?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Dermestes maculatus accounts for about 71.5% of dried fish infestation in most of the producing areas with a substantial loss in both dry weight and nutritional value. The study aimed to evaluate the repellent effect of oils extracted from Garlic (Allium sativum), Coconut (Cocos nucifera), Ginger (Zingiber officinale), Jatropha (Jatropha curcas), and Neema (Azadirachta indica) on Dermestes maculatus larvae in smoke-dried Protopterus annectens. The extracted oils from each plant were applied to the fish at varying concentrations of 0.001, 0.004, 0.016, 0.064, and 0.256 ml/g fish in triplicates. Late instar larvae of D. maculatus were introduced into Kilner jars containing fish treated with these oils and repellency was monitored for 24 hrs. Repellency was highest (87%) in A. sativum oil and lowest (59%) in A. indica oil at the highest concentration (0.256 ml/g of fish) within 24 hrs. of exposure. Furthermore, an increasing trend in repellency was observed with increasing concentrations of oils from all the plants used. The order of repellency performance was A. sativum > C. nucifera > Z. officinale > J. curcas > A. indica, starting with the highest to the lowest. Therefore, this study demonstrates the repellent properties of the oils in offering effective protection against infestation and damage by D. maculatus, suggesting that the oils can be utilized in post-harvest preservation of smoke-dried fish.
IMPACT STATEMENT
Smoked-dried fish, a vital source of animal protein and essential nutrients, significantly contributes to food security, employment, income, and foreign exchange, supporting sustainable development. However, a major challenge is the presence of Dermestes maculatus, a pervasive pest causing substantial losses in stored dry fish. This study focuses on the repellent effect of essential oils from selected plants known for their active ingredients, to combat D. maculatus infestation in smoked-dried Proptoterus annectens. The use of plant extracts as compared to chemicals presents a more sustainable and safe approach to preserving the nutritional value of fish products. Our results suggest the use of essential oils as a recommended strategy for preventing and controlling insect infestation in smoked-dried fish.
Reviewing Editor:
1. Introduction
Fish is an important food item and a key contributor to food and nutrition security globally (FAO, Citation2022; Maulu et al., Citation2021). Global demand for fish products has continued to rise due to the growing demand caused by a rapidly growing population (FAO, Citation2022). Hence, fish and fish products represent one of the most traded food items globally (Maulu et al., Citation2020). However, fish losses, estimated at 10% of the total production, remain a major concern in the fisheries and aquaculture value chain (FAO, Citation2022; Ward & Signa, Citation2017). The losses occur in quantity and quality, resulting mainly from inefficiencies in the value chain, the high perishability nature of fish, the lack of appropriate infrastructure, and the lack of skills and knowledge in postharvest fish management among the actors (FAO, Citation2022; Maulu et al., Citation2020). Smoke drying is one of the most common fish preservation methods, particularly in developing countries because it is relatively cheaper and takes a relatively short time (Adeyeye, Citation2018). However, smoke-dried fish are very vulnerable to infestation by pests which deteriorates the quality and therefore, poses risks to human health and safety besides causing economic losses (Adeyeye et al., Citation2015; Fafioye et al., Citation2002).
Common pests found on dried fish include beetles, flies, and mites (FAO., Citation1989). In Nigeria, fly genera such as Calliphora, Chrysomia, Lucillia, and Musca and the beetles, Dermestes maculatus Degeer and Necrobia rufipes Degeer have been reported as pests of dried fish (Osuji, Citation1985). Dermestes maculatus is commonly acknowledged as a global nuisance in stored goods, particularly in products that contain animal protein. D. maculatus feeds on hides, skins, feathers, and horns, and it is a well-known pest of dried fish. In fact, losses in quality and quantity of dried fish during storage have largely been attributed to D. maculatus infestation (Fasakin & Aberejo, Citation2002). Approximately 71.5% of losses are attributed to D. maculatus infestations in dried fish within most production areas, resulting in a significant decline in dry weight ranging from 43% to 62.7%, as indicated by Osuji (Citation1974). Lale and Sastawa (Citation1996) and Odeyemi et al. (Citation2000) estimate that susceptibility to pest infestation and the deterioration of smoked fish products lead to losses of around 50% during storage. This results in a decrease in the nutritional quality and market value of smoked fish. Various studies assessing the quantitative losses caused by Dermestes sp. in dried fish suggest a range of estimates, from negligible to a 50% reduction in weight, depending on factors such as storage duration, salt content, moisture levels, climatic conditions, and overall hygiene during processing and storage, as reported by Fasunwon et al., (Citation2011).
Synthetic pesticides used to preserve dried and smoked fish pose significant risks to the environment and human health. It is imperative to explore alternative methods to mitigate the negative effects of these chemicals. Khan and Khan (Citation2001) found that fish curers often use prohibited insecticides like dichlorvos, malathion, gamaxine, endrine, and DDT, contravening recommendations from Codex Alimentarius and FAO/WHO Joint Meeting Pesticide Residue Committee (JMPRC). A promising solution involves the use of plant-derived pest control agents, as suggested by Akinwumi (Citation2011), who reported 100% toxicity of various plant powders on both adult and larvae stages of D. maculatus, providing a potential avenue for pest control in fish preservation. However, several natural plant products, including oils derived from plant materials, have not been tested as protectants of dried fish from insect infestation. Furthermore, existing studies have focused on a few fish species. Therefore, this study aimed to investigate the repellent effects of the oils of Garlic (Allium sativum), Neem (Azadirachta indica), Coconut (Cocos nucifera), Jatropha (Jatropha curcas) and Giner (Zingiber officinale) and on the larvae of D. maculatus.
2. Materials AND METHODS
2.1. Source and processing of plant materials
Azadirachta indica and Jatropha curcas seeds were obtained from the National Research Institute of Chemical Technology, Zaria, Kaduna State while rhizomes of Zingiber officinale, bulbs of Allium sativum and fruits of Cocos nucifera were purchased from Sabon-gari market, Zaria, Kaduna State. The plant materials were sun-dried for two weeks, pulverized using mortar and pestle, and then stored in pre-labeled new plastic bags. Oils were extracted from the resulting powders using the Soxhlet procedure with n-Hexane.
2.1.1. Extraction of oils
The percentage yields of the oils were 22.6%, 60%, 20%, 40%, and 49% for A. sativum, C. nucifera, Z. officinale, J. curcas, and A. indica, respectively.
2.1.1.1. Allium sativum
Ethanol served as the solvent in a four-hour Soxhlet extraction at 70 °C. After evaporation, refluxing was conducted for three hours over a water bath to recover the oil and eliminate residual solvent. The resulting garlic oil was stored in a refrigerator at 4 °C until use.
2.1.1.2. Zingiber officinale
Ginger was washed, sun-dried, ground into a powder, and stored in a sealed container. Then 10 g of the powdered sample was placed in a Soxhlet extractor with 250 ml of methanol for a three-hour oil extraction. Following extraction, the methanol was removed using a rotary evaporator, leaving only the essential oil.
2.1.1.3. Jatropha curcas
10 g of the seeds were washed, dried, and subjected to a six-hour extraction using hexane as the solvent. The solvent was then removed through vacuum evaporation and heat exposure in an oven set at 50 °C. The resulting oil was stored in a bottle until required for use.
2.1.1.4. Cocos nucifera
Cocos nucifera fruit was cut, dried for 14 days, and ground with an electric blender (Crown Star, MC-211). Then 100 g of the dried material was extracted with 250 ml of 70% ethanol in a Soxhlet apparatus for approximately 8 hrs at 70 °C. The solvent was then distilled out using the air-oven method to eliminate any residual solvent.
2.1.1.5. Azadirachta indica
Neem seed oil was extracted with n-hexane for 3 hrs. Samples were periodically collected, and centrifuged to separate the solid fraction, and the filtrate was heated and evaporated at 50 °C to obtain solvent-free oil. The oil was stored in a sealed bottle until use.
2.1.1.6. Determination of percentage yield
The percent oil yield of each plant used in the present study was calculated according to the formula:
Weight of sample
2.2. Gas chromatography/mass Spectrometry Analysis
The oils of all plant samples were analyzed on a Hewlett-Packard (Model: 6890 N Gas Chromatography/Mass Spectrometry) system (Agilent Technologies) (Plate XI) coupled to a mass spectrometer (Model: HP5973). Separations were carried out using a DB-5 capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness). The injector and detector temperatures were maintained at 220 °C and 280 °C respectively. The column temperature was initially kept at 60 °C for 2 minutes and then gradually increased to 240 °C at the rate of 50 °C/min. Helium was used as carrier gas at a constant flow of 1.0 mL/min and an injection volume of 1 µL was employed. The Mass Spectrometry scan parameters included an electron impact ionization voltage of 70 eV, a mass range of 40-750 m/z, and a scanning interval of 0.5 s. Samples diluted in n-hexane were injected manually in the splitless mode. The identification of the components was based on a comparison of their mass spectra with those of the National Institute of Standards and Technology (NIST) libraries provided with the computer-controlling GC-MS system. The fatty acids profiles and metabolites of the oils used in this study are shown in and , respectively.
Table 1. Fatty acid composition (%) of the plant oils of Allium sativum, Cocos nucifera, Jatropha curcas, Zingiber officinale and Azadirachta indica.
Table 2. Metabolites (%) detected in the plant oils of Allium sativum, Cocos nucifera, Jatropha curcas, Zingiber officinale and Azadirachta indica.
2.3. Culture of dermestes maculatus larvae
The source of D. maculatus was naturally infested smoked catfish. It was cultured in a Kilner jar wrapped with muslin cloth and kept at tropical storage conditions, 30 ± 2 °C temperature, and 65 ± 5% relative humidity. The muslin cloth allowed for ventilation and prevented the entry or exit of beetles and other insects. Pieces of water-soaked cotton wool were supplied in the jar to induce oviposition. After 2 weeks, the parent adults were removed from the fish, and adult insects from the stock culture were placed on newly disinfested fish to create a new generation. After emergence, larvae from mass rearing were used for the repellency test.
2.4. Collection of fish
Smoke-dried Protopterus annectens fish were purchased from Sabon-gari market, Zaria, and were identified using taxonomic keys.
2.5. Bioassay
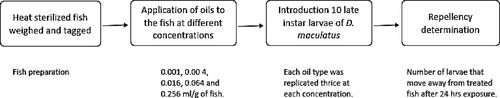
A rectangular container (18 cm × 12 cm × 10 cm) was used for the repellent test (). The heat-sterilized fish was weighed and tagged. The oil was applied to the fish at concentrations of 0.001 ml/g, 0.00 4 ml/g, 0.016 ml/g, 0.064 ml/g and 0.256 ml/g of fish. The treated fish was placed at the one end of an experimental rectangular glass container while the control (untreated fish) was placed at the other end. 10 late instar larvae of D. maculatus were introduced at the side of the jars outside the 1 cm diameter of the fish. The experiment was replicated thrice. The repellency of the larvae to the oil was determined by counting the number of larvae that moved away from the treated fish after a 24-hour exposure period (Egwunyenga et al., Citation1998).
2.6. Statistical Analysis
All repellency data were subjected to one-way analysis of variance (ANOVA) to determine any significant difference among treatments. The statistical software for social sciences (SPSS) was used for statistical analysis of the data. Duncan Multiple Range Test (DMRT) was employed to identify significant differences between the means. Data were presented as mean ± standard deviation, and the differences were regarded as significant at P ≤ 0.05.
3. Result
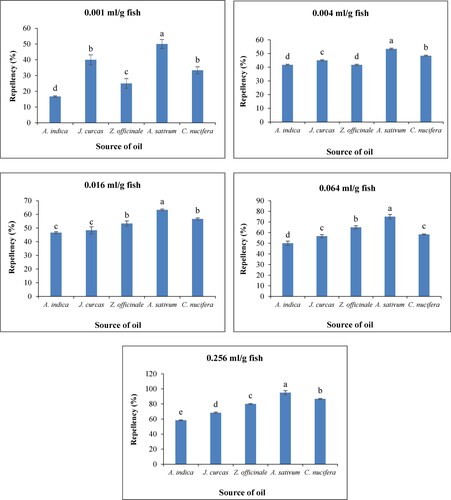
The results of the repellency performance of the different oils at different concentrations against D. maculatus in smoke-dried West African Lungfish (Protopterus Annectens) are shown in . As indicated, there were significant differences (P < 0.05) in repellency effect among the oils at each concentration during 24 hrs. of exposure to the insects. The highest (95%) degree of repellency of D. maculatus larvae was obtained with A. sativum oil at all concentrations, which repelled the larvae significantly compared to the other oils even at low concentrations, while A. indica oil showed the lowest repellency (58.33%) at the highest concentration. Z. officinale (80%) and C. nucifera (86.67%) showed a greater repellency than J. curcas oil (68.33%) against larvae of D. maculatus. The repellent activity of all five plant oils increased with an increase in concentration. Overall, the degree of repellency by the different oils used in this study was in the order, starting with the highest: A. sativum > C. nucifera > Z. officinale > J. curcas > A. indica. Interestingly to note is that there was a linear increase in repellency with increasing concentration of all oils and the trends were generally the same for each oil.
Figure 2. Repulsion of D. maculatus larvae by the Oils of Azadirachta indica, Jatropha curcas, Zingiber officinale, Allium sativum, Cocos nucifera at different concentrations: 0.001, 0.004, 0.016, 0.064, and 0.256 ml/g of fish (n = 20).Note. Data are expressed as mean ± standard deviation. Different alphabetical letters in the same figure indicates significant differences (P ≤ 0.05).
4. Discussion
In the present study much higher oil yield was obtained for A. sativum compared to that reported by Nilesh et al. (Citation2021) who recorded 16.55% from garlic powder by soxhlet extraction method using ethanol as a solvent. Furthermore, the percentage oil yield from Z. officinale obtained in this study was higher than the 15.2% reported by Mohammed et al. (Citation2022). However, the percentage of oil yield from C. nucifera obtained in the present study was lower than 83.23% reported by Oseni et al. (Citation2017) when n-hexane was used as a solvent for oil extraction. For J. curcas, our percent oil yield was within the ranges of 41.2–56.7% w/w reported by Zimilar et al. (Citation2021). The reasons for the variations in percent oil yield percentages may be attributed largely to the extraction methods used. For example, Hussein et al. (Citation2021) reported 42, 49, and 62% of extracted neem oil yield for cold water, hot water, and n-hexane extraction methods, respectively. Therefore, the cold-water extraction method is likely to yield lower oil percentages compared to the n-hexane extraction method used in the present study.
The use of plant extracts in controlling pests in food products could be a more sustainable approach to reducing food losses and wastage. This is because plant extracts are generally safe and environmentally sustainable compared to the use of chemicals that could promote antimicrobial resistance (Chen et al., Citation2023). The oils in this study showed different repellent activities at all the different concentrations used, this may be because of the differences in the active compounds present in the oils. Plant bioactive compounds such as phenolic acids and flavonoids naturally present in plant extracts have shown positive effects in controlling pests in food products (Chen et al., Citation2023). The repellent effect of the oils on the hide beetles could be attributed to the presence of metabolites in the plant oils. Most of the metabolites found in plant oils are known to demonstrate certain interference in the central nervous system through cutaneous or respiratory absorption, leading to death (Moyes et al., Citation2017). A study in dry African catfish (Clarias gariepinus) to control D. maculatus using four different plant extracts from Albizia lebbeck, Anacardium occidentale, Citrullus colocynthis, Citrullus vulgaris, and Khaya senegalensis also showed different repellency even at the same concentration (Akpotu & Adebote, Citation2013). Similarly, Ngamo et al. (Citation2007) reported different repellency of essential oils obtained from different plants in controlling pests in food. The better repellency observed for A. sativum, C. nucifera, and Z. officinale on the larvae of D. maculatus in the present study could be attributed to their compounds and strong smell. For example, A. sativum is reported to contain diallyl sulfphide, diallyl di-sulphide, and diallyl trisulphide compounds that have shown antagonistic effects against numerous pests (Lai et al., Citation2013; Hamada et al., Citation2018). Besides, compounds, such as flavonoid and allicin are known to have a pungent smell that repels insects (Kodera et al., Citation2002). The ability of C. nucifera to repel D. maculatus larvae could be due to the presence of fatty acids in the oils such as lauric acid that are reported to have antimicrobial properties (Gasco et al., Citation2018). Furthermore, a study by Barrozo et al. (Citation2021) demonstrated the strong repellent and acaricidal activity from 205 C. nucifera free fatty acids and catnip oil and their potential for controlling immature phases of Amblyomma sculptum. While the effects of C. nucifera against pests that damage fish products have not previously been reported, its effects on several other insects such as biting flies, bed bugs, ticks, and mosquitoes have been reported (Zhu et al., Citation2018). In Z. officinale oil, compounds such as sesquiterpene hydrocarbons, monoterpene hydrocarbons a-curcumene, and phenolic with properties that repel pests have been reported (Hasan et al., Citation2012). A study by Ayeloja and George (Citation2016) also found that Z. officinale had the best repellency against D. maculatus in dried C. gariepinus. Amusan and Okorie (Citation2002) attributed the repellent properties of Piper guineense and Dennetia tripetala to their pungent smell and reported that the smell and pepperish taste could asphyxiate insects by blocking their spiracles. J. curcas seeds extracts contain active compounds such as lectins, phytates, saponins, phorbol esters, and trypsin inhibitors that have been used to control pests of many stored products (Sharma & Trivedi, Citation2002; Silva et al., Citation2012). These compounds may be attributed to the repulsion of D. maculatus in the current study. The repulsion of D. maculatus larvae by A. indica oil could be attributed to the chemosensory impact of neem oil on the olfactory or gustatory receptors and the active compounds of neem, particularly allelochemical azadirachtin well-known for anti-insect properties (Mordue (Luntz) & Blackwell, Citation1993).
The reasons for the differences in efficacy of different oils at the same concentration observed in this study may be due to the differences in the composition and variety of bioactive compounds present in the different plants. This is also supported by the comprehensive review of the mechanism of plant extracts in controlling pests in food products by Chen et al. (Citation2023). This could especially be true given that the repellence of D. maculatus increased with the increase in concentration of all the plant oils. Further studies may be necessary to establish the optimum concentration of the oils from each plant extract since a linear increase in repellency was observed in the present study. The rating of the plant oils as having good repulsive properties in this study is partly in agreement with Egwunyenga et al. (Citation1998) who also attributed the repellency of D. maculatus to Dennetia tripetala from smoked fish to olfactory and gustatory sensations. Some plant oils have been observed to interfere with basic metabolites and the biochemical, physiological, and behavioral functions of insects. Insects inhale, ingest, or absorb essential oils; the rapid action of these oils against some pests is indicative of neurotoxicity as there is evidence of interference with the neuromodulator octopaminen (Khater, Citation2012). Some essential oils are larvicidal and can delay development and suppress the emergence of adult insects (Khater & Shalaby, Citation2008).
5. Conclusion
This study has demonstrated the efficacy of using plant oils to repel the larvae of Dermestes maculatus in dried West African Lungfish (Protopterus Annectens). Although all oils showed increased repellency levels with increasing concentrations, A. sativum, and C. nucifera oils showed the highest efficacy at all concentrations. Therefore, plant oils can be used for the protection of smoke-dried fish against insect pest infestation, as good alternatives to synthetic insecticides. Further studies may be required to determine the optimum concentration levels for each plant oil used in this study. Furthermore, as these oils show the potential to preserve fish products thereby reducing losses, there is a need to investigate how long the products would remain protected from pests.
Acknowledgment
The authors acknowledge the support of Mr. Dipo Aribido from the National Research Institute of Chemical Technology (NARICT), Zaria, Kaduna State, Nigeria for the support rendered during the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
We confirm that all the data generated during this study have been included within this document.
Additional information
Notes on contributors

Folasade Damilola Amulejoye
Dr. Folasade Amulejoye, currently serving as a lecturer at the Olusegun Agagu University of Science and Technology in Okitipupa, Ondo State, Nigeria, is dedicated to advancing knowledge in the field of aquatic sciences. Her research focuses on fish nutrition, breeding, and fisheries management.
Sahya Maulu
Sahya Maulu is a PhD Researcher in Fish nutrition and health at the University of Plymouth in the United Kingdom. His main areas of expertise include novel feed ingredients in aquaculture, fish nutrition, and fish health. However, his research interests cut across the fields of Aquaculture, Fisheries, Agriculture, and climate change.
References
- Adeyeye, S. A. O., Oyewole, O. B., Obadina, A. O., Omemu, A. M., Adeniran, O. E., Oyedele, H. A., & Abayomi, S. O. (2015). Quality and safety assessment of traditional smoked fish from Lagos State, Nigeria. International Journal of Aquaculture, 5(15), 1–8. https://doi.org/10.5376/ija.2015.05.0015
- Adeyeye, S. O., Fayemi, E. O., & Adebayo-Oyetoro, O. A. (2018). Amino acid, vitamin and mineral profiles of smoked fish as affected by smoking methods and fish type. Journal of Culinary Science & Technology, 1542–8052. https://doi.org/10.1080/15428052.2017.1418693
- Akinwumi, F. O. (2011). Evaluation of some plant materials for control of smoked fish pest, Dermestes maculatus Degeer (Coleoptera: Dermestidae) in Clarias gariepinus (Pisces: Clariidae). Asian Research Publishing Network Journal of Agricultural and Biological Science, 6(7), 65–69.
- Akpotu, J. O., & Adebote, D. A. (2013). Repellency Effect of Five plant extracts Against the larvae of Dermestes maculatus on smoke Dried Clarias gariepinus. Research Journal of Chemical and Environmental Science, 1(4), 1–4.
- Amusan, A. A. S., & Okorie, T. G. (2002). The use of Piper guineense fruit oil (PFO) as proctectant of dried fish against Dermestes maculatus (DeGeer) infestation. Global Journal of Pure Applied Science, 8, 197–201.
- Ayeloja, A. A., & George, F. O. A. (2016). Insecticidal Effects of Natural Preservatives on Insect Pests of Smoked African Mud Catfish, Clarias gariepinus (Burchell, 1822). Journal of Food Processing & Technology, 12, 1–5.
- Barrozo, M. M., Zeringóta, V., Borges, L. M. F., Moraes, N., Benz, K., Farr, A., & Zhu, J. J. (2021). Repellent and acaricidal activity of coconut oil fatty acids and their derivative 2 compounds and catnip oil against Amblyomma sculptum. Veterinary Parasitology, 300, 109591. https://doi.org/10.1016/j.vetpar.2021.109591
- Chen, Y., Xing, M., Chen, T., Tian, S., & Li, B. (2023). Effects and mechanisms of plant bioactive compounds in preventing fungal spoilage and mycotoxin contamination in postharvest fruits: A review. Food Chemistry, 415, 135787. https://doi.org/10.1016/j.foodchem.2023.135787
- Egwunyenga, O. A., Alo, E. B., & Nmorsi, O. P. G. (1998). Laboratory evaluation of the repellency of Dennettia tripetala Baker (Anonaceae) to Dermestes maculatus (Coleoptera: Dermestidae). Journal of Stored Products Research, 34, 195–199.
- Fafioye, O. O., Efuntoye, M. O., & Osho, A. (2002). Studies on the infestation of five traditionally smoked-dried fresh-water fish in Ago-Iwoye, Nigeria. Mycopathologia, 154(4), 177–179. https://doi.org/10.1023/a:1016331418893
- FAO. (1989). A field guide to the types of insects and mites infesting cured fish. Corporate Document Repository, Food and Agricultural Organization, Geneva, Technical paper 303.
- FAO. (2022). The State of World Fisheries and Aquaculture 2020. Sustainability in Action. FAO.,
- Fasakin, E. A., & Aberejo, B. A. (2002). Effect of smoked pulverized plant material on the developmental stages of fish beetle, Dermestes maculatus Degeer in smoked catfish (Clarias gariepinus) during storage. Bioresource Technology, 85(2), 173–177. https://doi.org/10.1016/s0960-8524(02)00058-5
- Fasunwon, B. T., Banjo, A. D., & Jemine, T. A, Tai Solarin University of Education. (2011). Effects of Dermestes maculatus on the nutritional qualities of two edible insects (Oryctes boas and Rhynchophorus phoenicis). African Journal of Food, Agriculture, Nutrition and Development, 11(48), 5600–5613. https://doi.org/10.18697/ajfand.48.9445
- Gasco, L., Gai, F., Maricchiolo, G., Genovese, L., Ragonese, S., Bottari, T., & Caruso, G. (2018). Fishmeal Alternative Protein Sources for Aquaculture Feeds. In Feeds for the Aquaculture Sector. Springer Briefs in Molecular Science. Springer. https://doi.org/10.1007/978-3-319-77941-6_1
- Hamada, H. M., Awad, M., El-Hefny, M., & Moustafa, M. A. (2018). Insecticidal Activity of Garlic (Allium sativum) and Ginger (Zingiber officinale) Oils on the Cotton Leafworm, Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). African Entomology, 26(1), 84–94. https://doi.org/10.4001/003.026.0084
- Hasan, H. A., Rasheed, R. A. M., Abd Razik, B. M., & Rasool, H. B. A. (2012). Chemical composition and antimicrobial activity of the crude extracts isolated from Zingiber officinale by different solvents. Pharmaceutica Analytica Acta, 3, 1000184.
- Hussein, J. B., Ilesanmi, J. O. Y., Yahuza, H. A., & Nkama, I. (2021). Effect of Extraction Methods and Storage Time on the Yield and Qualities of Neem Seed (Azadirachta indica A. Juss) Oil. Nigerian Journal of Technological Development, 18(1), 55–62. https://doi.org/10.4314/njtd.v18i1.8
- Khan, M. A. J., & Khan, R. J. (2001). Insect infestation and preventive measures in dry fish storage of Chittagong, Bangladesh. Institute of Marine Sciences, University of Chittagong, Bangladesh. Online journals of Biological Sciences, 1(10), 963–965.
- Khater, H. F., & Shalaby, A. A. (2008). Potential of biologically active plant oils for the control of mosquito larvae Culex pipiens (Diptera: Culicidae) from an Eyptian locality. Pharmacologia, 50(2), 107–112.
- Khater, H. F. (2012). Prospects of Botanical Biopesticides in Insect Pest Management. Pharmacologia, 31(12), 641–656.
- Kodera, Y., Ichikawa, M., Yoshida, J., Kashimoto, N., Uda, N., Sumioka, I., Ide, N., & Ono, K. (2002). Pharmacokinetic study of allixin, a phytoalexin produced by garlic. Chemical & Pharmaceutical Bulletin, 50(3), 354–363. https://doi.org/10.1248/cpb.50.354
- Lai, K. C., Hsu, S. C., Kuo, C. L., Yang, J. S., Ma, C. Y., Lu, H. F., Tang, N. Y., Hsia, T. C., Ho, H. C., & Chung, J. G. (2013). Diallyl sulfide, diallyl disulfide, and diallyl trisulfide inhibit migration and invasion in human colon cancer colo 205 cells through the inhibition of matrix metalloproteinase-2, -7, and -9 expressions. Environmental Toxicology, 28(9), 479–488. https://doi.org/10.1002/tox.20737
- Lale, N. E. S., & Sastawa, B. M. (1996). The effects of sun drying on the infestation of the African catfish (Clarias gariepinus) by post-harvest insects in the Lake Chad District of Nigeria. International Journal of Pest Management, 42(4), 281–283. https://doi.org/10.1080/09670879609372007
- Maulu, S., Nawanzi, K., Abdel-Tawwab, M., & Khalil, H. S. (2021). Fish nutritional value as an approach to children’s nutrition. Frontiers in Nutrition, 8, 780844.https://doi.org/10.3389/fnut.2021.780844
- Maulu, S., Hasimuna, O. J., Monde, C., & Mweemba, M. (2020). An assessment of post-harvest fish losses and preservation practices in Siavonga District, Southern Zambia. Fisheries and Aquatic Sciences, 23(1), 9. https://doi.org/10.1186/s41240-020-00170-x
- Mordue (Luntz), A. J., & Blackwell, A. (1993). Azadirachtin: an update. Journal of Insect Physiology, 39(11), 903–924. https://doi.org/10.1016/0022-1910(93)90001-8
- Mohammed, H. H., Laftah, W. A., Ibrahim, A. N., & Che Yunus, M. A. (2022). Extraction of essential oil from Zingiber officinale and statistical optimization of process parameters. RSC Advances, 12(8), 4843–4851. https://doi.org/10.1039/d1ra06711g
- Moyes, C. L., Vontas, J., & Martins, A. J. (2017). Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negi Trop Dis, 11(7), 1–20.
- Ngamo, T. S. L., Ngatanko, I., Ngassoum, M. B., Mapongmestem, P. M., & Hamce, T. (2007). Persistence of insecticidal activities of crude essential oils of three aromatic plants towards four major stored product insect pests. African Journal of Agricultural Research, 2(4), 173–177.
- Nilesh, D., Proshanta, G., & Rakesh, K. G. (2021). Extraction and characterization of essential oil of garlic (Allium sativa L.). International Journal of Chemical Studies, 9(1), 1455–1459.
- Odeyemi, O. O., Owoade, R. A., & Akinkurolere, O. (2000). Toxicity and population suppression effects of Parkia clappatoniana on dried fish pests (Dermestes maculatus and Necrobia rufipes). Global Journal Pure Applied Science, 6(2), 191–195.
- Oseni, N. T., Fernando, W. M., Coorey, R., Gold, I., & Jayasena, V. (2017). Effect of extraction techniques on the quality of coconut oil. African Journal of Food Science, 11(3), 58–66.
- Osuji, F. N. C. (1974). Beetle infestation of dried fish purchased from a Nigerian market, with special reference to Dermestes maculatus Degeer. Nigerian Journal of Entomology, 1(1), 69–79.
- Osuji, F. N. C. (1985). Outline of stored products. Entomology for the tropics (1st ed., pp. 103). Fourth Dimension Publishers.
- Sharma, N., & P. C., Trivedi, P. C. (2002). Screening of leaf extracts of some plants for their nematicidal and fungicidal properties against Meloidogyne incognita and Fusarium oxysporum. Asian Journal of Experimental Sciences, 16, 21–28.
- Silva, G. N., Faroni, L. R., Sousa, A. H., & Freitas, R. S. (2012). Bioactivity of Jatropha curcas L. to insect pests of stored products. Journal of Stored Products Research, 48, 111–113. https://doi.org/10.1016/j.jspr.2011.10.009
- Ward, A., & Signa, D. (2017). Reducing post-harvest fish losses for improved food security, SMARTFISH Programme.
- Zhu, J. J., Cermak, S. C., Kenar, J. A., Brewer, G., Haynes, K. F., Boxler, D., Baker, P. D., Wang, D., Wang, C., Li, A. Y., Xue, R. D., Shen, Y., Wang, F., Agramonte, N. M., Bernier, U. R., de Oliveira Filho, J. G., Borges, L. M. F., Friesen, K., & Taylor, D. B. (2018). Better than DEET Repellent Compounds Derived from Coconut Oil. Scientific Reports, 8(1), 14053. https://doi.org/10.1038/s41598-018-32373-7
- Zimilar, H. E., Mandlate, J. S., Artur, E. M., Muiambo, H. F., & Uamusse, A. A. (2021). Vortex-assisted solid-liquid extraction for rapod screening of oil content in Jatropha seed: an alternative to the modified soxhlet method. South African Chemical Institute, 75, 1–5.