 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The study aimed to compare the growth-promoting and innate immune gene expression of phyto-biotics powder; moringa leaf, ginger, garlic, and a combination of the three, as well as a control diet fed to Clarias gariepinus. Relative to the juveniles in the control diet, growth and nutrient utilization indices of juveniles in phyto-additive diets increased, with fish fed with garlic having higher growth and feed utilization in terms of weight gain: 381.6 ± 71, feed conversion ratio: 2.7 ± 0.39, specific growth rate: 0.849 ± 1.72 and relative growth rate: 2.932 ± 0.23 when compared to the control fish fed diet as well as moringa and a combination of the phyto-biotics fed supplements. The expression of the innate immune gene TnF-α was observed to be downregulated in C. gariepinus fed with phyto-biotics fed supplements; moringa, ginger, garlic, and their combination having a fold change of; 0.124 ± 0.034, 0.196 ± 0.145, 0.288 ± 0.088, 0.097 ± 0.30, respectively while upregulated in fish fed with a control fish-fed diet having a fold change of 1.436 ± 0.791. This finding indicates that the use of garlic and ginger supplements can enhance growth performance and nutrient utilization while providing anti-inflammatory benefits in Clarias gariepinus.
Reviewing Editor:
Introduction
Over the past two decades, several research groups have been engaged in understanding the relation between dietary/nutritional factors and metabolic or physiological effects at the molecular level. These studies have contributed toward a better understanding of specie metabolic pathways and toward assessing the nutritional control of the expression of genes corresponding to supplemental feed (Panserat & Kaushik, Citation2010).
According to recent research findings, proper nutrition can aid in the treatment of a variety of diseases. Nutrition genomics is a field of study that focuses on in-depth nutritional studies (nutrigenomics) (Abd El-Gawad et al., Citation2020). This field of study is concerned with the characterization of gene products, their physiological function, and interactions. Nutrition genomics investigates the effect of nutrition on gene expression (downregulation or upregulation). To investigate the significance of diet formulation, it is necessary to first understand the molecular basis of organisms’ physiological responses to dietary components.
In fish nutrition, particularly for aquaculture species, formulated feeds differ substantially from the natural diet due to recent changes in nutrient sources, particularly protein sources, which are now dominated by terrestrial derived ingredients (Hoseinifar et al., Citation2016). Furthermore, many feeds are now incorporated into health management and referred to as functional feeds, which are thought to improve fish health, reduce disease outbreaks, and ultimately improve post-infection recovery (Martin & Król, Citation2017).
Most common phytochemicals are Moringa (Moringa oleifera), Garlic (Allium sativum) and Ginger (Zingiber officinale) which are both perennial crops, natural and are readily available, affordable and have been reported to promote various health activities (Ayoade et al., Citation2022). According to Ashad et al. (Citation2014), phytochemicals and their constituents play an important role in disease management through modulation of biological activities. Ginger is considered potential chemopreventive agent due to its therapeutic role in the control of bacteria disease in farmed fish. Major ingredients in ginger includes; tarpenes and oleoresin (ginger oil) with antibacterial and antifungal properties (Payung et al., Citation2017). Ginger powder contains volatile compounds such as gingerol, zingiberol, shogaol and zingiberene that exhibit anti-inflammatory and immunological that is beneficial to fish health (Ratna, Citation2015).
Nutritional and phytochemical analyses revealed that garlic contains about 17 amino acids, 33 sulfur compounds, several enzymes, minerals and vitamins. Studies have also revealed its antiviral, antibacterial, antifungal, antioxidant, antiprotozoal and an effective immuno-stimulant, growth promoter and improve flesh quality owing to the presence of various organ sulfur compounds (Nyadjeu et al., Citation2021). An active compound in garlic known as, allicin (dially thiosulfinate), responsible for most of the pharmacological properties of garlic and is the most abundant compound representing not less than 70% of all thiosulfinates present in crushed garlic (Batiha et al., Citation2020).
Garlic also provides odor stimulation to increase the response of fish feed. It has been also reported that dietary garlic can improve the antioxidant status of rainbow trout (Mohebbi et al., Citation2012). Moreover, garlic has the ability of catalase activity in serum and lowering the levels of plasma glucose in fish (Nwabueze, Citation2012). It increases the welfare of fish and can help in the control of pathogens, specially bacteria and fungi. The garlic has several beneficial effects including antioxidant, antihypertensive and antimicrobial properties (Fazlolahzadeh et al., Citation2011).
Moringa oleifera is a potential alternative plant protein source in aquaculture. Its leaves contain phenolics and flavonoids which have various biological activities, including antioxidant, anticarcinogenic, immunodulation, and hepatoprotective properties (Sherif et al., Citation2014). Moringa contains low concentration of harmful factors in its seed and its protein contents are higher than proteins of soybean seeds and other legumes (Ferreira et al., Citation2008).
However, some limitations related to plant protein used in fish feed are well documented. Anti-nutritional factors (ANFs) present in the plant-based feed are the primary constraint, an example being phytate, which is phosphate (P) in its main storage form (Nutrient Requirements of Fish – NRC, Citation2001).
Understanding the interaction between fish nutrients and its immune system is critical for the new product development to increase aquaculture production efficiency (Biller-Takahashi & Urbinati, 2013). A group of proteins in fish called cytokines are involved in signaling molecules and immunomodulating proteins. They permit intercellular communication to facilitate immune response in both the innate and adaptive immune systems (Palanisamy et al., Citation2012). Cytokines are commonly thought to play a role in the creation of immunological and inflammatory responses. One of these cytokines is Tumor Necrosis Factor Alpha (TNF-), which is involved in Innate immune response in fish and is involved in the creation of cytokines.
TNF-α is a pleiotropic and potent cytokine produced by macrophages and other cells in response to inflammation, infection, and other physiological stressors (Saeij et al., Citation2003). TNF-α induces a variety of systemic and cellular responses, including leukocyte activation and migration, and phase reactions (Baud & Karin, Citation2001). TNF-α mRNA appears to be transcribed in a variety of cells and is heavily controlled post-transcriptionally (Smith et al., Citation2019). TNF-α promotes the release of other cytokines and has a wide range of biological roles, including the control of cell differentiation, tissue renewal and remodeling, as well as the regulation of proinflammatory responses (Sebastián et al., Citation2012). The mechanism of action of innate immune cytokine is inflammation. Inflammation is a biological response of the immune system that can be triggered by a variety of factors, including pathogens, damaged cells and toxic compounds (Chen et al., Citation2017). Feed supplements used in feed formulation having anti-oxidative and inflammatory active components triggering inflammatory response will be of interest in Nutrition, it is of an advantage to fish immune system.
Natural plant dietary supplementation in aquafeed could be used as an effective way for increasing the immune-competency and disease resistance of fish. Results have suggested that M. oleifera leaf powder-supplemented diets could enhance the immune response of O. niloticus fry and prevent disease caused by Aeromonas hydrophila (Abd El-Gawad et al., Citation2020). Dietary inclusion effect of ginger and garlic have also been shown to induce growth in Clarias gariepienus (Nyadjeu et al., Citation2021) and feed formulated with these supplements fed to Common carp has shown significant increase in RBCs, WBCs, PCV, Hb and total plasma protein (Ajeel & Al-Faragi, Citation2013), hence the inclusion of these supplements in fish fed diet has always been of interest as evidenced by several researchers. However, there is dearth of information on the supplements physiological effect on innate immune related gene expression which necessitated this study.
Materials and methods
Processing of additives
Freshly harvested moringa leaves (M. oleifera), ginger roots (Z. officinale) and garlic bulbs (A. sativum) were washed under a running clean tap water and wiped with a clean kitchen towel. Garlic and ginger were individually peeled to remove fore-skin before being diced with a kitchen knife. Additives were sundried for a period of 72 h, then milled to powder using a laboratory milling machine (Model: HK - 860) and sieved with a hand sieve to obtain 2 kg each of the powders which were then stored in an airtight cellophane bag and refrigerated at 4 °C for further analysis.
Experimental diets and acclimatization of experimental fish
Five iso-nitrogenous diets were formulated to contain 35% crude protein with each additive powder added at 2 g/100g. Diet ‘A’ (2% moringa inclusion), Diet ‘B’ (2% ginger inclusion), Diet ‘C’ (2% garlic inclusion), Diet ‘D’ (mixture of all three additives: 2% inclusion) and Diet ‘CT’ (0% additives). Tables of experimental feed ingredients and proximate composition of the additives powder are shown in and .
Table 1. Formulation of experimental diets.
Table 2. Percentage proximate composition of moringa leaf, ginger and garlic powder.
At the end of the feeding trial, the weight gain, mean weight gain, total weight gain, relative growth rate (RGR), specific growth rate (SGR) and feed conversion ratio (FCR) were estimated from fortnightly measurements made using the following formula:
Weight Gain = difference between the initial weight and final weight gain.
Mean Weight Gain = Mean Final Weight - Mean Initial Weight
Total Weight Gain = Final Average Weight – Initial Average Weight
Percentage Weight Gain (PWG): This was calculated using the formula:
PWG = (Total Weight Gained)/(Initial Weight) × 100
Specific Growth Rate (SGR): This was calculated from the relationship of the difference in the weight gain of fish within the experimental period.
SGR (%) = (Log W2 − Log W1 × 100)/T
Where
W2= final body weight
W1= initial body weight of fish
T = duration of study in days
Relative Growth Rate (SGR): This was calculated as:
RGR (%) = Final weight – initial weight/initial weight
Feed conversion ratio (FCR)
From the weight gained and feed consumed by each group of fish, the feed conversion ratio (FRC) was calculated as: FCR = Feed Intake/Net weight gain.
Feeding trial/fish sample collection
After acclimatization, three hundred (300) catfish juveniles of average weight of 6.7 ± 0.4 g were collected from the fish hatchery unit of the Nigerian Institute for Oceanography and Marine Research for the experiment before they were divided into five groups (four representing fish fed with phyto-biotic leaf-based diet; and control without). They were distributed in an equal number of 20 pieces into a fifteen 80 L plastic tanks. Each treatment was replicated thrice in a completely randomized design and fed thrice daily at 5% body weight between (10:00h, 13:00h and 16:00h).
The feeding trial was carried out for a period of 10 weeks during which the corresponding weight of fish was recorded on a biweekly basis. The experiment was conducted in water recirculatory system (WRS) plastic tank with a water exchange rate of 70 L flow per minute.
Tissue samples were collected from each group after feeding trial which lasted for Ten weeks. In the molecular Biology Laboratory NIOMR, Tissues samples (1 g) were collected from the ventral region (Cordero et al., Citation2017) of Clarias gariepinus and immersed in 1 ml of RNALater buffer, contained in well labeled 2 ml Eppendorf tube to maintain RNA stability, and stored in the Refrigerator at −20° C until further use.
Gene identification, primer design and PCR efficiency
The Gene involved in immune response was selected based on concurrent literature and their transcripts were retrieved from NCBI. Each sequence was evaluated using Blastx search of NCBI. Primer pairs for qPCR were designed using NCBI primer Blast and UCSC tool was used to confirm the primer. All primers profile was optimized using gradient PCR standardized and checked on agarose gel.
RNA extraction and cDNA synthesis
Total RNA was isolated using JENA mini kit (Jena Bioscience, Germany) according to the manufacturer’s protocol. The purity and concentration of RNA extract was determined using a Thermo fisher 1000 C Nanodrop spectrophotometer. Reverse Transcription Polymerase Chain Reaction (RtPCR) was carried out using Fire script RT cDNA synthesis kits (FIREScript®, Estonia), using Oligo-dt primer (with the thermal profile: 65˚ C for 5 mins for denaturation, followed by Primer annealing at 25˚ C for 10 mins and 85˚ C for 5 min to inactivate RT enzyme).
The housekeeping Gene, Glyceradehyde 3 Phosphate dehydrogenase (GAPDH) was used to quantify complementary DNA (Primer sequence shown in ), amplification was done with initial min denaturation at 94 °C followed by 30 cycles of following: denaturation at 94 °C for 30 s annealing at 54 °C for 30s, extension at 72 °C for 30s.
Table 3. Primers used for nucleic acid quantification and qPCR in this study.
Quantitative real time-PCR
The study utilized quantitative real-time PCR to measure immune regulatory gene expression, with β-Actin serving as the housekeeping gene. The primer sequences for the genes analyzed are provided in , and the qPCR reaction was performed in triplicate with a final volume of 10 μl. SYBR green was used with a light Cycler 480 (Roche) in accordance with the manufacturer’s instructions to quantify mRNA levels. Gel electrophoresis was employed to verify proper amplification, and melting curve analysis confirmed the specificity of qPCR reactions. Normalization was carried out using the β-Actin reference gene, and expression was presented as relative to the non-treated control group. Gel electrophoresis was also used to verify correct amplification, and all CT values were exported in spreadsheet format.
Gene expression was measured by the relative quantification method, which was carried out using the 2-ΔΔCT method as stated by Livak and Schmittgen (Citation2001) is shown below:
Step 1: Normalization of ΔCT values of target genes with the reference gene:
Step 2: Normalization of CT values of fed supplements with the control diet
Statistical analysis
All data were tested for normality before being analyzed in this study. GraphPad Prism software 20.1 was used to carry out one-way analysis of variance (ANOVA) to test for significant differences (p < .05) among fed treatment groups and control. Duncan’s multiple range test was used to compare differences among the treatment means when significant F-values were observed at (p < .05) level. The data were presented as mean ± SD.
Results
Growth performance indices revealed that cultured fish in treatment ‘C’ had the highest total weight gain (381.6 ± 71 g) while fish in treatment ‘D’ had the lowest total weight gain (32.8 ± 26.8 g). These values are significantly different (p < .05) from other treatments. Feed conversion ratio (FCR) values of C. gariepinus fed with different dietary inclusions of moringa, ginger, and garlic basal diets are presented in . It was noted that feed consumption translated into flesh increase for most of the treatments. In general, fish fed on diet ‘C’, ‘B’ and ‘CT’ each containing 2% of garlic, ginger, and control diet inclusion had the best FCR with values of 2.7 ± 0.39, 3 ± 0.84 and 2.8 ± 0.14, respectively, and are significantly different (p < .05) when compared to treatments ‘A’ and ‘D’.
Table 4. Growth performance and nutrient utilization of experimental fish.
RNA and cDNA quantification
The concentration and absorbance values of RNA isolates in this study met the optimum concentration of 50 ng/µl and purity required for downstream application, shows the mean concentration and purity (Δ260/280). The quantified cDNA gel capture () shows GAPDH PCR amplicons on 2% agarose gel electrophoresis of reverse transcribed RNA sample (n = 3).
Figure 1. Gel image capture cDNA quantification of samples done in triplicate, GAPDH with expected amplicons band size of 450 bp.
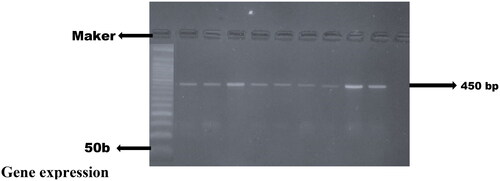
Table 5. Nucleic acid quantification showing the Δ260/280 values and concentrations in ng/µl of RNA isolated from Clarias gariepinus.
Gene expression
shows gene expression fold change of the innate immune gene TNF-α in cultured C. gariepinus fed with control (CT) and phyto-biotics fish fed a leaf-based diet with moringa (A), ginger (B), garlic (C), and a combination of the leaf fed (D). The experimental data are expressed as the mean ± standard deviation (SD) and were processed using GraphPad prism 9 for Windows. One-way ANOVA was used to compare control (CT) group and phyto-biotics fish fed leaf-based diet. The differences between groups were considered statistically significant when p < .05. The expression of the TNF-α gene was upregulated in experimental fish fed with the control-fed group having a fold change of 1.436 ± 0.791, and TNF-α expression was downregulated in phyto-biotics fish fed leaf-based diet, with ‘C’ fed group having a higher fold change of 0.288 ± 0.088 compared with ‘D’ being a combination of the three supplements with TNF-α fold change of 0.097 ± 0.30.
Table 6. The gene expression level of different experimental fish fed with different fed supplements.
Discussion
The physiological properties of a gene are determined by its expression (up/down-regulated) which is a determining factor of translation and protein functional effect. Mechanisms by which gene regulation can be accomplished are factors that bind in a competitive and antagonistic manner to hormone receptors. Earlier research has found that supplementing medicinal plant products in the diet improves a variety of activities such as growth, immunity, appetite, and digestion stimulation (Nyadjeu et al., Citation2021; Shalab et al., Citation2006). Despite the global acceptance of plant additives, there is still opinionated information on the genetic effects of phyto-biotics used in diet formulation for C. gariepinus. A dearth of information has also been noticed on the significance of these phyto-biotics; moringa leaf, garlic, ginger, and their combination as additives in the diet formulation of C. gariepinus (Adeniji et al., Citation2019). The results of this study showed that C. gariepinus fingerlings fed with a basal diet supplemented with ginger/garlic powder developed and utilized feed more efficiently in terms of weight gain, feed conversion ratio, specific growth rate as well as relative growth rate as this parameter were seen to be significantly higher in C. gariepinus fingerlings fed with this diet, when compared to C. gariepinus fed with phyto-biotics fish-fed diet containing moringa or a combination of both supplements. The low performance with moringa could be attributed to the antinutrients such as saponins, phytate and tannins present in moringa leaf. Saponin has an adverse effect on biological membrane by increasing the permeability of intestinal mucosal cell aiding the free transportation of active nutrients, while phytate inhibits absorption of nutrient thereby reduces the availability of minerals and protein digestibility (Abd El-Gawad et al., Citation2020). The findings of this study support the study results of Oh et al. (Citation2022) who found that incorporating ginger (Zingiber officinale) residue from juice extracts improves the growth performance of juvenile black rockfish (Sebastes schlegelii). Iheanacho et al. (Citation2017) reported improvement in BWG & SGR in C. gariepinus juvenile when exposed to different concentrations (0.25, 0.50, 0.75 and 1.0 g/35 mgL) of ginger as compared to the control. Abdel-Hakim et al. (Citation2010) also reported that incorporating garlic into diets for growing Nile tilapia significantly improved weight gain and specific growth rate. However, several studies are on the contrary with our findings; Nwabueze (Citation2012) reported that garlic supplemented diet did not have any significant effect on weight gain of C. gariepinus in comparison with control group, Faisal (Citation2003) reported significant decrease of mean corpuscular volume (MCH) and mean corpuscular hemoglobin (MCV) in Cat fish (Clarias garepins) fed with garlic. Although Talpur et al. (Citation2013) suggested that the growth was dose-dependent and highest supplementation of ginger and garlic at 5 and 10 g/kg feed was most favorable for the growth and survival of Asian sea bass. Similarly, Lee et al. (Citation2014) showed that adding of garlic powder by inclusion level 3% could positively affect growth performance in fingerling starlet sturgeon. Temitope (Citation2012) also concluded that growth rate of T. zillii fed the diets having 20 g inclusion level of garlic/kg basal diet had higher growth rate than other group fed (0, 5, 10, 15 g garlic kg−1 basal diet).
Modulation of the innate immune response is carried out mainly through the proinflammatory cytokines secreted by immune cells, especially TNF-α and IL-1β thus, gene expression patterns of this cytokine could be used to judge changes in an immune response. The nutrigenomics investigation in this study revealed significant TNF-α downregulation in C. gariepinus fed with phyto-biotics fish fed diet, indicating that the fish fed phyto-biotics leaf-based diet compromised the action of the innate immune gene TNF-α, despite being shown to have phytochemical properties improving fish health. This could be due to the experimental period of (10 weeks) was insufficient for the innate immune gene to express. The fact that the inclusion level of phyto-biotics (2%) was not varied to different concentration could be attributable to the downregulation of the innate immune gene TNF-α. Thus, variation of the inclusion is encouraged. Since TNF-α plays a crucial role in the rapid inflammatory response against environmental pathogens, this indicates that the garlic and ginger supplements possess anti-inflammatory properties. Similarly, Ahmadifar et al. (Citation2019) found that feeding ginger at 1–3% had no significant effects on TNF-α, IL-1β or IL8 gene expression. Abdel-Tawwab et al. (Citation2021) found a significant up-regulation in the levels of mRNA expression of IL-1β and IL-4 genes alongside with downregulation of TNF-α and HSP70 transcripts in response to garlic and chitosan powder treatment against Zearalenone induced toxicity in European seabass.
This is also in line with the findings of Nyadjeu et al. (Citation2021) and Megbowon et al. (Citation2013) as well as antioxidant and antimicrobial Wankhede et al. (Citation2022). The conflicting results in downregulation of fish supplemented with garlic, ginger and Moringa may be attributed to dose, period of study. This is confirmed by the results recorded by Fazelan et al. (Citation2020) who suggested that supplementation with 10 g garlic, ginger/kg mitigated oxidative stress, immunosuppression and improved the defense to disease in common carp reared under high stocking density through up regulating gene expression levels of TNF-α, IL-1β and IL-8. Shalaby et al. (Citation2006) also demonstrated that the best performance and high specific growth rate in Nile tilapia fish was obtained by a diet containing 30 g garlic powder per kilogram diet and improvement in FBW was more prominent at higher incorporation levels.
On the other hand, the upregulation seen in fish fed with the control diet presumes there is a significant expression of TNF-α, which is advantageous in pathogen resistance. Comparatively, in this study, phyto-biotics leaf-based diet had a positive significant effect on growth parameters (weight gain), whereas a non-significant effect was seen in the expression of the innate immune gene, as the weight gain and TNF-α expression of C. gariepinus fed with the control and supplements fed diet (CT, A, B, C, D) were found to be; 352.96 ± 28.3 vs 1.436 ± 0.791, 88.61 ± 21.3 vs 0.124, 345.4 ± 134 vs 0.197, 381.6 ± 71 vs 0.289, 32.8 ± 26.8 vs 0.097 ± 0.303, respectively. In addition, garlic has more potent growth performance and antioxidant effects than ginger. Fish diet integrated with 2% phytobiotics supplementation to fish diet had the most benefits on C. gariepinus fish growth performance as boost the body weight, weight gain, specific growth rate and improve also feed conversion ratio. However, had no significant effect on immunostimulation and general health of C. gariepinus fish.
Conclusion
To summarize, the study found that the addition of phyto-biotic supplements Ginger and Garlic had a significant positive effect on the growth performance of C. gariepinus juvenile having growth parameters factors compared to the control. However, it was observed that the TNF-α innate immune gene was downregulated in fish fed the supplements, while an upregulation was observed in the control group that did not receive phyto-biotic supplements. As TNF-α plays a crucial role in the rapid inflammatory response against environmental pathogens, this indicates that the garlic and ginger supplements possess anti-inflammatory properties. Overall, these findings suggest that the use of ginger and garlic supplements can enhance growth performance and nutrient utilization while providing anti-inflammatory benefits in C. gariepinus.
Recommendation
This study elucidated the relationship between growth parameters and innate immune expression in phyto-biotics fed supplements, noticing a positive significance in growth performance with a downregulation in innate immune gene (TNF-α) expression, the authors recommend incorporation of phyto-biotics fed supplements having inflammatory properties to upregulate TNF-α gene expression to facilitate defense against environmental pathogens.
Authors’ contribution
This work was carried out in collaboration among all authors. Authors Megbowon I, Ukenye E.A, Joseph J. B, Usman, Sokenu, Adeleke, Edah B and Omatah designed the study, involved in the feeding experiment, sample collection, performed laboratory analysis and wrote the first draft of the manuscript while authors Oguntade and Akinwale supervised the research, performed the data analysis and reviewed the first draft of the manuscript. All authors made input, read and approved the final manuscript.
Acknowledgments
Authors appreciate the support and assistance rendered by other staff of Biotechnology Department of Nigerian Institute for Oceanography and Marine Research (NIOMR) Lagos, Nigeria and the Inqaba Biotechnology Laboratory, South Africa, while conducting this research.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abd El-Gawad, E. A., El Asely, A. M., Soror, E. I., Abbass, A. A., & Austin, B. (2020). Effect of dietary Moringa oleifera leaf on the immune response and control of Aeromonas hydrophila infection in Nile tilapia (Oreochromis niloticus) fry. Aquaculture International, 28(1), 1–10. https://doi.org/10.1007/s10499-019-00469-0
- Abdel-Hakim, N., Lashin, M., Ashry, A., & Al-Azab, A. D. (2010). Effect of fresh or dried garlic as a natural feed supplement on growth performance and nutrients utilization of the Nile Tilapia (Oreochromis niloticus). Egyptian Journal of Aquatic Biology and Fisheries, 14(2), 19–38. https://doi.org/10.21608/ejabf.2010.2058
- Abdel-Tawwab, M., Khalil, R. H., Diab, A. M., Khallaf, M. A., Abdel Razek, N., Abdel-Latif, H. M., & Khalifa, E. (2021). Dietary garlic and chitosan enhanced the antioxidant capacity, immunity, and modulated the transcription of HSP70 and cytokine genes in zearalenone-intoxicated European seabass. Fish Shellfish Immunology, 113, 35–41. https://doi.org/10.1016/j.fsi.2021.03.012
- Adel, M., Dawood, M. A., Gholamhosseini, A., Sakhaie, F., & Banaee, M. (2021). Effect of the extract of lemon verbena (Aloysia citrodora) on the growth performance, digestive enzyme activities, and immune-related genes in Siberian sturgeon (Acipenser baerii). Aquaculture, 541, 191–219.
- Adeniji, C. A., Wusu, D., & Falana, E. O. (2019). Individual and combined effects of moringa leaf and garlic powder on growth and plasma biochemical indices of Clarias gariepinus Juveniles. American Journal of Food Science and Technology, 7(5), 137–145. https://doi.org/10.12691/ajfst-7-5-1
- Ahmadifar, E., Sheikhzadeh, N., Roshanaei, K., Dargahi, N., & Faggio, C. (2019). Can dietary ginger (Zingiber officinale) alter biochemical and immunological parameters and gene expression related to growth, immunity and antioxidant system in zebrafish (Danio rerio)? Aquaculture, 507, 341–348. https://doi.org/10.1016/j.aquaculture.2019.04.049
- Ajeel, S. G., & Al-Faragi, J. K. (2013). Effect of ginger, (Zingiber officinale) and garlic, (Allium sativum) to enhance health of common carp, Cyprinus carpio. The Iraqi Journal of Veterinary Medicine, 37(1), 59–62. https://doi.org/10.30539/iraqijvm.v37i1.332
- Ashad, H. R., Fahad, M. A., & Salah, M. A. (2014). Active ingredients of ginger as potential candidates in the prevention and treatment of diseases through modulation of biological activities. International Journal of Physiology, Pathophysiology and Pharmacology, 6(2), 125–136.
- Ayoade, W. G., Amoo, I. A., Lajide, L., & Ajayi, M. G. (2022). Phytochemicals and antioxidant potential of ginger (Zingiber officinale) and garlic (Allium sativum) extracts. GSC Biological and Pharmaceutical Sciences, 19(1), 226–234. https://doi.org/10.30574/gscbps.2022.19.1.0144
- Batiha El-Saber, Gaber, Amany Magdy Beshbishy, Lamiaa G. Wasef, Yaser H. A. Elewa, Ahmed A. Al-Sagan, Mohamed E. Abd El-Hack, Ayman E. Taha, Yasmina M. Abd-Elhakim, & Hari Prasad Devkota. (2020). Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients, 12(3), 872. https://doi.org/10.3390/nu12030872
- Baud, V., & Karin, M. (2001). Signal transduction by tumor necrosis factor and its relatives. Trends in Cell Biology, 11(9), 372–377. https://doi.org/10.1016/s0962-8924(01)02064-5
- Biller-Takahashi, J. D., & Urbinati, E. C. (2014). Fish immunology: The modification and manipulation of the innate immune system: Brazilian studies. Anais da Academia Brasileira de Ciencias, 86(3), 1484–1506. https://doi.org/10.1590/0001-3765201420130159
- Chen, L., Deng, H., Cui, H., Fang, J., Zuo, Z., Deng, J., Li, Y., Wang, X., & Zhao, L. (2017). Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 9(6), 7204–7218. https://doi.org/10.18632/oncotarget.23208
- Cordero, H., Cellballlos-Francisco, D., Cuesta, A., & Esteban, M. A. (2017). Dorso-ventral skin characterization of the farmed fish gilthead seabream (Sparus aurata). PLoS One, 12(6), e0180438. https://doi.org/10.1371/journal.pone.0180438
- Faisal, A. S. R. (2003). Adverse effects of some antimicrobial agents used in fish [PhD thesis]. Faculty of Veterinary Medicine, Cairo University.
- Fazelan, Z., Vatnikov, Y. A., Kulikov, E. V., Plushikov, V. G., & Yousefi, M. (2020). Effects of dietary ginger (Zingiber officinale) administration on growth performance and stress, immunological, and antioxidant responses of common carp (Cyprinus carpio) reared under high stocking density. Aquaculture, 518, 734833. https://doi.org/10.1016/j.aquaculture.2019.734833
- Fazlolahzadeh, F., Keramati, K., Nazifi, S., Shirian, S., & Seifi, S. (2011). Effect of garlic (Allium sativum) on hematological parameters and plasma activities of ALT and AST of Rainbow trout in temperature stress. Australian Journal of Basic & Applied Sciences, 5, 84–90.
- Ferreira, P. M. P., Farias, D. F., Oliveira, J. T. D. A., & Carvalho, A. D. F. U. (2008). Moringa oleifera: Bioactive compounds and nutritional potential. Revista de Nutrição, 21, 431–437.
- Hoseinifar, S. H., Ringø, E., Shenavar Masouleh, A., & Esteban, M. Á. (2016). Probiotic, prebiotic and synbiotic supplements in sturgeon aquaculture: A review. Reviews in Aquaculture, 8(1), 89–102. https://doi.org/10.1111/raq.12082
- Iheanacho, S., Ogunji, J. O., Ogueji, E. O., Nwuba, L. A., Nnatuanya, I. O., Ochang, S. N., Mbah, C. E., Ibrahim, B. U., & Haruna, M. (2017). Comparative assessment of ampicillin antibiotic and ginger (Zingiber officinale) effects on growth, haematology and biochemical enzymes of Clarias gariepinus Juvenile. Journal of Pharmacognosy and Phytochemistry, 6(3), 761–767.
- Lee, D. H., Lim, S. R., Han, J. J., Lee, S. W., Ra, C. S., & Kim, J. D. (2014). Effects of dietary garlic powder on growth, feed utilization and whole body composition changes in fingerling Sterlet sturgeon, Acipenser ruthenus. Asian-Australasian Journal of Animal Sciences, 27(9), 1303–1310. https://doi.org/10.5713/ajas.2014.14087
- Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods, 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262
- Martin, S. A., & Król, E. (2017). Nutrigenomics and immune function in fish: New insights from omics technologies. Developmental and Comparative Immunology, 75, 86–98. https://doi.org/10.1016/j.dci.2017.02.024
- Megbowon, I., Adejonwo, O. A., Adeyemi, Y. B., Kolade, O. Y. & A. A. A., Adetoye. (2013). Effect of garlic on growth performance, nutrient utilization and survival of an ecotype cichlid, ‘Wesafu’. IOSR Journal of Agriculture and Veterinary Science, 6(3), 10–13. https://doi.org/10.9790/2380-0631013
- Mohebbi, A., Nematollahi, A., Ebrahimi Dorcheh, E., & Goodarzian Asad, F. (2012). Influence of dietary garlic (Allium sativum) on the antioxidative status of rainbow trout (Oncorhynchus mykiss). Aquaculture Research, 43(8), 1184–1193. https://doi.org/10.1111/j.1365-2109.2011.02922.x
- Nutrient Requirements of Fish – NRC. (2001). Nutrient requirements of fish and shrimp. National Academy Press.
- Nwabueze, A. A. (2012). The effect of garlic (Allium sativum) on growth and haematological parameters of Clarias gariepinus (Burchell, 1822). Sustainable Agriculture Research, 1(2), 222–228. https://doi.org/10.5539/sar.v1n2p222
- Nyadjeu, P., Yemdjie, D. D. M., Ndjuissi, N. A. T., Nguenang, G. N., Dedou, N. Y. C., & Tabi-Tomedi, M. E. (2021). Effect of Zingiber officinale and Allium sativum powders as natural feed additives promoting growth, feed utilization and whole-body composition in Clarias gariepinus fry. Food and Nutrition Sciences, 12(06), 526–543. https://doi.org/10.4236/fns.2021.126040
- Oh, H. Y., Lee, T. H., Lee, D. Y., Lee, C. H., Joo, M. S., Kim, H. S., & Kim, K. D. (2022). Dietary supplementation with ginger (Zingiber officinale) residue from juice extraction improves Juvenile Black Rockfish (Sebastes schlegelii) growth performance, antioxidant enzyme activity and resistance to Streptococcus iniae infection. Animals, 12(5), 546. https://doi.org/10.3390/ani12050546
- Palanisamy, V., Jakymiw, A., Van Tubergen, E. A., D’Silva, N. J., & Kirkwood, K. L. (2012). Control of cytokine mRNA expression by RNA-binding proteins and microRNAs. Journal of Dental Research, 91(7), 651–658. https://doi.org/10.1177/0022034512437372
- Panserat, S., & Kaushik, S. (2010). Regulation of gene expression by nutritional factors in fish. Aquaculture Research, 41(5), 751–762. https://doi.org/10.1111/j.1365-2109.2009.02173.x
- Payung, C. A., Tumbol, R. A., & Manoppco, H. (2017). Dietary ginger (Zinigiber officinale) enhance resistance of Nile tilapia (Oreochromis niloticus) against Aeromonas hydrophila. Aquaculture, Aquarium, Conservation and Legislation, 10(4), 962–968.
- Ratna, S. S. (2015). Medicinal use of ginger (Zinigiber officinale) improves growth and enhances immunity in aquaculture. International Journal of Chemical Studies, 3, 83–87.
- Saeij, J. P., Stet, R. J., de Vries, B. J., van Muiswinkel, W. B., & Wiegertjes, G. F. (2003). Molecular and functional characterization of carp TNF: A link between TNF polymorphism and trypano tolerance. Developmental and Comparative Immunology, 27(1), 29–41. https://doi.org/10.1016/s0145-305x(02)00064-2
- Sebastián, R., Kevin, M., Felipe, R., Daniela, T., Ana-María, S., & Mónica, I. (2012). Fish cytokines and immune response. In New advances and contributions to fish biology.
- Shalab, Y. A. M., Khattab, Y. A., & Abdel-Rahman, A. M. (2006). Effects of garlic (Allium sativa) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus). Journal of Venomous Animals and Toxins Including Tropical Disease, 12, 172–201.
- Shalaby, A. M., Khattab, Y. A., & Abdel, R. A. M. (2006). Effects of garlic (Allium sativum) and chloramphenicol on growth performance, physiological parameters and survival of Nile tilapia (Oreochromis niloticus). Journal of Venomous Animals and Toxins Including Tropical Diseases, 12(2), 172–201. https://doi.org/10.1590/S1678-91992006000200003
- Sherif, A. H., El-Gomol, A. M. ,& Tolan, A. E. (2014). Incorporation of Moringer oleifera in Nile tilapia (Oreochromis niloticus) diet and its effect on growth performance and immune status. Journal of Vetinary Science, 1(1), 8.6–8.14.
- Smith, N. C., Rise, M. L., & Christian, S. L. (2019). A comparison of the innate and adaptive immune systems in cartilaginous fish, ray-finned fish, and lobe-finned fish. Frontiers in Immunology, 10, 2292. https://doi.org/10.3389/fimmu.2019.02292
- Talpur, A., Ikhwanuddin, M., & Abol-Munafi, A. B. (2013). Nutritional effects of ginger (Zingiber officinale Roscoe) on immune response of Asian sea bass, Lates calcarifer (Bloch) and disease resistance against Vibrio harveyi. Aquaculture, 401, 46–52. https://doi.org/10.1016/j.aquaculture.2013.02.04
- Temitope, J. (2012). Effect of garlic (Allium sativum) on growth, nutrient utilization, resistance and survival of Tilapia zillii (Gervais 1852) fingerlings. Journal of Agricultural Science. 4(2), 269–274.
- Wankhede, S. D., Dutta, N., Tambe, M. B., Kaur, N., Jadhav, S. E., & Pattanaik, A. K. (2022). Effect of dietary inclusion of Moringa oleifera foliage on nutrient metabolism, metabolic profile, immunity and growth performance of goat kids. Emerging Animal Species, 3, 100005.

