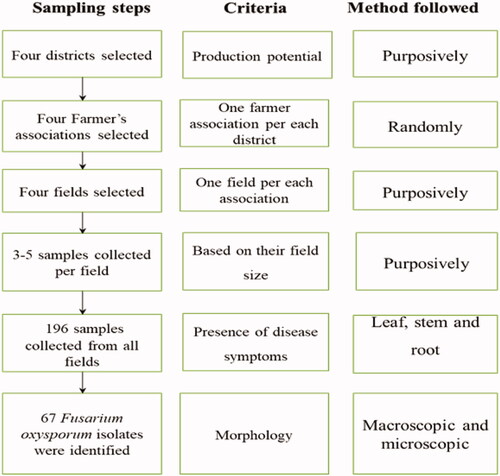
Disease infected plants in each quadrant were collected for diagnostic use. During the survey period in each infected field, plant samples were collected from different plant parts such as root, stem, and leaf which showed suspected typical wilting symptoms of different diseases (Abebe & Abera, Citation2019). The typical wilting symptoms including leaf yellowing, dropping, and partial or complete plant wilting (Akbar et al., Citation2018). Based on field size and disease incidence, 3–5 samples were collected per field (Altinok et al., Citation2019). A total of 196 samples were collected and maintained separately in labeled paper bags. The collected samples were placed in an icebox and used for morphological characterization. The characterization was made at Injibara University, College of Natural and Computational Sciences, Microbiology and Parasitology Laboratory. The samples were preserved at 4 °C until isolation in the laboratory (Akbar et al., Citation2018; Endriyas et al., Citation2020). The sample collection was performed between September and October 2021.
Disease incidence and severity
The incidence and severity of the disease were assessed from quadrants at approximately 20-50 m far from each other (Assefa et al., Citation2015). The incidence of wilt disease was estimated using the formula;
where X, is the number of infected plants and N is the total number of plants observed.
The severity of the disease was determined using a 0 to 4 indexing scale (Paz-Lago et al., Citation2000). The scales are 0 = healthy plants; 1 = initiation of wilt symptoms; 2 = wilting, yellowing, and browning up to 50%; 3 = plants wilted, yellow-brown discoloration more pronounced, and entire plants started dying (75%); 4 = complete drying and dying of the entire plant due to wilt. Therefore, the percentage of disease severity was computed as follows:
where, v = numerical/rate value of infected plants; n = number of infected plants in each scale; N = total number of plants examined and 4 = the highest infection rate.
Isolation of FOC isolates
The collected samples were thoroughly washed with running tap water. The washed parts were cut into small pieces (5 mm long) (Endriyas et al., Citation2020) and surface sterilized by soaking in 70% ethanol and 0.5% sodium hypochlorite solution (NaOCl) for 30 seconds and 1 min, respectively (Oljira & Berta, Citation2020). The surface sterilized samples were rinsed for 1 min. in three successive changes of sterile distilled water and dried on filter paper (Altinok et al., Citation2019; Biri & Gomathinayagam, Citation2021). Then, the samples were placed on PDA plates (9 cm diameter) supplemented with chloramphenicol (0.25 g/l) to restrict bacterial growth (Demissie et al., Citation2020; Kebede et al., Citation2020). All plates were labeled, sealed with parafilm and incubated at 25 °C for 7 days to allow the development of mycelial growth of Fusarium (Getaneh et al., Citation2021; Hafizi et al., Citation2013). When the mycelia emerged, the colonies were sub-cultured onto a fresh sterile PDA slants to obtain a pure culture. Sub-culturing was made till a distinct/pure culture was obtained and a pure culture of each isolate was stored on PDA slants at 4 °C for further work (Hami et al., Citation2021).
Data collection and characterization methods
Data related to the prevalence of disease incidence, and symptoms of wilt disease in the field were collected through field observation and interviews with farmers. Cultural and morphological characters of the fungal pathogen data were observed and recorded under laboratory conditions. Colony color, pigmentation, margin, radial growth, shape, and mycelial growth pattern were recorded from the 7th day after subculture following standard manuals (Campbell & Johnson, Citation2013; Leslie & Summerell, Citation2008; Nelson et al., Citation1983; Shahnazi et al., Citation2012). Microscopic data were recorded using a compound microscope (400x) (Biri & Gomathinayagam, Citation2021) by preparing specimens from the 14 days grown mycelium. The prepared specimens were stained with lactophenol cotton blue. Observations on presence of macro-and micro-conidia, number of septations in macro-conidia, presence and absence of chlamydospore, apical cell morphology, basal cell morphology, macro-conidia morphology, micro-conidia morphology, and sporulation capacity of the isolates were recorded (Mohammed et al., Citation1998). Macroconidia and microconidia were differentiated based on length and presence or absence of septation, while chlamydospores were differentiated based on their absence or presence (Akbar et al., Citation2018; Campbell & Johnson, Citation2013; Leslie & Summerell, Citation2008; Nelson et al., Citation1983).
Data analysis
The percentage data of pepper wilting record in disease incidence (DI) and disease severity index (DSI) will be transformed using Arcsin transformation function in order to ensure homogeneity of the variance and normalize the distribution of data variability. Then the normalized data were analyzed using descriptive statistics such as mean, standard deviation, frequency, and percentages procedures of statistical package for the social sciences (SPSS) version 22. The data on colony characteristics and microscopic studies were analyzed by descriptive statistics, because to analyze using inferential statistics they don’t have dependent and independent variable for qualitative and quantitative data recorded during the laboratory evaluations.
Results
Disease symptoms, incidence, and severity
During the assessment, it was observed that most of the pepper plants got dried up before maturation and fruit harvest. shows the proportion of isolates in the districts. The observed symptoms of infected plants included wilting, vascular discoloration, yellowing and shedding of leaves, and complete death of plants ().
Figure 3. Symptoms of pepper wilt in the assessed field. (a) Wilted pepper plants; (b) Dead plants due to disease; (c) Dropped leaves and flowers; (d) Dark-brown vascular tissue discoloration 30 cm above the base of plants. The photos were taken during the experiment by the first author– Tadesse Tilahun.

Table 2. Number of samples collected and the proportion of isolates in the district and study area.
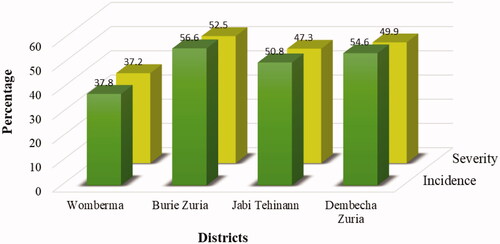
Generally, Burie Zuria district recorded the highest disease incidence (56.6%) and severity (52.5%) whereas Womberma district recorded the lowest disease incidence (37.8%) and severity (37.2%). Disease incidence and severity recorded a direct relationship. Both were significantly correlated at (p < 0.05) level of significance ().
Table 3. Statistical test/analysis of incidence and severity.
Isolation and pure culture preparation
A total of 67 (34.18% of the total samples) isolates had the characteristics described for FOC from the 196 samples collected in the study area (), based on the colony characteristics on PDA and microscopic examination on a compound microscope. Accordingly, a total of 24, 10, 19, and 14 isolates collected from Womberma, Burie Zuria, Jabi Tehinan and Dembecha Zuria districts respectively, were also considered as FOC. The highest disease incidence was recorded in Burie Zuria district (56.6%) and the lowest in Womberma district (37.8%; ).
Morphological characterization of FOC isolates
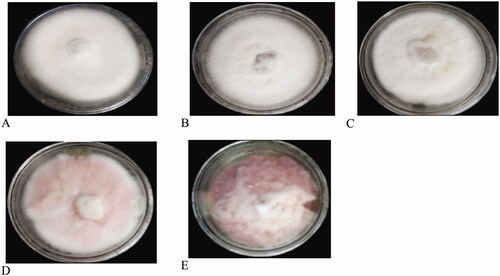
For differentiation of the isolates, the morphological characteristics such as mycelial growth pattern (elevation), colony color, shape, and margin of the colony were evaluated on the 7th day after incubation (DAI) (). According to the fungal radial growth, most of the isolates were fast-growing. The mycelial growth (in cm) of the isolates ranged between 2 to 4.5 after 7 days of incubation. In three districts (Burie Zuria, JabiTehinan, and Dembecha Zuria) most of the isolates (6, 17, and 10), respectively, recorded 3-3.5 cm mycelial growth (diameter in cm). In Womberma district, a larger number of isolates (15) had 3.6-4.5 cm radial growth. From 67 isolates, a few numbers (6) of isolates had lesser radial growth (2-2.9 cm) in all the districts. During incubation, the colony produced different colours on the plates such as light pink, pink, white, creamy white, and cottony white (). Maximum number of isolates (29) produced white color and only one isolate in JabiTehinan district recorded creamy white mycelial color. Light pink colony color was also observed in 28 isolates, which was equivalent to the occurrence of white color. The results of the mycelial growth pattern revealed two different patterns namely adherent smooth/flat and fluffy growths. Fifty isolates produced an adherent growth pattern and 17 had a fluffy pattern. In Womberma district, the majority of the isolates (19) had adherent type of growth pattern and lesser number of isolates (7) produced an adherent growth pattern in Dembecha Zuria district. The maximum number of isolates with fluffy pattern (7) were recorded in Dembecha Zuria district. Regarding the colony shape, 34 isolates had filamentous and 33 with circular shape.
Figure 5. Colony color of some representative pictures of the culture (a) White, (b) Cottony white, (c) Creamy white, (d) Light Pink, (e) Pink.
In Womberma district, maximum number of isolates (17) recorded filamentous colony shape whereas the circular shape colony (15) were recorded in JabiTehinan district. Based on the margin of the colony, 49 isolates had an entire margin whereas 18 isolates had a filiform margin. Both Womberma and JabiTehinan districts had an equal number of isolates (16) that have an entire margin. Buria Zuria had the least number of isolates (6) with entire margin. All the characteristics are mentioned in and pictorial evidence are described in and .
Figure 6. Colony shape (A&B), margin (C&D), and growth (E&F) pattern of FOC cultures. (a) Circular, (b) Filamentous, (c) Entire, (d) Filiform, (e) Fluffy, (f) Adherent smooth.
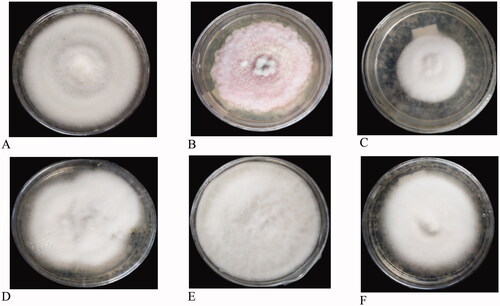
Figure 7. Comparison of each colony characteristics of FOC isolates in West Gojjam Zone of Ethiopia.
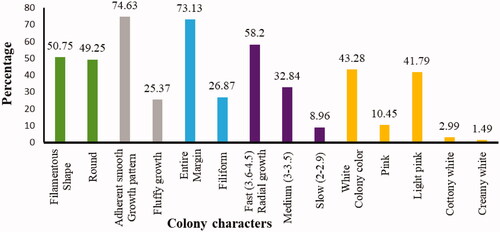
Table 4. The number of FOC isolates with morphological characteristics.
Microscopic features of Fusarium oxysporum isolate
The microscopic examination was focusing on the morphological features like the shape, presence or absence, and size of the conidia, and the presence or absence of the chlamydospore (). It was revealed that all the isolates produced two types of conidia i.e. micro-and macro-conidia. The observed microconidia were oval and kidney like-shaped. In all the isolates, the number of microconidia was more than that of macroconidia. The macroconidia were curved and straight-shaped, with three to five septations. Furthermore, all the isolates produced chlamydospores in single and pair forms at either terminal or intercalary positions ().
Figure 8. Microscopic characteristics of FOC isolates. (a) Oval to kidney-shaped microconidia, (b) Slightly curved, thick-walled, and septated macroconidia, (c) Intercalary chlamydospore, (d) Paired terminal chlamydospore.
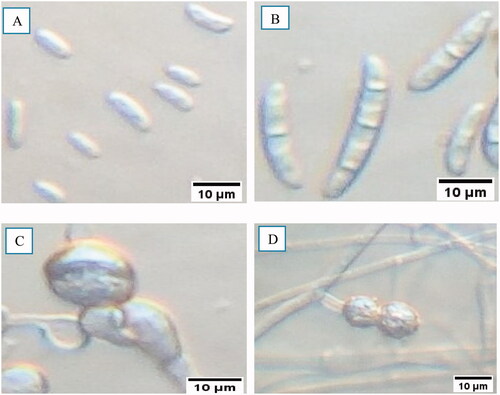
Table 5. Microscopic features of FOC isolates collected from diseased pepper plants.
There were variations in the number of septations in the macroconidia. The number of septa varied from 1 to 5. Maximum number of isolates (65.7%) produced macro conidia with 3 septations. The least frequent septations was 4 which was recorded in 2 isolates (2.99%) of the total isolates (). Based on the ability of isolates to produce conidia within the microscopic field (400X), the isolates were divided into three categories viz. poor sporulation (1-10 spores in the microscopic field), medium sporulation (11-100 conidia in the microscopic field), and good sporulation (>100 conidia in the microscopic field (Rajendran et al., Citation2018). From the total isolates, 54 (80.6%) of the isolates produced good sporulation while 11 isolates (16.4%) produced sporulation at a medium level. Poor sporulation was observed in two isolates (3%) of the total isolates, viz. 4WBMr2 and 2BZZs2.
The occurrence of FOC in different plant parts
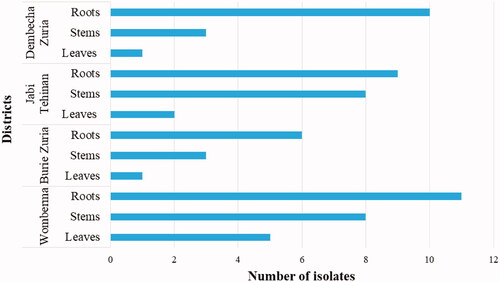
From the collected 196 samples, FOC could be isolated only from 67 samples (). The isolated FOC was found distributed throughout all parts of the pepper plant. However, the FOC disease was more prevalent in the roots (36) followed by stems (22). Less incidence was observed on the leaves (9). In all the districts, the incidence of FOC was observed on roots.
Discussion
In this study, the symptom, incidence, severity, distribution, and morphological variability of FOC were investigated using standard procedures. Accordingly, during the field observation various disease symptoms like wilting, vascular discoloration, and complete plant death were observed. Most of these symptoms were similar to the symptoms of FOC and this result is in line with the findings of Velarde-Félix et al. (Citation2018) who stated that symptomatic plants had wilting, flower abortion, vascular discoloration, and dead plants.
The incidence and severity of disease showed variability within and across the districts. The average disease incidence of 37.8%, 56.6%, 50.8%, 54.6%, and severity of 37.2%, 52.5%, 47.3%, and 49.9%, respectively, was in Womberma, Burie Zuria, Jabi Tehinan and Dembecha Zuria Districts. Assefa et al. (Citation2015), reported that disease incidence and severity varied from season to season and from location to location. This existing variation in disease incidence and severity among districts might be attributed to the difference in the prevalent climatic conditions in the districts. Joshi et al. (Citation2012) and Momol et al. (Citation2001) described that wilt disease is influenced by environmental conditions like soil temperature, soil moisture, and soil type that influenced the growth and development of soil microbial populations. In addition, Pandey et al. (Citation2019) reported that environmental factors such as temperature and relative humidity had a direct positive correlation between the development of Alternaria alternata and chili fruit rot diseases under natural conditions. Hence in this study, Burie zuria district which have low altitude, recorded the highest disease incidence (56.6%) and severity (52.5%). Alehegn et al. (Citation2022) reported that possible risks of wilt pressure would occur more frequently at lower altitude ranges than at higher altitudes. The lowest disease incidence (37.8%) and severity (37.2%) was recorded in Womberma district. Similarly, Rao (Citation2014) reported that symptoms of wilt disease are more visible at higher temperatures than at lower temperatures in a cropping season. Disease incidence and severity had a directly proportional relationship, when disease incidence increased severity also increased.
From the total collected 196 wilted pepper samples, based on their morphological characters, 67 (34.18%) isolates were identified as FOC, the highest isolate was recorded from Womberma (24) district whereas the lowest was from Burie zuria district (10). This variability for the occurrence of FOC isolates may be due to the variations in environmental conditions in the districts.
The identified isolates had different cultural and morphological variability. Hafizi et al. (Citation2013) reported that the colony appearances of FOC on PDA were highly variable. According to the fungal radial growth, isolates were grouped into three growth types such as slow growers (2-2.9 cm), medium growers (3-3.5 cm), and fast growers (3.6-4.5 cm). This result showed the presence of great variability in growth rate among different isolates of Fusarium associated with pepper vascular wilt. This variation might be due to the inherited characteristics of the pathogen. A similar result was also reported by Endriyas et al. (Citation2020) who reported that based on the fungal radial growth, isolates were grouped as sluggish growers (2.4-2.9 cm), intermediate growers (3-3.5 cm), and fast growers (3.6-4.5 cm). In our study, 6 isolates viz. 1WBMr1, 2WBMr3, 2BZZs2, 1BZF1, and 1DBA1 (8.96%) of the total isolates were considered as slow growers. The other 22 (32.83%) isolates were medium growers and the remaining 39 (58.21%) isolates were the fastest growers.
During incubation, colony color on PDA showed predominately light pink to white mycelia. Fusarium isolates could showed sparse to abundant mycelia and red, white, purple, brown, and pink colonies on PDA medium (Leslie & Summerell, Citation2008; Isaac et al., Citation2018). Similarly, Rajendran et al. (Citation2018) have also confirmed the presence of different colony colors like light pink, pink, dark pink, creamy white, pale white with pink, creamy white with pink, milky white, and pinkish white. The occurrence of different colour in Fusarium species may be due to the presence of specific pigments such as carotenoid and polyketide molecules derived pigments which is produced as a byproduct during mycelial growth and enzymatic activities (Demissie et al., Citation2020). The results of the mycelial growth pattern showed two different patterns namely adherent smooth/flat and fluffy growths. Most of the mycelial growth pattern was adherent type 50 (74.6%), whereas 17 (25.4%) of the isolates showed fluffy patterns. Regarding the colony shape, out of 67 isolates, 34 (50.75%) had filamentous and 33 (49.25%) had circular shape. This study is in line with that of Endriyas et al. (Citation2020) who stated that the presence of 21 round and 28 filamentous shape isolates. Based on the margin of the colony 49 isolates (73%) showed entire and 18 (27%) of the isolates revealed filiform margin.
Morphological features like shape, presence or absence, and size of the conidia, and the presence or absence of the chlamydospore were studied. All of the isolates produced two types of conidia such as micro and macroconidia. In most isolates, the number of microconidia was more than that of macroconidia. The macroconidia were curved and straight-shaped, with three to five septations. Similar results were observed by Angel et al. (Citation2016) who reported that the isolates showed hyaline, oval, and kidney-shaped microconidia without septations and three to five septatations macroconidia with curved and straight shapes and gradually pointed edges. In our experiment, we observed 41 straight and 26 curved shaped isolates macroconidia and microconidia have 48 oval and 19 kidney shaped isolates. All the isolates have tapered apical cell morphology with foot-shaped, pointed, and blunt end basal morphology. Furthermore, isolates produced chlamydospores in single and pairs forms at both terminal and intercalary positions. This study was supported by Rajendran et al. (Citation2018) who reported that chlamydospores were usually developed singly or in pairs, but they can also be found in clusters or short chains sometimes.
There were variations in the number of septations for macroconidia. The observed number of septa varied from one to five. Jaywant (Citation2016) reported that FOC isolates have 1-5 septa per conidia. Repeatedly occurred number of septate was three, which accounts for 44 (65.67%) of the total isolates, and the least occurred septate was four in two isolates (2.99%) of the total isolates. Similar reports showed that the isolates of Fusarium oxysporum have most frequently three septa per conidia (Endriyas et al., Citation2020; Jaywant, Citation2016). Based on the ability of isolates to produce spores within the microscopic field (400X), the isolates were divided into three categories such as poor sporulation (1-10 spores in the microscopic field), medium sporulation (11-100 spores in the microscopic field), and good sporulation (>100 spores in the microscopic field (Rajendran et al., Citation2018). From the total isolates, fifty-four (80.6%) of the isolate produced good sporulation while eleven isolates (16.4%) produced sporulation at a medium level. Poor sporulation was observed in the two isolates (3%) of the total isolates, viz. 4WBMr2 and 2BZZs2. Even though the disease was found in every part of the plants, the rates of occurrence of FOC varied in different parts of plants root (36), stem (22), and leaf (9). This may be due to the soil-borne nature of the pathogen that increased its occurrence in the root. This study generated baseline information on the presence, distribution, and variability of FOC isolates in the study areas which can be used to prepare wilt management strategy. Further, this study can used as a benchmark pathogenicity testing, screening of resistant pepper variety and breeding of resistant pure lines.
Conclusion and recommendation
Fusarium oxysporum f. sp. capsici (FOC) is the most prominent pathogen resulting in qualitative and quantitative loss of pepper yield by wilting pepper plants before maturation and fruit harvest. A total of 67 FOC isolates were characterized using morphological approach which revealed the presence of different morphotypes. The macroscopic characteristics observed included colony color, colony shape, radial growth, and growth pattern showed great variability.
There were also microscopic features variability in macro-and micro conidia morphology, basal morphology, level of conidia production, and number of septations in the macroconidia. All the isolates produced two types of conidia such as micro and macroconidia. The distribution of the pathogen was more prevalent in the roots might be due to the soil born nature of the pathogen. The results of this study can be utilized to prepare wilt management strategy and as a benchmark for carrying out pathogenicity tests, screening of resistant pepper variety and breeding of resistant pure lines. However, pathogenicity tests and molecular identification through phylogenetic reconstruction should be conducted.
Authors’ contributions
TT: conceived, designed, performed the experiments, analysed and interpretation of data; drafted the manuscript; SA: take part in the design of the experiment, commented on the drafted manuscript, and revised the manuscript; TT: take part in the lab experiment, data analysis, and interpretation of data; MT: supervised the overall experiment, commented on the drafted manuscript, and approved the version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The research proposal was presented to the Institute of Biotechnology, Bahir Dar University and approved for data collection. An official letter was written by the Bahir Dar University, with a detailed description of the objective of the study.
Consent for publication
All authors agreed for the manuscript to be published.
Acknowledgments
The authors would like to acknowledge Debre Tabor University, Ethiopia, for providing the first author with study leave.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The data that support the findings of this study are available in [Science Data Bank at http://doi.org/10.57760/sciencedb.0306.], reference number [10.57760/sciencedb.03069].
Additional information
Funding
Notes on contributors

Tadesse Tilahun
Tadesse Tilahun, the first author, was born in 1988 in Ethiopia. He received a Bachelor of Sciences (BSc) degree in Applied Biology from Hawassa University, and MSc in Applied Genetics from Haramaya University, Ethiopia. He has worked as a university lecturer for two years at Samara University and for 5 years at Debre Tabor University, Ethiopia. As a lecturer, he has taught several courses including Principles of Genetics, Plant and Animal Breeding, Forest Genetics, Taxonomy, Biochemistry, and Biotechnology for undergraduate programs. He is currently a PhD candidate in Plant Biotechnology at Bahir Dar University, Ethiopia. He has published more than three articles and other group members published more than 50 articles in national and international journals. His research interest includes genetics, molecular breeding, and plant biotechnology.
References
- Abebe, A., & Abera, M. (2019). Pepper disease assessment and identification in major growing districts of West Gojam Zone in Northwestern Ethiopia. International Journal of Sustainable Agricultural Research, 6(1), 1–15. https://doi.org/10.18488/journal.70.2019.61.8.20
- Abebe, B. D., Tigabu, D. K., Abebe, D. K., & Asmamaw, A. A. (2019). Analysis of red pepper marketing: evidence from northwest Ethiopia. Journal of Economic Structures, 8(24), 1–14.
- Akbar, A., Hussain, S., Ullah, K., Fahim, M., & Ali, G. S. (2018). Detection, virulence and genetic diversity of Fusarium species infecting tomato in Northern Pakistan. PloS One., 13(9), e0203613. https://doi.org/10.1371/journal.pone.0203613
- Alehegn, M., Fininsa, C., Terefe, H., Dejene, M., & Mohammed, W. (2022). Agro-ecological factors influencing geographic distribution and epidemic development of hot pepper wilt complex disease in Northwestern Ethiopia. International Journal of Pest Management, 1–15. https://doi.org/10.1080/09670874.2022.2132546
- Altinok, H. H., Yüksel, G., & Altinok, M. A. (2019). Pathogenicity and phylogenetic analysis of Fusarium oxysporum f. sp. capsici isolates from pepper in Turkey. Canadian Journal of Plant Pathology, 42(2), 279–291. https://doi.org/10.1080/07060661.2019.1641749
- Angel, R.-C., Michel, L.-M., Lidcay, H.-I., Aminael, S.-R., & Roldán, T.-G. (2016). Morphological and molecular identification of Fusarium species associated with vascular wilt of babaco (Vasconcellea heilbornii var. pentagona Badillo). Revista de Protección Vegetal, 31(3), 184–193.
- Assefa, M., Dawit, W., Lencho, A., & Hunduma, T. (2015). Assessment of wilt intensity and identification of causal fungal and bacterial pathogens on hot pepper (Capsicum annuum L.) in Bako Tibbe and Nonno districts of West Shewa zone, Ethiopia. International Journal of Phytopathology, 4(1), 21–28. https://doi.org/10.33687/phytopath.004.01.0972
- Belay, F., & Tsehaye, Y. (2020). Variability, association and path coefficient analysis of green pod yield and yield components of hot pepper (Capsicum annuum L.) landraces at Mereb Lehke, Northern Ethiopia. Journal of Plant Breeding and Crop Science, 12(1), 58–69.
- Biri, B., & Gomathinayagam, P. (2021). Response of hot pepper (Capsicum annuum L.) to major fungal diseases under field and greenhouse conditions in Horo Guduru Wollega, Oromia, Ethiopia. African Journal of Agricultural Research, 17(6), 923–932.
- Burgess, L., & Summerell, B. (1992). Mycogeography of Fusarium: survey of Fusarium species in subtropical and semi-arid grassland soils from Queensland, Australia. Mycological Research, 96(9), 780–784. https://doi.org/10.1016/S0953-7562(09)80448-6
- Campbell, C. K., & Johnson, E. M. (2013). Identification of pathogenic fungi. John Wiley & Sons.
- CSA. (2006). Central statistical agency, agricultural sample survey 2005/2006 report on area and production of crops, Addis Ababa, Ethiopia. Statistical Bulletin, 1, 361.
- CSA. (2006). Central statistical agency, agricultural sample survey. In Report on Area and Production of Crops. Statistical Bulletin. Addis Ababa.
- CSA. (2020). Central statistical agency, the federal democratic republic of Ethiopia, central statistical agency agricultural sample survey, report on area and production of major crops. Statistical Bulletin, 1, 1–137.
- Demissie, S., Megersa, G., Meressa, B. H., & Muleta, D. (2020). Resistance levels of Ethiopian hot pepper (Capsicum spp.) varieties to a pathogenic Fusarium spp. and in vitro antagonistic effect of Trichoderma spp. Archives of Phytopathology and Plant Protection, 54(11-12), 647–663. https://doi.org/10.1080/03235408.2020.1853494
- Dessie, A. B., Abate, T. M., Mekie, T. M., & Liyew, Y. M. (2019). Crop diversification analysis on red pepper dominated smallholder farming system: Evidence from Northwest Ethiopia. Ecological Processes, 8(1), 1–11. https://doi.org/10.1186/s13717-019-0203-7
- Dias, G., Gomes, V., Moraes, T., Zottich, U., Rabelo, G., Carvalho, A., Moulin, M., Gonçalves, L., Rodrigues, R., & Da Cunha, M. (2013). Characterization of Capsicum species using anatomical and molecular data. Genetics and Molecular Research: GMR, 12(4), 6488–6501. https://doi.org/10.4238/2013.February.28.29
- Endriyas, G., Daniel, T., & Getachew, A. (2020). Cultural, morphological and pathogenic variability among isolates of Fusarium oxysporum f. sp. capsici causing wilt of Hot Pepper in Central Rift Valley. Ethiopia. Journal of Plant Pathology & Microbiology, 11(6), 1–12.
- Faisal, H., & Muhammad, A. (2011). Pest and diseases of chilli crop in Pakistan: A review. International Journal of Biology and Biotechnology, 8(2), 325–332.
- Fasikaw, B., & Yemane, T. (2020). Variability, association and path coefficient analysis of green pod yield and yield components of hot pepper (Capsicum annuum L.) landraces at Mereb Lehke, Northern Ethiopia. Journal of Plant Breeding and Crop Science, 12(1), 58–69. https://doi.org/10.5897/JPBCS2019.0840
- Fekadu, M., & Dandena, G. (2006). Review of the status of vegetable crops production and marketing in Ethiopia. Uganda Journal of Agricultural Sciences, 12(2), 26–30.
- Ferniah, R. S., Daryono B, S., Kasiamdari R, S., & Priyatmojo, A. (2014). Characterization and pathogenicity of Fusarium oxysporum as the causal agent of Fusarium wilt in chili (Capsicum annuum L.). Microbiology Indonesia, 8(3), 121–126. https://doi.org/10.5454/mi.8.3.5
- Gabrekiristos, E., Teshome, D., & Ayana, G. (2020). Cultural, morphological and pathogenic variability among isolates of Fusarium oxysporum f. sp. capsici causing wilt of hot pepper in Central Rift Valley, Ethiopia. Journal of Plant Pathology and Microbiology, 11, 499.
- Getaneh, G., Tefera, T., Lemessa, F., Ahmed, S., Fite, T., & Jandouwe, V. (2021). Genetic diversity and population structure of Didymella rabiei affecting chickpea in Ethiopia. Journal of Fungi, 7(10), 820. https://doi.org/10.3390/jof7100820
- Getnet, M. (2019). Characterization of Fusarium species from hot pepper (Capsicum anuum L.) and In vitro antagonistic effect of Trichoderma species [Thesis]. Jimma University.
- Gobie, W. (2019). A seminar review on red pepper (Capsicum) production and marketing in Ethiopia. Cogent Food & Agriculture, 5(1), 1647593. https://doi.org/10.1080/23311932.2019.1647593
- Hafizi, R., Salleh, B., & Latiffah, Z. (2013). Morphological and molecular characterization of Fusarium. solani and F. oxysporum associated with crown disease of oil palm. Brazilian Journal of Microbiology: [Publication of the Brazilian Society for Microbiology], 44(3), 959–968. https://doi.org/10.1590/s1517-83822013000300047
- Hami, A., Rasool, R. S., Khan, N. A., Mansoor, S., Mir, M. A., Ahmed, N., & Masoodi, K. Z. (2021). Morpho-molecular identification and first report of Fusarium equiseti in causing chilli wilt from Kashmir (Northern Himalayas). Scientific Reports, 11(1), 1–14.
- Isaac, M. R., Leyva-Mir, S. G., Sahagún-Castellanos, J., Câmara-Correia, K., Tovar-Pedraza, J. M., & Rodríguez-Pérez, J. E. (2018). Occurrence, identification, and pathogenicity of Fusarium spp. associated with tomato wilt in Mexico. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 46(2), 484–493. https://doi.org/10.15835/nbha46211095
- Jaywant, K. S. (2016). Pathogenic variability and management of Fusarium wilt of chilli (Capsicum annuum L.). PhD.
- Joshi, M., Srivastava, R., Sharma, A. K., & Prakash, A. (2012). Screening of resistant varieties and antagonistic Fusarium oxysporum for biocontrol of Fusarium wilt of chilli. Journal of Plant Pathology & Microbiology, 03(05), 1–6. https://doi.org/10.4172/2157-7471.1000134
- Kebede, M., Adugna, G., & Hundie, B. (2020). Identification of Fusarium species responsible to cause wheat head blight in Southwestern Ethiopia. Research Journal of Plant Pathology, 3(1), 3.
- Kibiru, K., Alemayehu, L., & Zewdu, T. (2021). Adaptability and performance evaluation of improved large pod hot pepper (Capsicum annum L.) varieties in West and Kellem Wollega Zones. American Journal of Agricultural Forestry, 9(6), 366–371.
- Kim, S., Park, M., Yeom, S.-I., Kim, Y.-M., Lee, J. M., Lee, H.-A., Seo, E., Choi, J., Cheong, K., Kim, K.-T., Jung, K., Lee, G.-W., Oh, S.-K., Bae, C., Kim, S.-B., Lee, H.-Y., Kim, S.-Y., Kim, M.-S., Kang, B.-C., … Choi, D. (2014). Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nature Genetics, 46(3), 270–278. https://doi.org/10.1038/ng.2877
- Leslie, J. F., & Summerell, B. A. (2008). The Fusarium laboratory manual. John Wiley & Sons.
- Mekonen, S., & Chala, A. (2014). Assessment of hot pepper (Capsicum species) diseases in southern Ethiopia. International Journal of Science and Research, 3(3), 91–95.
- Mengist, Y., Tadesse, D., & Birara, A. (2019). Assessment of prevalence, incidence and severity of red pepper disease in Capsicum frutescens L. at Central Gondar, Ethiopia. Journal of Academia and Industrial Research, 8(3), 45.
- Mohammed, S. A. S., Rana, M. J., & Reem, M.-R Y. (1998). Laboratory manual for mycology.
- Momol, T., Pradhanang, P., & Lopes, C. A. (2001). Bacterial wilt of pepper. University of Florida.
- Motbainor, A., Worku, A., & Kumie, A. (2016). Level and determinants of food insecurity in East and West Gojjam zones of Amhara Region, Ethiopia: a community based comparative cross-sectional study. BMC Public Health., 16(1), 503. https://doi.org/10.1186/s12889-016-3186-7
- Nelson, P. E. (1992). Taxonomy and biology of Fusarium moniliforme. Mycopathologia, 117(1-2), 29–36. https://doi.org/10.1007/BF00497276
- Nelson, P. E., Toussoun, T. A., & Marasas, W. (1983). Fusarium species: an illustrated manual for identification. Pennsylvania State University Press.
- Oljira, T., & Berta, S. (2020). Isolation and characterization of wilt-causing pathogens of local growing pepper (Capsicum annuum L.) in Gurage Zone, Ethiopia. International Journal of Agronomy, 2020, 1–8. https://doi.org/10.1155/2020/6638683
- Pandey, M., Dwivedi, P. K., Mishra, R. P., & Mukesh, S. (2019). Effect of atmospheric temperature, relative humidity and rainfall on disease development of Alternaria alternata causing Alternaria leaf spot and fruit rot of Chili under Natural Conditions. International Journal of Current Microbiology and Applied Sciences, 8(01), 2860–2864. https://doi.org/10.20546/ijcmas.2019.801.300
- Paz-Lago, D., Borges, A. A., Gutiérrez, A., Borges, A., Cabrera, G., Ramírez, M. A., & Falcón, A. (2000). Tomato-Fusarium oxysporum interactions: II. Chitosan and MSB induced resistance against fol in young tomato plants. Ministerio de Educación Superior (Cuba), 21(4), 17–20.
- Rajendran, M., Sankarasubramanian, H., Gandhi, K., & Thiruvengadam, R. (2018). Comparative proteomic analysis of different isolates of Fusarium oxysporum f.sp. lycopersici to exploit the differentially expressed proteins responsible for virulence on tomato plants. Frontiers in Microbiology, 9(420), 1–13.
- Rao, J. K. (2014). Studies on survey of Fusarium wilt of pea in Eastern Uttar Pradesh. International Journal of Life Sciences, 2(4), 359–362.
- Rivera-Jiménez, M. N., Zavaleta-Mancera, H. A., Rebollar-Alviter, A., Aguilar-Rincón, V. H., García-de-los-Santos, G., Vaquera-Huerta, H., & Silva-Rojas, H. V. (2018). Phylogenetics and histology provide insight into damping-off infections of ‘Poblano’pepper seedlings caused by Fusarium wilt in greenhouses. Mycological Progress, 17(11), 1237–1249. https://doi.org/10.1007/s11557-018-1441-2
- Shahnazi, S., Meon, S., Vadamalai, G., Ahmad, K., & Nejat, N. (2012). Morphological and molecular characterization of Fusarium spp. associated with yellowing disease of black pepper (Piper nigrum L.) in Malaysia. Journal of General Plant Pathology, 78(3), 160–169. https://doi.org/10.1007/s10327-012-0379-5
- Sharma, S., Katoch, V., & Banyal, D. K. (2021). Review on harnessing biotechnological tools for the development of stable bacterial wilt-resistant solanaceous vegetable crops. Scientia Horticulturae, 285(2021), 110158. https://doi.org/10.1016/j.scienta.2021.110158
- Teshome, B. (2012). IIntegrated approach and plant extract management options against pepper wilt (Fusarium oxysporum f. sp. capsici) at Bako. MSc Haramya University.
- Velarde-Félix, S., Garzón-Tiznado, J. A., Hernández-Verdugo, S., López-Orona, C. A., & Retes-Manjarrez, J. E. (2018). Occurrence of Fusarium oxysporum causing wilt on pepper in Mexico. Canadian Journal of Plant Pathology, 40(2), 238–247. https://doi.org/10.1080/07060661.2017.1420693