 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Essential oils (EOs) are volatile compounds that can serve as alternatives to chemical pesticides for the management of plant pests, including fungal pathogens. The current study was carried out to investigate the chemical composition and antifungal activities of EO extracted from three plants, Thymus vulgaris, Coriandrum sativum and Cymbopogon martini, grown in southwestern Ethiopia. EOs were extracted from the leaves of each plant by hydro distillation using a Clevenger apparatus. The chemical composition of the oils was analyzed by Gas chromatograph-Mass Spectrometry (GC-MS), and their inhibitory effects were tested against mycotoxigenic fungi isolated from maize kernels belonging to Aspergillus and Fusarium. Chemical analysis revealed the presence of 32 compounds in C. sativum with hexanedioic acid, bis (2-ethylhexyl) ester (46.9%) and 2-Decenal (E)-(12.6%) being the dominant compounds. T. vulgaris contained 25 compounds, of which thymol (34.4%) and o-cymene (17.5%) were the major components. Twenty-five compounds were detected in C. martinii of which geraniol (51.4%) and geranyl acetate (14.5%) were dominant. The EOs of the tested plants had very high antifungal activity (up to 100% efficacy) against Aspergillus flavus, A. niger, Fusarium graminearum and F. verticillioides in vitro and on maize grains. The anti-fungal activities of these EOs were dependent on their major components, including thymol, hexanedioic acid, bis (2-ethylhexyl) ester and geraniol. This study confirmed the potential of EOs as bio-fungicides for the management of mycotoxigenic fungi associated with maize. This could reduce the health impacts of mold and toxigenic compounds produced in maize in a sustainable and environmentally friendly manner.
Reviewing Editor:
1. Introduction
Maize (Zea mays L) is the most important staple food crop in sub-Saharan Africa. In Ethiopia, maize is the most widely cultivated and consumed crop covering approximately 2.55 million hectares of land and contributing to more than 10 million farmers as a food security crop (USDA (United States Department of Agriculture), Citation2022). However, maize grains are susceptible to toxigenic fungal contamination both in the field, and during post-harvest handling, processing and storage conditions (Liu et al., Citation2016). The problem is more profound in developing countries, such as Ethiopia, where effective pre- and post-harvest management practices are not widely used. Good agricultural practices encourage alternative and safe disease management techniques using bio-pesticides and other biological methods, making the search for potential bioactive compounds a viable alternative pest management strategy for effective and sustainable food production (Kesraoui et al., Citation2022).
Certain plant essential oils (EOs) limit toxigenic fungal growth and mycotoxin production during the preservation of grains (Anjorin et al., Citation2013) and hence, they are currently considered promising alternatives to synthetic pesticides. EOs are complex volatile compounds obtained from aromatic and medicinal plants (Kesraoui et al., Citation2022). They are naturally produced in different plant parts as secondary metabolites (Swamy et al., Citation2016). Different plant EOs have shown promising results in the management of various pathogenic fungi (Chang et al., Citation2022; Gurjar et al., Citation2012). Different effective bio-pesticide compounds are found in EOs, including aldehydes, phenolics, terpenes, and other antimicrobials that can effectively manage different pathogens (Swamy et al., Citation2016). EO-based products are safe, bioactive, biodegradable, ecologically and economically viable products (Chang et al., Citation2022). Therefore, plant products in the form of plant extracts and EOs are used to decrease chemical residue effects and fungicide risks (Anjorin et al., Citation2013). In line with this, various plant EOs play a pivotal role in plant disease management, involving a range of antimicrobial activities in vitro (Chang et al., Citation2022) and in vivo (Mandal & Mandal, Citation2015).
In Ethiopia, maize crops are stored for 6–12 months for household consumption and sale at higher prices. Different studies have revealed the prevalence of Aspergillus, Fusarium and Penicillium fungal species in stored maize grains (Ayalew, Citation2010; Dubale et al., Citation2014; Garbaba et al., Citation2018). Moreover, major mycotoxins have been detected in stored maize grains from different parts of Ethiopia (Getachew et al., Citation2018; Yilma et al., Citation2019) at varied intensities. Thus, it is important to manage post-harvest mold contamination in maize. Marín et al. (2020) applied propionate formulation and arrested mold growth on maize grains, consequently reducing mycotoxin production. Wang et al. (Citation2019) confirmed that complex EOs significantly reduced fungal contamination of maize grains and subsequently reduced mycotoxin contamination.
Ethiopia is considered one of the centers of origin and diversity for many plant species. The country is rich in medicinal and spice plants that can potentially cure many different diseases in humans (Nigussie et al., Citation2021) and animals traditionally (Moges & Moges, Citation2019). However, their potential in controlling plant pathogens in general and toxigenic fungal pathogens has yet to be explored.
Therefore, the present study was carried out with the objectives of investigating the major bioactive components of EOs of three traditional spices and medicinal plants i.e. thyme (Thymus vulgaris), coriander (Coriandrum sativum), and palmarosa (Cymbopogon martini), which are cultivated in Ethiopia, and evaluating their effects on potentially toxigenic fungi isolated from maize grains.
2. Materials and methods
2.1. Plant material collection
Three plant species, C. sativum (L.), T. vulgaris (L.) and C. martini (L) (), were collected from the College of Agriculture and Veterinary Medicine (JUCAVM) farm site, Jimma University, and the local market, in September 2022. The JUCAVM farm site is located at an altitude of 1726.2 m, 7° 41’ 9’’N and 36° 49’ 49” E. The samples were collected early in the morning and stored in a freezer (−10 °C).
Table 1. Description of plants used for EO extraction.
2.2. EOs extraction
EOs from C. martinii, T. vulgaris and C. sativum leaves were extracted using the hydro distillation method in a Clevenger apparatus following the procedure described by Phuc et al. (Citation2019). Briefly, fresh leaves were washed with water and chopped manually into small pieces. One hundred fifty grams of chopped leaves was added to each distillation flak (1000 mL) containing 400 mL of distilled water and extraction was carried out by heating the flask at 100 °C for 3 h. After the distillation was completed, the water was discarded until the oil layer remained. Finally, the oil products were dehydrated using sodium sulfate (Na2SO4) and stored in a sealed glass at 4 °C until use. In this process, EOs were extracted from fresh leaves without drying to minimize the risk of losing antimicrobial activity, as confirmed by Benbelaid et al. (Citation2013).
2.3. Gas chromatograph-Mass spectrometry (GC-MS) analysis
Gas chromatography (GC) 7890 (Agilent Technologies Palo Alto, CA, USA) fitted with a mass spectrometry (MS) detector (Agilent 5977 A MS) and DB-5MS fused silica capillary columns (30x0.25x0.25 µm) were used to analyze the EOs with a flow rate of 1 mL/min and helium as the carrier gas. The oven temperature was initially set at 60 °C for 3 min, gradually increased to 160 °C at a rate of 5 °C/min, held for 1 min, increased at a rate of 10 °C/min to 185 °C, held for 1 min, and finally increased at a rate10 oC/min to 280 °C. The sample injector temperature was maintained at 280 °C and the sample was injected in split mode (100:1 ratio) with a volume of 1 µL. GC-MS data were measured using the same gas chromatograph coupled with an MS 5977 detector. The employed settings were ionization voltage at 70 eV, MS, transfer temperature, 280 °C; ion source temperature, 230 °C; quadruple temperature, 150 °C; and solvent delay, 4 min. Data were acquired in scan mode, scanning from 50 to 550 amu (atomic mass units).
2.4. Isolation and inoculum preparation of toxigenic fungi
Toxigenic fungi were isolated from naturally contaminated maize kernels collected from farmers’ stores in southwestern Ethiopia. The kernels were first surface-sterilized with 1% sodium hypochlorite for one minute, rinsed three times in sterile distilled water for 30 s, and dried on tissue paper aseptically. From the surface-disinfected samples, 10 kernels were plated aseptically on Petri dishes (9 cm diameter) containing potato dextrose agar (PDA) with three replicates and incubated at 25 °C for 7 days.
Fungal colonies growing on the PDA plates were further transferred to new PDA plates, and single spores have been prepared and incubated at 25 °C for 5 days. Pure Aspergillus, and Fusarium were morphologically identified at the genus level.
Then Aspergillus genera were grown on specific media on Czapek Dox Agar (CZDA), Malt Extract Agar (MEA), Czapek Yeast Extract Agar (CYA) at 25 °C for 7 days based on procedure described by Klich (Citation2002). Fusarium genera were grown on Synthetischer nährstoffärmer agar (SNA) at 25 °C for 7 days exposed to a 12:12-hour light/dark regime and allowed to grow for identification to species level (Leslie & Summerell, Citation2006).
Isolates were identified to the species level based on the micro- and macro-morphological characteristics of the standard key following standard isolation procedures and identification keys (Klich, Citation2002; Pitt & Hocking, Citation2009). Finally, four major potentially toxigenic pathogenic fungi (Aspergillus niger, Aspergillus flavus, Fusarium graminearum and Fusarium verticillioides), which are frequently associated with maize grains were used as test fungi in the current study.
2.5. Testing inhibitory effects of EOs on potentially toxigenic fungi (in vitro test)
The selected fungal species were regenerated from fresh PDA plates and incubated for seven days at 25 °C to determine the inhibitory effects of the EO. The inhibitory effects of EOs on the radial mycelium growth of the selected fungi were tested using the poisoned food method on PDA plates (Balouiri et al., Citation2016). Each EO was added to a molten medium at three concentrations: 0.5 μL/mL, 1 μL/mL and 1.5 μL/mL and sterile distilled water was used as a negative control. PDA amended with the various concentrations was poured separately into 90 mm diameter petri plates aseptically. Finally, from each of the grown fungal species, 5 mm disk of fungal cultures was transferred to the center of the Petri dishes. Each treatment combination (i.e., different concentrations of EOs and fungal species) was repeated three times, and the plates were arranged in a completely randomized design. The radial growth of the fungal isolates was measured at 24 h interval, and antifungal activity of the treatments was determined using the following formula (Balouiri et al., Citation2016).
Where:DC- Diameter of control test
DS- Diameter of the treated test
2.6. Testing antifungal activities of selected EOs on maize kernels (in vivo test)
To test the effects of EOs on maize grain, A. flavus was selected as it is a very common and highly toxigenic fungus. Three different doses of EOs (5, 10 and 15 µL/plate) were applied to maize kernels artificially inoculated with A. flavus. Initially, normal-looking undamaged maize kernels were sterilized and dried in an oven at 55 °C for 30 min. The kernels were then inoculated by suspending them at 106 a kernel weight of for five minutes. Four inoculated maize kernels were then placed in each Petri plate (35 mm diameter) on sterilized tissue paper. EOs from C. martinii, T. vulgaris, and C, sativum were used at rates of 5, 10 and 15 µL/plate as treatments. The smaller plates were kept inside larger petri plates (90 mm diameter) on sterilized tissue paper moistened with 10 mL of double-distilled sterilized water to maintain humidity, which was then covered with parafilm. Plates were incubated for 15 days at 28 °C. All treatments were repeated three times. After the incubation period, inoculated maize kernels were washed with sterile water and spore production was measured as spore/g of maize grain with hemocytometry (Xiang et al., Citation2020)
2.7. Identification of chemical components of tested EOs
The chemical components of the EOs from the tested plants were identified based on their retention times (Rt) under the same temperature-programmed conditions. The EOs were co-injected with authentic standards. Compounds were identified by comparing their RTs and mass spectra with those of the National Institute of Standards and Technology (NIST) and WELIY, and by comparing the given mass spectral data with literature data (Adams, Citation2007). The relative amounts of each component of EO and its fractions were expressed as a percentage of the peak area relative to the total peak area.
2.8. Data analysis
All experiments were repeated three times, and the experimental data were analyzed using Microsoft Excel. Results are expressed as mean ± standard error of colony growth diameter and number of spores grown on maize kernel to compare the inhibition rate of each EOs concentration.
3. Results and discussion
3.1. EOs composition
The chemical composition of the EOs from the three selected plants, C. martini, T. vulgaris and C. sativum, revealed the presence of different compounds (; ). Twenty-five different compounds were identified from T. vulgaris, another 25 from C. martini and 32 compounds from C. sativum (Appendix ). From these, depending on relative percentage obtained from the peak area of the GC-MS, selected compounds with output greater than 1% were presented in .
Table 2. Chemical compositions of the EOs from selected aromatic plants.
Table 3. Proportion of volatile compound classes in T. vulgaris, C. martini and C. sativum EOs.
The major components of T. vulgaris EO were thymol (34.4%), o-cymene (17.5%), and gamma-terpinene (16.8%) (). This finding was largely in agreement with the results of Hudaib et al. (Citation2002), except for o-cymene. A higher amount of o-cymene was detected in the present study than that reported by Al-Asmari et al. (Citation2017), who reported low levels of o-cymene in Saudi Arabia. Previous studies have shown T. vulgaris EO composition depends on numerous factors, such as the environment, altitude and cultivation practices (Hudaib and Aburjai, Citation2008). In this study the identified volatile compounds in T. vulgaris EO were grouped into seven different chemical classes i.e. (40%) Monoterpene, (20%) oxygenated monoterpene, (4%) aldehayde, (4%) esters, (12%) sesquiterpenes, (8%) oxygenated sesquiterpenes and (12%) others (). Among these, monoterpenes represent the main class of compounds, accounting for (60%), followed by sesquiterpenes (20%). As suggested by Zieli (Citation2020) monoterpenes and their derivatives are key ingredients for biological active compounds.
Coriander sativum EOs’ dominant compounds were hexanedioic acid, bis(2-ethylhexyl) ester (46.9%), 2-Decenal,(E)- (12.6%), and linalool (8.3%) (). These results are largely in agreement with Dharmalingam and Nazni (Citation2013), except for hexanedioic acid, bis (2-ethylhexyl) ester, which was too high compared to other investigations. In this study, EOs were extracted from C. sativum leaves during the flowering stage. Bhuiyan et al. (Citation2009) from Bangladesh and Dharmalingam and Nazni (Citation2013) from India also identified hexanedioic acid, bis (2-ethylhexyl) ester, from Coriander sativum EO at the flowering stage. In addition to that, as Mandal and Mandal (Citation2015) reported, C. sativum EO extracted from different parts have different chemical compositions. In the current study, the most dominant component of C. sativum was hexanedioic acid, bis (2-ethylhexyl) ester, which is a plasticizer derivative (Ghisari & Bonefeld-Jorgensen, Citation2009). However, it has been reported from various EOs from leaves of Adenophyllum porophyllum (Hernández-Ceja et al., Citation2021), flower of Bergenia ciliata (Ferdosi et al., Citation2021), leaves and fruits of Chrysophyllum albidum (Nartey et al., Citation2021). Antifungal activity of hexanedioic acid, bis(2-ethylhexyl) ester against Fusarium species Elleuch et al. (Citation2010) and Colletotrichum gloeosporioides Hernández-Ceja et al. (Citation2021) were reported previously. The major volatile compound class of C. sativum EO were Monoterpenes (31.3%), aldehyde (31.3%) and others (25%) (). As Yang et al. (Citation2023) suggested, the presence of aldehyde in EOs increases antifungal activities.
The major component of the EO from C. martinii was geraniol (51.4%), followed by geranyl acetate (14.5%) and trans-ß-ocimene (11.7%) (), which was more or less in line with the reports of Daba et al. (Citation2022) and Dangol et al. (Citation2023). As Chen and Viljoen (Citation2010a) suggested, geraniol is the responsible component of C. martinii EO for the antifungal properties. The volatile compound class of C.martinii EO were oxygenated monoterpene (20%), monoterpene (28%), sesquiterpenes (20%), oxygenated sesquiterpenes (8%) and esters (16%) (). The dominant class of compounds were monoterpenes with 48% and sesquiterpenes (28%).
In the current study, the three EOs have different chemical compositions that were categorized under seven different chemical classes, i.e. monoterpene, oxygenated monoterpene, aldehyde, esters, sesquiterpenes, oxygenated sesquiterpenes and others (Appendix ). Among them monoterpenes were the dominant class in the three EOs i.e. T. vulgaris (60%), C. martini (48%) and C. sativum (31.3%). Aldehydes accounted for (31.3%) of the EOs in C. sativum. As confirmed by several studies, the antifungal properties of Eos were linked to the functionality of their oil components, terpenes/terpenoids (monoterpenes and sesquiterpenes) (Abdi-Moghadam et al., Citation2023), which are known for disturbing the cell membrane, causing cell death, inhibiting sporulation and germination (Nazzaro et al., Citation2017). In this study, high percentage of monoterpenes and sesquiterpenes were detected in T. vulgaris and C. martini, which were responsible for their antifungal properties.
Table 4. Colony growth diameter (mean ± SE) of potentially toxigenic fungi treated with three different doses of EOs after five days of incubation.
The EOs composition of aromatic plants can be significantly affected by cultivar and location (Isa et al., Citation2006; Satyal et al., Citation2016); planting time, planting conditions, plant parts harvested, harvesting time, and extraction methods (Wei et al., Citation2019); plant growth stage and the environment (Chang et al., Citation2009; Nurzynska-Wierdak, Citation2013). Seasonal changes (Santos-Gomes & Fernandes-Ferreira, Citation2001), genotypes (Badr et al., Citation2021), plant parts harvested (Satyal & Setzer, Citation2020), plant (Farias et al., Citation2023) and other exogenous and endogenous factors (Barra, Citation2009) are also known to affect their chemical composition. These factors might have contributed to variations in the EOs composition of the plants tested in the current study, as compared to some previous reports.
3.2. Effects of EOs on toxigenic fungi
EOs extracted from the three plants tested in the current experiment inhibited the mycelial growth of potentially toxigenic fungi at different degrees compared to the control (). However, only T. vulgaris EOs inhibited the growth of all the tested toxigenic fungi at all doses. Alonso-Gato et al. (Citation2021) and Arraiza et al. (Citation2018) reported that EOs from T. vulgaris and C. martini were effective in reducing the growth of selected toxigenic and plant pathogenic fungi. As reported by Oliveira et al. (Citation2020), Thymes EOs completely inhibited A.flavus and aflatoxin production. Composite EOs of cinnamon, oregano and lemongrass were also effective against A.flavus and decreased aflatoxin production on maize (Xiang et al., Citation2020). Cinnamaldehyde and citral EO inhibited A.flavus growth and reduce aflatoxin production in broth and poultry feed (Han et al., Citation2022). T. vulgaris was found to have strong antifungal activities against the mycelial growth of phytopathogenic fungi in previous studies too (Cosic et al., Citation2010; Mohammadi et al., Citation2015). As several study results show, several EOs including those from T. vulgaris had promising results in controlling growth of A.flavusand aflatoxin production.
The relatively lower efficacy of C. martini EOs, especially against A. flavus, is in agreement with the results of Gemeda et al. (Citation2014). However, C. martini EOs inhibited the growth of A. niger, F. graminearum and F. verticillioide completely at all doses. As the previous result showed, C. martini EOs inhibited the growth of F. graminearum and reduce their mycotoxin production (Perczak et al., Citation2019).
EOs from T. vulgaris completely inhibited mycelial growth of the four fungal species tested in the current experiment. On the other hand, EOs from the other two plants, C. sativum and C. martini had slight differences in their efficacy against A. flavus growth depending on their concentration (with higher concentrations being more effective). Dene et al. (Citation2023) and Kačániová et al. (Citation2020) also reported the strong antifungal activity of C. sativum against Botrytis ceneria and Penicillium expansum. In the current study also C. sativum inhibited the growth of A. niger, F. graminearum and F. verticillioides completely.
A. flavus was the fungus most resistant to EOs at all tested concentrations. However, it was highly sensitive to T. vulgaris EOs at all applied doses. A. niger, F. graminearum and F. verticillioides were highly sensitive to the EOs of all three plants at all doses. In this study, EOs that were richer in monoterpenes compounds were more effective against all the tested fungi than others. Results remained the same even though T. vulgaris had more monoterpenes compounds (60%) than C. sativum and C. martini. As confirmed by Drioiche et al. (Citation2022), antimicrobial activities of Thymus EOs were associated with phenolic and terpene compounds. As suggested by Hu et al. (Citation2017) the antimicrobial properties of EO mainly depend on their chemical constituents and the quantity of their major single compounds. Thus, in current study, the major chemical constituents of the three EOs were thymol, hexanedioic acid, bis (2-ethylhexyl) ester and geraniol compounds were more likely to be responsible for inhibition of fungi growth. As the chemical compounds of these EOs vary, so do their antifungal activities.
3.3. Effects of EOs on A. flavus in-vivo assay
The efficacy of EOs from T. vulgaris, C. sativum and C. martini against A. flavus was tested by using maize kernels as substrates. Compared to the negative control, EOs from all the test plants markedly reduced the spore production of the fungus at a rate of 5,10 and 15 µL/plate (). Furthermore, T. vulgaris completely inhibited fungal spore production at all the tested concentrations. EOs from the same plant also caused complete inhibition of fungal growth in vitro regardless of dose and fungal species ( and ). This may be due to the high concentration of terpene compounds in T. vulgaris. In contrast, oils from C. sativum and C. martini had the same effect only at the highest concentration. These results are comparable to those reported by Wang et al. (Citation2019) and Xiang et al. (Citation2020).
Table 5. Spores number (mean ± SE) of A. flavus on maize grains treated with three doses of EOs after 15 days.
4. Conclusion
The present study confirmed the presence of various compounds and volatile compound classes in EOs extracted from three aromatic plants (T. vulgaris, C. sativum and C. martini) cultivated in Ethiopia. Monoterpenes are the major components of volatile compounds classes of the three EOs.The EOs from these plants were dominated by thymol, hexanedioic acid, bis (2-ethylhexyl) ester, and geraniol compounds, suggesting the antifungal potential of these chemicals against potentially toxigenic fungal species, such as A. flavus, A. niger. F. graminearum and F. verticillioides.
Although the EOs of all the tested plants showed very high efficacy in inhibiting the growth and spore production of the tested fungi, oils from T. vulgar were the most effective. In addition, oils from the same plant caused total suppression of spore production by A. flavus in maize grains, and the results remained the same across doses.
These findings confirmed the role of EOs from the three plants may play in controlling toxigenic fungi. Thus, oils from these plants may provide attractive alternatives to synthetic chemicals. However, future studies aiming to evaluate the efficacy of the pure compounds on maize kernel and mycotoxin production are needed to further confirm the results.
Authors contribution
Birhane Atnafu contributed to the design of the study, data collecting and analysis, and writing the draft manuscript. Alemayehu Chala, Chemeda Abedeta and Fikre Lemessa contributed to the study design, data analysis and review of the draft manuscript. Abdi Mohammed and Safa Oufensou were involved in the critical review and editing of the draft manuscript. All the authors have read and approved the manuscript.
Availability of data and material
The data sets used in this manuscript are fully available upon request from the corresponding author.
Acknowledgement
The authors are grateful to Jimma University College of Agriculture and Veterinary Medicine for providing the necessary support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abdi-Moghadam, Z., Mazaheri, Y., Rezagholizade-Shirvan, A., Mahmoudzadeh, M., Sarafraz, M., Mohtashami, M., Shokri, S., Ghasemi, A., Nickfar, F., Darroudi, M., Hossieni, H., Hadian, Z., Shamloo, E., & Rezaei, Z. (2023). The significance of essential oils and their antifungal properties in the food industry: A systematic review. Heliyon, 9(11), 1. https://doi.org/10.1016/j.heliyon.2023.e21386
- Adams, R. P. (2007). Identification of essential oil components by gas chromatography/mass spectrometry. 4th edn. Allured Publishing Corporation.
- Al-Asmari, A., Athar, M., Al-Faraidy, A., & Almuhaiza, M. (2017). Chemical composition of essential oil of Thymus vulgaris collected from Saudi Arabian market. Asian Pacific Journal of Tropical Biomedicine, 7(2), 147–11. https://doi.org/10.1016/j.apjtb.2016.11.023
- Alonso-Gato, M., Astray, G., Mejuto, J., & Simal-Gandara, J. (2021). Essential oils as antimicrobials in crop protection. Antibiotics (Basel, Switzerland), 10(1), 34. https://doi.org/10.3390/antibiotics10010034
- Anjorin, T. S., Salako, E. A., & Makun, H. A. (2013). Control of toxigenic fungi and mycotoxins with phytochemicals : Potentials andchallenges. In mycotoxin and food safety in Developing Countries. InTech. https://doi.org/10.5772/53477
- Arraiza, M., Gonzalez-Coloma, A., Andres, M., Berrocal-Lobo, M., Dominguez-Nunez, J. A., Jr, Navarro-Rocha, J., & Calderon-Guerrero, C. (2018). Antifungal effect of essential oils. Potential of Essential Oils, 146–164 https://doi.org/10.5772/intechopen.78008
- Ayalew, A. (2010). Mycotoxins and surface and internal fungi of maize from Ethiopia. African Journal of Food, Agriculture, Nutrition and Development, 10(9), 10.4314/ajfand.v10i9.62890.
- Badr, A., El-Shazly, H., Sakr, M., Farid, M., Hamouda, M., Elkhateeb, E., & Ahmad, H. S. (2021). Genetic diversity and volatile oil components variation in Achillea fragrantissima wild accessions and their regenerated genotypes. Journal of Genetic Engineering and Biotechnology, 19(1), 166. https://doi.org/10.1186/s43141-021-00267-3
- Balouiri, M., Sadiki, M., & Ibnsouda, S. (2016). Methods for in vitro evaluating antimicrobial activity : A review. Journal of Pharmaceutical Analysis, 6(2), 71–79. https://doi.org/10.1016/j.jpha.2015.11.005
- Barra, A. (2009). Factors affecting chemical variability of essential oils: A review of recent developments. Natural Product Communications, 4(8), 1934578X0900400. https://doi.org/10.1177/1934578X0900400827
- Benbelaid, F., Abdoune, M., Khadir, A., & Bendahou, M. (2013). Drying effect on yield and antimicrobial activity of essential oils. International Journal of Medicinal Aromatic Plants, 3(1), 93–101.
- Bhuiyan, M., Begum, J., & Sultana, M. (2009). Chemical composition of leaf and seed essential oil of Coriandrum sativum L. from Bangladesh. Bangladesh Journal of Pharmacology, 4(2), 150–153. https://doi.org/10.3329/bjp.v4i2.2800
- Chang, X., Alderson, P., & Wright, C. (2009). Enhanced UV-B radiation alters basil (Ocimum basilicum L.) growth and stimulates the synthesis of volatile oils. Journal of Horticulture and Forestry, 1(2), 27–31.
- Chang, Y., Harmon, P. F., Treadwell, D. D., Carrillo, D., Sarkhosh, A., & Brecht, J. K. (2022). Biocontrol potential of essential oils in organic horticulture systems : From farm to fork. Frointiners in Nutrition, 8, 1–26. https://doi.org/10.3389/fnut.2021.805138
- Chen, W., & Viljoen, A. (2010). Geraniol—a review of a commercially important fragrance material. South African Journal of Botany, 76(4), 643–651. https://doi.org/10.1016/j.sajb.2010.05.008
- Cosic, J., Vrandecic, K., Postic, J., Jurkovic, D., & Ravlic, M. (2010). In vitro antifungal activity of essential oils on growth of phytopathogenic fungi. Poljoprivreda, 16(2), 25–28.
- Daba, A., Tadesse, M., Habte, G., Negawo, A. T., & Berecha, G. (2022). Phytochemical composition of essential oils from aromatic plants inherited with bioherbicidal activity in arabica coffee production system of Ethiopia. Journal of Agriculture and Food Research, 10, 100368. https://doi.org/10.1016/j.jafr.2022.100368
- Dangol, S., Poudel, D. K., Ojha, P. K., Maharjan, S., Poudel, A., Satyal, R., Rokaya, A., Timsina, S., Dosoky, N. S., Satyal, P., & Setzer, W. N. (2023). Essential oil composition analysis of Cymbopogon species from Eastern Nepal by GC-MS and Chiral GC-MS, and antimicrobial activity of some major compounds. Molecules (Basel, Switzerland), 28(2), 543. https://doi.org/10.3390/molecules28020543
- Dene, L., Lauzike, K., Rasiukeviciute, N., Chrapaciene, S., Brazaityte, A., Virsile, A., Vastakaite-Kairiene, V., Miliauskiene, J., Sutuliene, R., Samuoliene, G., & Valiuskaite, A. (2023). Defense response of strawberry plants against Botrytis cinerea influenced by coriander extract and essential oil. Frontiers in Plant Science, 13, 1–10. https://doi.org/10.3389/fpls.2022.1098048
- Dharmalingam, R., & Nazni, P. (2013). Phytochemical evaluation of Coriandrum L flowers. International Journal of Food and Nutritional Sciences, 2(4), 2320–7876. http:/www.ijfans.com/currentissue.html
- Drioiche, A., Zahra Radi, F., Ailli, A., Bouzoubaa, A., Boutakiout, A., Mekdad, S., Al Kamaly, O., Saleh, A., Maouloua, M., Bousta, D., Sahpaz, S., El Makhoukhi, F., & Zair, T. (2022). Correlation between the chemical composition and the antimicrobial properties of seven samples of essential oils of endemic thymes in Morocco against multi-resistant bacteria and pathogenic fungi. Saudi Pharmaceutical Journal, 30(8), 1200–1214. https://doi.org/10.1016/j.jsps.2022.06.022
- Dubale, B., Solomon, A., Geremew, B., Sethumadhava, R., & Waktole, S, Jimma Agricultural Mechanization Research Center. (2014). Mycoflora of grain maize (Zea mays L) stored in traditional storage containers (Gombisa and sacks) in selected Woredas of Jima Zone, Ethiopia. African Journal of Food, Agriculture, Nutrition and Development, 14(62), 8676–8694. https://doi.org/10.18697/ajfand.62.11900
- Elleuch, L., Shaaban, M., Smaoui, S., Mellouli, L., Karray-Rebai, I., Fourati-Ben Fguira, L., Shaaban, K., & Laatsch, H. (2010). Bioactive secondary metabolites from a new terrestrial streptomyces sp. TN262. Applied Biochemistry and Biotechnology, 162(2), 579–593. https://doi.org/10.1007/s12010-009-8808-4
- Farias, J. P., Barros, A. L. A. N., de Araújo-Nobre, A. R., Sobrinho-Júnior, E. P. C., Alves, M. M. d M., Carvalho, F. A. d A., da Franca Rodrigues, K. A., de Andrade, I. M., Silva-Filho, F. A. e., Moreira, D. C., Lima, D. F., Lucarini, M., Durazzo, A., Arcanjo, D. D. R., & de Souza de Almeida Leite, J. R. (2023). Influence of plant age on chemical composition, antimicrobial activity and cytotoxicity of Varronia curassavica Jacq. essential oil produced on an industrial scale. Agriculture, 13(2), 373–386. https://doi.org/10.3390/agriculture13020373
- Ferdosi, M. F. H., Khan, I., Javaid, A., Saeed, H., Butt, I., & Munir, A. (2021). GC-MS analysis and bioactive components of flowers of Bergenia ciliata, a weed of rock crevices in Pakistan. Journal of Weed Science Research, 27(4), 527–535. https://doi.org/10.28941/pjwsr.v27i4.1012
- Garbaba, C., Diriba, S., Ocho, F., & Hensel, O. (2018). Potential for mycotoxin-producing fungal growth in various agro-ecological settings and maize storage systems in southwestern Ethiopia. Journal of Stored Products Research, 76, 22–29. https://doi.org/10.1016/j.jspr.2017.12.001
- Gemeda, N., Woldeamanuel, Y., Asrat, D., & Debella, A. (2014). Effect of Cymbopogon martinii, Foeniculum vulgare, and Trachyspermum ammi essential oils on the growth and mycotoxins production by Aspergillus species. International Journal of Food Science, 2014, 874135–874139. https://doi.org/10.1155/2014/874135
- Getachew, A., Chala, A., Hofgaard, I. S., Brurberg, M. B., Sulyok, M., & Tronsmo, A.-M. (2018). Multimycotoxin and fungal analysis of maize grains from south and southwestern Ethiopia. Food Additives & Contaminants. Part B, Surveillance, 11(1), 64–74. 10.1080/19393210.2017.140869829258380
- Ghisari, M., & Bonefeld-Jorgensen, E. C. (2009). Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicology Letters, 189(1), 67–77. https://doi.org/10.1016/j.toxlet.2009.05.004
- Gurjar, M. S., Ali, S., Akhtar, M., & Singh, K. S. (2012). Efficacy of plant extracts in plant disease management. Agricultural Sciences, 03(03), 425–433. https://doi.org/10.4236/as.2012.33050
- Han, B., Fu, G. W., & Wang, J. Q. (2022). Inhibition of essential oils on growth of Aspergillus flavus and aflatoxin B1 production in broth and poultry feed. Toxins, 14(10), 655. https://doi.org/10.3390/toxins14100655
- Hernández-Ceja, A., Loeza-Lara, P. D., Espinosa-García, F. J., García-Rodríguez, Y. M., Medina-Medrano, J. R., Gutiérrez-Hernández, G. F., & Ceja-Torres, L. F. (2021). In vitro antifungal activity of plant extracts on pathogenic fungi of blueberry (Vaccinium sp.). Plants (Basel, Switzerland), 10(5), 852–864. https://doi.org/10.3390/plants10050852
- Hu, Y., Zhang, J., Kong, W., Zhao, G., & Yang, M. (2017). Mechanisms of antifungal and anti-aflatoxigenic properties of essential oil derived from turmeric (Curcuma longa L.) on Aspergillus flavus. Food Chemistry, 220, 1–8. https://doi.org/10.1016/j.foodchem.2016.09.179
- Hudaib, M., & Aburjai, T. (2008). Volatile components of Thymus vulgaris L. from wild‐growing and cultivated plants in Jordan. Flavour and Fragrance Journal, 22(4), 322–327. 10.1002/ffj.1800
- Hudaib, M., Speroni, E., Di Pietra, A., & Cavrini, V. (2002). GC/MS Evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. Journal of Pharmaceutical and Biomedical Analysis, 29(4), 691–700. https://doi.org/10.1016/S0731-7085(02)00119-X
- Isa, T., Ozlem, G., & Nermin, S. (2006). Yield, essential oil content and composition of coriandrum sativum varieties (var. Vulgare alef and var. Microcarpum dc.) grown in two different locations. Journal of Essential Oil Research, 18(2), 189–193. https://doi.org/10.1080/10412905.2006.9699063
- Kačániová, M., Galovičová, L., Ivanišová, E., Vukovic, N. L., Štefániková, J., Valková, V., Borotová, P., Žiarovská, J., Terentjeva, M., Felšöciová, S., & Tvrdá, E. (2020). Antioxidant, antimicrobial and antibiofilm activity of coriander (Coriandrum sativum L.) essential oil for its application in foods. Foods (Basel, Switzerland), 9(3), 282. https://doi.org/10.3390/foods9030282
- Kesraoui, S., Andrés, M. F., Berrocal-Lobo, M., Soudani, S., & Gonzalez-Coloma, A. (2022). Crop protection. Plants, 11(16), 2144. https://doi.org/10.3390/plants11162144
- Klich, M. (2002). Identification of Common Aspergillus Species. Centraalbureau voor Schimmelcultures.
- Leslie, J., & Summerell, B. (2006). The Fusarium Laboratory Manual. Blackwell Publishing. https://doi.org/10.1002/9780470278376
- Liu, Z., Zhang, G., Zhang, Y., Jin, Q., Zhao, J., & Li, J. (2016). Factors controlling mycotoxin contamination in maize and food in the Hebei province, China. Agronomy for Sustainable Development, 36(2), 1–10. https://doi.org/10.1007/s13593-016-0374-x
- Mandal, S., & Mandal, M. (2015). Coriander (Coriandrum sativum L.) essential oil: Chemistry and biological activity. Asian Pacific Journal of Tropical Biomedicine, 5(6), 421–428. https://doi.org/10.1016/j.apjtb.2015.04.001
- Marín, S., Magan, N., Abellana, M., Canela, R., Ramos, A. J., & Sanchis, V. (2000). Selective effect of propionates and water activity on maize mycoflora and impact on fumonisin B1 accumulation. Journal of Stored Products Research, 36(2), 203–214. https://doi.org/10.1016/S0022-474X(99)00043-0
- Moges, A., & Moges, Y. (2019). Ethiopian common medicinal plants : Their parts and uses in traditional medicine. Ecology and Quality Control. https://doi.org/10.5772/intechopen.86202
- Mohammadi, A., Hashemi, H., & Seyed, M. (2015). Comparison of antifungal activities of various essential oils on the Phytophthora drechsleri, the causal agent of fruit decay. Iranian Journal of Microbiology, 7(1), 31–37.
- Nartey, D., Gyesi, J. N., & Borquaye, L. S. (2021). Chemical composition and biological activities of the essential oils of Chrysophyllum albidum G. Don (African Star Apple). Biochemistry Research International, 2021, 9911713–9911711. https://doi.org/10.1155/2021/9911713
- Nazzaro, F., Fratianni, F., Coppola, R., & De Feo, V. (2017). Essential oils and antifungal activity. Pharmaceuticals (Basel, Switzerland), 10(4), 86. https://doi.org/10.3390/ph10040086
- Nigussie, D., Davey, G., Beyenee, T., Beyene, B., Malcolm, L., Belete, A., Fekadu, A., & Makonnen, E. (2021). Antibacterial and antifungal activities of Ethiopian medicinal plants : a systematic review. Frontiers in Pharmacology, 12, 633921. https://doi.org/10.3389/fphar.2021.633921
- Nurzynska-Wierdak, R. (2013). Essential oil composition of the coriander (Coriandrum sativum L.) herb depending on the development stage. Acta Agrobotanica, 66(1), 53–60. https://doi.org/10.5586/aa.2013.006
- Oliveira, R. C., Carvajal-Moreno, M., Correa, B., & Rojo-Callejas, F. (2020). Cellular, physiological and molecular approaches to investigate the antifungal and anti-aflatoxigenic effects of thyme essential oil on Aspergillus flavus. Food Chemistry, 315, 126096. https://doi.org/10.1016/j.foodchem.2019.126096
- Perczak, A., Gwiazdowska, D., Marchwińska, K., Juś, K., Gwiazdowski, R., & Waśkiewicz, A. (2019). Antifungal activity of selected essential oils against Fusarium culmorum and F. graminearum and their secondary metabolites in wheat seeds. Archives of Microbiology, 201(8), 1085–1097. https://doi.org/10.1007/s00203-019-01673-5
- Phuc, N., Thy, L., Lam, T., Yen, V., & Lan, N. (2019). Extraction of jasmine essential oil by hydrodistillation method and applications on formulation of natural facial cleansers. IOP Conference Series: Materials Science and Engineering, 542(1), 012057. https://doi.org/10.1088/1757-899X/542/1/012057
- Pitt, J., & Hocking, A. (2009). Fungi and food spoilage. (3rd ed.). Springer. https://doi.org/10.1007/978-0-387-92207-2
- Santos-Gomes, P., & Fernandes-Ferreira, M. (2001). Organ-and season-dependent variation in the essential oil composition of Salvia officinalis L. cultivated at two different sites. Journal of Agricultural and Food Chemistry, 49(6), 2908–2916. https://doi.org/10.1021/jf001102b
- Satyal, P., Murray, B., McFeeters, R., & Setzer, W. (2016). Essential oil characterization of Thymus vulgaris from various geographical locations. Foods (Basel, Switzerland), 5(4), 70. https://doi.org/10.3390/foods5040070
- Satyal, P., & Setzer, W. (2020). Chemical compositions of commercial essential oils from Coriandrum sativum fruits and aerial parts. Natural Product Communications, 15(7), 1934578X2093306. https://doi.org/10.1177/1934578X20933067
- Swamy, M. K., Akhtar, M. S., & Sinniah, U. R. (2016). Antimicrobial properties of plant essential oils against human pathogens and their mode of action : An updated review. Evidence-Based Complementary and Alternative Medicine, 2016, 3012462–3012421. https://doi.org/10.1155/2016/3012462
- USDA (United States Department of Agriculture). (2022). World agricultural production. Global Market Anlysis. Circular Series, WAP, 7-23, 59–65. https://doi.org/10.32317/2221-1055.201907059
- Wang, L., Liu, B., Jin, J., Ma, L., Dai, X., Pan, L., Liu, Y., Zhao, Y., & Xing, F. (2019). The complex essential oils highly control the toxigenic fungal microbiome and major mycotoxins during storage of maize. Frontiers in Microbiology, 10, 1643–1656. https://doi.org/10.3389/fmicb.2019.01643
- Wei, J., Liu, Z., Zhao, Y., Zhao, L., Xue, T., & Lan, Q. (2019). Phytochemical and bioactive profile of Coriandrum sativum L. Food Chemistry, 286, 260–267. https://doi.org/10.1016/j.foodchem.2019.01.171
- Xiang, F., Zhao, Q., Zhao, K., Pei, H., & Tao, F. (2020). The efficacy of composite essential oils against Aflatoxigenic fungus Aspergillus flavus in Maize. Toxins, 12(9), 562. https://doi.org/10.3390/toxins12090562
- Yang, S., He, M., Li, D., Shi, J., Peng, L., & Jinjing, L. (2023). Antifungal activity of 40 plant essential oil components against Diaporthe fusicola from postharvest kiwifruits and their possible action mode. Industrial Crops and Products, 194, 116102. https://doi.org/10.1016/j.indcrop.2022.116102
- Yilma, S., Sadessa, K., & Kebede, D. (2019). Fungal infections and aflatoxin contamination in maize grains collected from west showa and east wallega zones, ethiopia. International Journal of Current Research and Review, 11(21), 16–22. https://doi.org/10.31782/IJCRR.2019.11213
- Zieli, M. (2020). Monoterpenes and their derivatives—Recent development in biological and medical applications. International Journal of Molecular Sciences, 21, 7078. https://doi.org/10.3390/ijms21197078
Appendix
Figure 1. GC profiles of essential oil from Thymus vulgaris (a), Coriandrum sativum (b) and Cymbopogon martini (c).
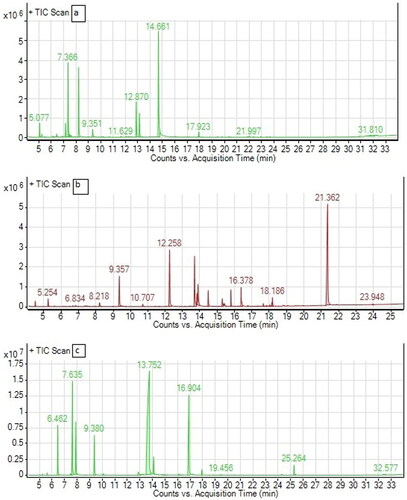
Table A1. Chemical Composition of T. vulgaris essential oil.
Table A2. Chemical composition of C. martini essential oil.
Table A3. Chemical composition of C. sativum essential oil.
Table A4. Volatile compound class of T. vulgaris, C.martini and C.sativum.

