?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Stigma maydis (SM) possesses remarkable nutritional value due to the presence of health-protective chemical constituents in it. Despite its commercial uses, there is a lack of information on changes in the nutritional profile of SM during Shenke 601 waxy corn development by following four different maturity stages (silking stage; blister stage; milky stage; dough stage). In this study, the ideal times for the development of seven active compounds (polysaccharides, saponin, rutin, luteolin, quercetin, kaempferol and chlorogenic acid) were at the silking stage. The total flavonoid and total phenol content increased as the SM matured. Except for the above-mentioned active compounds, the content of major nutritional components of dough stage was higher than that of three other stages. The extract of Stigma maydis exhibited good α-amylase, α-glucosidase and pancreatic lipase inhibition activities. The ethanolic SM extracts showed better inhibitory effects than aqueous SM extracts with a dose–effect relationship. The hypoglycemic activity of ethanolic extract was higher than that of aqueous extract at each stage. This provided an important basis for the application of SM.
Highlights
Polysaccharides decreased during the maturation of the Stigma maydis (SM).
The total phenolic and total flavonoid contents of SM increased as SM gradually matured.
SM extract exhibited good hypoglycemic activity.
Ethanolic SM extracts showed better hpyerglycemic effects with a dose-effect relationship.
REVIEWING EDITOR:
1. Introduction
Stigma maydis (Zea mays subsp. mays L., SM) is elongated stigmas from the female flowers of maize, available in abundance from a waste product of corn (Singh et al., Citation2022a). It was a well-known functional food and herbal medicine in China, American and many parts of the world derives from SM contains many bioactive compounds such as vitamins, potassium, calcium, sodium salts, volatiles oils, magnesium, proteins, carbohydrates and alkaloids, steroids, flavonoids and other phenolic compounds with beneficial effects on human heal (Sarepoua et al., Citation2015; Singh et al., Citation2023a). The bioactivities of SM constituents were widely reported in the literatures, including treating nephritis, gout, prostatitis, urinary, edema, diuretic, cystitis infections, hyperglycemia reduction, neurological, obesity and cardiovascular disorders (Chaiittianan et al., Citation2016; Gulati et al., Citation2023; Guo et al., Citation2019; Kaur et al., Citation2023; Sabiu et al., Citation2016; Singh et al., Citation2023b; Wang et al., Citation2016).
Attributable to different functional effects of Stigma maydis, it has been widely applied in medicines and healthy foods in many other countries, including American, Turkey and France (Hasanudin et al., Citation2012). According to the State Intellectual Property Office of China announcement, more than 500 types of functional foods and pharmaceutical formulations related to SM have been patented (CNIPA). Stigma maydis can be utilized commercially as an ingredient to produce a wide variety of healthy products such corn silk beverage, corn silk tea and corn silk tablet. Liquid and powders of SM extracts were mainly form to apply into the currently related products on the general e-commerce sited (Amazon. ca) (Zhang et al., Citation2020b).
The phytochemicals of SM are mainly affected by many factors such as regional characteristics, climatic conditions, cultivation condition, raw material variety and maturity (Singh et al., Citation2022b; Znidarcic, Citation2012). The ripening process of corn is roughly divided into four stages as follows (). The silking stage, the blister stage, the milky stage and the dough stage. A recent study showed that Stigma maydis of dough stage corn had the highest content of total flavonoid, total phenolic and total anthocyanin (Sarepoua et al., Citation2013).
Figure 1. Four maturity stages of corn. (a) Silking stage; (b) blister stage; (c) milky stage; (d) dough stage.

In this study, nutrition content (moisture, carbohydrate, protein, fat and ash), minerals, saponin, flavone (rutin, luteolin, quercetin, apigenin, kaempferol and L-epicatechin) and polyphenol (gallic acid, vanillic acid, caffeic acid and chlorogenic acid) of Stigma maydis were analyzed. Furthermore the hypoglycemic properties of aqueous and ethanol extracts were also compared in different maturity stages.
2. Material and methods
2.1. Stigma maydis sample collection
Four maturity stages of Shenke 601 waxy corn (the silking stage, organ differentiation and morphogenesis were mainly carried out during silking stage; the blister stage, corn filaments darken and begin to dry out, and the kernels are white and blistered with clear, transparent liquid, when the kernels are up to 85% water; the milky stage, fast grouting with a linear increase in dry weight; and the dough stage, the rate of grain filling was decreased and dry weight gain was slow) were picked at Zhuanghang experiment station of Shanghai academy of agricultural sciences, Shanghai, China (30°53’27’’N, 121°22’47’’E) in the rainy season during the middle of June and July (). Baby corn which was harvested after anthesis 9–11 days at the silking stage. Blister stage was harvested after silking stage for 10–13 days. The corn was harvested at the physiological maturity stage (the milky stage, 7–9 days after blister stage) for seed production, and the corn of the dough stage was harvested after milky stage for 8–10 days. The maturity stages of SM are characterized in .
Table 1. Characteristics of different maturity stages of SM.
The husks of four maturity stages corns were eliminated and silks were detached from its fruit. The samples of fresh SM were washed with distilled water, and then the samples were put into the -80 °C refrigerator for 30 min, and then put into the freeze-dryer (Henan Brother Instrument & Equipment Co., China) for freeze-drying until the moisture of the samples was 10%. Dry samples of four maturity stages were smashed into powder and stored in a glass desiccator for further bioactive materials and hypoglycemic activity analysis.
2.2. Chemicals and reagents
The standards such as L-epicatechin, rutin, luteolin, quercetin, apigenin, kaempferol, gallic acid, perchloric acid, oleanolic acid, trans-ferulic acid, 4-hydroxybenzoic acid, hydrocinnamic acid, trans-cinnamic acid, vanillic acid, Vanillin, p-hydroxycinnamic Acid, benzoic acid, protocatechualdehyde, 3,4-dihydroxybenzoic acid, caffeic acid, syringic acid, 4-hydroxy-3,5-dimethoxycinnamic acid, salicylic acid, neochlorogenic acid, cryptochlorogenic acid, chlorogenic acid, isochlorogenic acid C, isochlorogenic acid A, iIsochlorogenic acid B were purchased from Sigma-Aldrich Chemical Co. Ltd. α-amylase (porcine pancreas, 12 μ/mg) and α-glucosidase (Yeast, 25.7 μ/mg) were obtained from Shanghai yuanye Bio-Technology Co., Ltd. Lipase (porcine pancreas, 15–35 μ/mg) was purchased from Aladdin Bio-Chem Technology Co., LTD. All the other reagents and chemicals used were of analytical grade from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.3. Determination of moisture, crude fat, protein and ash contents
Moisture was measured by direct drying method, protein was determined by Kjeldahl nitrogen determination, and ash was determined by combustion in a muffle furnace and then weighed. Crude fat was extracted using the Automatic Soxhlet apparatus (SOXTEC 8000, Shanghai Scientific Instruments and Materials Co., Ltd., China).
2.4. Determination of mineral content
Samples (10–50 mg) were digested by HNO3 (1:20, w/w), as described by Wang et al. (Citation2015). Minerals were determined using ICP-OES (PerkinElmer Avio200, MA, USA).
2.5. Determination of polysaccharide content
Smashed samples powder (10 g) were defatted with ethanol (80%) overnight and extracted twice with distilled water (1:20, w/v) at 80 °C, 2 h for each time. After centrifugation, the supernatant then was collected and diluted to 500 mL in a volumetric flask to obtain the final solution in order to determine the polysaccharide content by phenol-sulfuric method (Dubois et al., Citation1956).
2.6. Determination of total flavone and flavonoid contents
Total flavones content was analyzed using a modified colorimetric aluminum chloride method with rutin hydrate as the standard by a previously described protocol by Sarepoua et al. (Citation2015). Briefly, A quantity of test solution (50 μL) was taken and 950 μL of corn silk extract diluted in methanol and 0.3 mL of 0.01 M aluminum chloride were added and left at RT for 10 min. The absorbance of the resulting mixture was read at 400 nm using a multiscan spectrum of ThermoFisher (Multiskan GO, USA). The total flavones content was determined by using rutin calibration curve (0.1-1 mg/mL concentrations in methanol).
The content of flavonoids analysis was conducted according to the most used method derived from Jaegle et al. (Citation2016). Briefly, extract 50 mg of pulverized SM powder with 2 mL of 100% methanol at 65 °C for 4 h. The mixture was centrifuged at 12000 rpm for 10 min to obtain a supernatant. The extract was then analyzed using UPLC-MS (AcQuity, Waters, USA), equipped with a HSS T3 capillary column (50 mm × 2.1 mm, 1.8 μm film thickness, Waters, USA) at 40 °C. The individual peaks of the flavonoid were identified by their characteristic GC retention times. The quantitative analysis of flavonoids was carried out using the triple quadrupole mass spectrometer equipped with electrospray ionization.
2.7. Determination of total phenolic contents
The total phenolic content (TPC) of extracts was determined using the Folin–Ciocalteu colorimetric method and gallic acid was used as a standard (Sarepoua et al., Citation2015). A quantity of sample (0.2 g) was mixed with 2 ml of ethanol and extracted under ultrasonic treatment at 60 °C for 30 minutes. The mixture was centrifuged at 12000 rpm for 10 min and obtained the supernatant. Corn silk extract (0.25 mL) was mixed with 0.25 mL of Folin-Ciocalteu reagent and 1 mL of 0.2% (w/v) Na2CO3 solution and left at 25 °C for 1 h, then measured at 760 nm. A standard calibration curve (concentrations between 0.16 and 0.0025 mg/mL) was used to determine the TPC.
2.8. Determination of phenolic acid contents
The identification and quantification of gallic acid, vanillic acid, chlorogenic acid and caffeic acid were analyzed according to methods with slight modifications and performed using UPLC-MS (AcQuity, Waters, USA) (Kilci & Gocmen, Citation2014). Both samples and standards were pre-filtered through a 0.45 µm nylon filter prior to analysis. A HSST3 capillary column (100 mm × 2.1 mm, 1.8 µm, Waters, USA) was used for the separation. The injection volume for samples and standards was 2 μL. The mobile phase for separation was as follows: 0.1% formic acid and water (Mobile Phase A), formic acid (0.1%) and acetonitrile (Mobile Phase B). The flow rate of the solvents was 0.3 mL/min using the following gradient elution: 0 min, 10% B; 2 min, 10% B; 6 min 60% B; 8 min 60% B; 10 min 10% B. The quantification of gallic acid, vanillic acid, chlorogenic acid and caffeic acid contents were calculated from the peak area recorded by the external standard method using calibration curves (R2 = 0.9995, 0.9993, 0.9999 and 0.9992, respectively).
2.9. Determination of total saponin contents
A quantity of sample (0.2 g) was extracted in 5 ml of 80% aqueous ethanol at 70 °C for 3 hours. After standing for several minutes, 0.125 mL supernatant was subsequently concentrated to a dry powder. 0.05 mL 7% vanillin-glacial acetic acid (w/v) and 0.2 mL ClHO4 were added and heated for 20 min under 70 °C, and then mixed with 1.25 mL glacial acetic acid. The absorbance of the resulting solution was read at 540 nm using a spectrophotometer and oleanolic acid was used as a standard.
2.10. Hypoglycemic activity
2.10.1. Sample preparations
Four maturity stages (silking stage; blister stage; milky stage; dough stage) of corn silks powders were equally assigned to each of the ethanolic or aqueous groups and placebo. The extraction was carried out according to previous studies (Andrade et al., Citation2021). For aqueous extraction by infusion, each maturity stages of corn silks powders (380 g) were extract with 7600 mL distilled water (1:20, w/v) at 60 °C for 60 min, named RW1, RW2, RW3 and RW4, respectively. For ethanolic extraction, sample (380 g) was extracted with 7600 mL of ethanol: water (1:20, w/v) for 60 min, RE1, RE2, RE3 and RE4 were named, respectively. The extracts were centrifuged at 12.000 rpm for 10 min using a centrifuge. The supernatants were collected, followed by filtering through Whatman No.1 filter paper (Wang et al., Citation2015). After concentration, the obtained extracts were lyophilized, respectively (). Then, the dried aqueous and ethanolic extracts were redissolved in water. Various concentrations of extracts were further diluted for the following hypoglycemic activity evaluations.
Figure 2. Four maturity stages of Stigma maydis extracts (a, aqueous extract of silking stage; b, aqueous extract of blister stage; c, aqueous extract of milky stage; d, aqueous extract of dough stage; e, ethanolic extract of silking stage; f ethanolic extract of blister stage; g, ethanolic extract of milky stage; h, ethanolic extract of dough stage).
2.10.2. RW and RE on ɑ-amylase, ɑ-glucosidase and pancreatic lipase inhibitory activity
The ɑ-amylase inhibitory activity assay was performed as previously reported (Guo et al., Citation2018). Briefly, polysaccharides solution (0.2 mL) was added to porcine pancreas ɑ-amylase solution (0.1 mL, 2.0 U/mL) and incubated at 37 °C. After 10 min, starch solution (0.3 mL, 1%) was mixed and incubated at 37 °C for 15 min. Then the reaction was stopped with 0.2 mL DNS reagent and heated for 10 min. After the mixture solution cooled, the absorbance of the reaction mixture was measured at 540 nm with acarbose as the positive control. The percentage inhibition of ɑ-amylase (AA) was calculated as follows:
(1)
(1)
where Acontrol, the absorbance of buffer; Acontrolblank, the absorbance of buffer in the absence of ɑ-amylase; Asample, the absorbance of the sample; Ablank, the absorbance of the sample in the absence of ɑ-amylase.
The ɑ-glucosidase inhibitory activity was measured by the method of Yuan et al. (Citation2018) with some modifications. Sample (50 μL) was added to 100 µL ɑ- glucosidase solution and incubated at 37 °C for 15 min. Then p-NPG (50 µL, 5 mM) was added to each mixtures and incubated for another 20 min. The absorbance of the reaction mixture was measured at 405 nm. Acarbose was used as the positive control. The ɑ-glucosidase inhibitory activity (AG) was expressed as inhibition rate was calculated as follows:
(2)
(2)
where Acontrol, the absorbance of buffer; Acontrolblank, the absorbance of buffer in the absence of ɑ- glucosidase; Asample, the absorbance of the sample; Ablank, the absorbance of the sample in the absence of ɑ-glucosidase.
The pancreatic lipase inhibitory activity of SM extracts was determined using the method of (Worsztynowicz et al., Citation2014; Zhang et al., Citation2020a), with minor modification. In the assay p-nitrophonol palmitate (p-NPP) as the substrate was used, which was hydrolyzed to p-nitrophonol (p-NP) by lipase. Tryptic lipase solution (10 mg, 1 mg/mL) was added to 10 mL of assay buffer (150 mM NaCl, pH 8.0, 1.3 mM CaCl2, and 13 mM Tris–HCl) and centrifuged for 10 min at 4000 rpm at 25 °C, then the supernatant was collected. Different concentration of samples (50 µL) was mixed with enzyme (50 µL) to incubate in 96 well plates at 37°Cfor 20 min, followed by the addition of 2 mg/mL p-NPB (50 µL) to initiate the enzyme reaction at 37 °C. The absorbance readings were thereafter taken at 405 nm using a micro-plate reader after 20 min. The pancreatic lipase inhibitory activity (PL) was expressed as % inhibition using the following expression:
(3)
(3)
where Acontrol was the absorbance of buffer, Acontrolblank was the absorbance of buffer in the absence of pancreatic lipase, Asample was defined as the absorbance of the sample, and Ablank was defined as the absorbance of the sample in the absence of pancreatic lipase.
2.11. Statistical analysis
The analytical data are reported as mean ± standard deviation of at least three independent extractions. SPSS software (version 22.0) was used for statistical analysis. The significance of differences between samples was analyzed by the Duncan test. Differences with p < 0.05 were considered significant.
3. Results and discussion
3.1. The contents of moisture, fat, protein, ash and polysaccharides
The contents of moisture, fat, protein, ash and polysaccharides in four maturity stages of Stigma maydis are presented in . There were statistical significant differences in terms of moisture, fat, protein, ash and polysaccharides within four maturity stages, with blister stage had a longer length (33.30 cm) and higher ash content (4.66%). With the growth of SM, the contents of moisture, polysaccharide and crude fat decreased, the content of crude protein increased. Firstly, the rise in protein and crude fat content during Stigma maydis growth was caused by a variety of reasons, including anabolic activity of the maize itself, fat storage, nutrient uptake and absorption, hormonal regulation, and regulation of gene expression (Zhou et al., Citation2020). Polysaccharide is one of the most abundant components of Stigma maydis with multiple pharmacological effects, such as antifatigue, antihepatoma, antiobesity, anticancer, anti-diabetic activities, hypoglycemic and neuroprotective effects (Zhang et al., Citation2020b). As shown in , the contents of polysaccharides were 8.17% in silking stage, which may be attributed to the carbohydrate conversion during the growth of SM, 4 times more than the amount of dough stage. The decrease in polysaccharide content in Stigma maydis was attributed to consumption by Stigma maydis respiration, and supplying the growth requirements of pollen tubes (Lu et al., Citation2023). On the other hand, the decline in water content during Stigma maydis growth was attributed to a combination of factors including transpiration, reduced water uptake by the root system, evapotranspiration dissipation and growth requirements.
Table 2. Length and moisture, fat, protein, ash and polysaccharides contents of Stigma maydis.Table Footnotea.
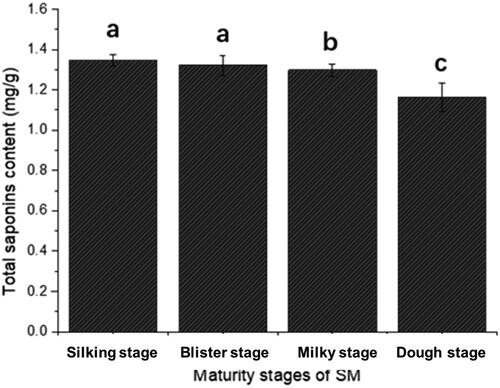
3.2. The contents of saponin
Saponin is another important bioactive compound in SM due to its multi-bioactivities and minimal potential toxicity. As shown in , the saponin content of silking stage and blister stage was 1.347 and 1.321 mg/g, respectively, also significantly higher than that of R4. The decrease in saponin content during Stigma maydis growth may be attributable to the production of gibberellins during the growth process of Stigma maydis. Gibberellins may play a regulatory role in the synthesis and accumulation of saponins in the process involved in the regulation of plant growth and metabolism (Adhikary & Dasgupta, Citation2023).
3.3. Minerals
The profiles of five macroelements and eighteen trace minerals of four maturity stages Stigma maydis are presented in . Except for Al, As, Bi, Ni, Pb, Sb and V, the other minerals content of dough stage were significantly higher than the other three maturity stages.
Table 3. The minerals content of Stigma maydis.Table Footnotea
The minerals detected can be classified into four groups based on their relationship with human health. Therefore, K, P, Ca, Mg and Na (macroelements), Fe, Zn, Cu, Co, Sr, Se, Cr and Mo (essential trace elements), Mn, Ni, Ba and V (possible trace elements), Bi, Sb, Ti, Al, As and Pb (potential toxicity elements) were analyzed (Wang et al., Citation2015). As for macroelements, K and P contents were significantly higher in dough stage. For three essential trace elements, Cu, Sr and Se, in dough stage were significantly higher than those in blister stage and milky stage. Zn, an important essential trace mineral possessing multifunctional in the human body metabolic process, was much higher in dough stage. The content of possible essential element (Mn) was almost doubled with the growth of SM. The contents of potential toxicity elements (Bi, Sb and Ti) in milky stage, blister stage and dough stage were obviously higher, respectively. Al, As and Pb contents in all four samples cannot be detected. In addition, it was found that some minerals increased as the growth period lengthened. Minerals undergo synthesis and accumulation processes in plants. For example, plants synthesize and accumulate calcium by absorbing and translocating calcium ions so that they function as structural support in cell walls, cell membranes, and organelles. Similarly, plants accumulate other minerals such as iron and magnesium. Thus, as the cornhusk grows, the synthesis and accumulation processes may lead to an increase in mineral content (Kaur et al., Citation2021b).
3.4. Total flavone and flavonoid contents
The maturity stage was the key factor that influenced the composition of the functional component of the Stigma maydis. As shown in , the total flavonoids reached a maximum at 2.444 mg/g when SM at the dough stage, which was significantly higher than the other three maturity stages. Flavonoids, as a large group of secondary metabolites in plants, are able to protect against certain harsh ecological environments as well as damage caused by animals and microorganisms (Vidal-Gutiérrez et al., Citation2020). In order to avoid damage from certain harsh environments (e.g., stimulation by bright light), maize plants enhanced the activity of several key enzymes for flavonoid synthesis (e.g., cinnamic acid-4-hydroxylase (CA4H), phenylalanine ammonia-lyase (PAL), and ligase of 4-coumaric acid CoA (4CL)) in the unpollinated period, and therefore increased their flavonoid synthesis to prevent UV damage to the plant (Li et al., Citation2021a).
Table 4. The total flavone and flavonoid contents of Stigma maydis.
According to thin-layer chromatography results, flavonoids from SM were mainly contained luteolin and apigenin, while the results from ultraviolet scanning showed that the flavonoids of Stigma maydis were mainly flavones and isoflavones (Li et al., Citation2021b). Flavonoids (rutin, luteolin, quercetin, apigenin, kaempferol, epicatechin) contents varied significantly among maturity stages (). The amounts of the major flavonoids were determined by standard curve quantitative analysis. In traditional healthcare, silk is mainly used from corn at the milky stage to dough stage when it takes brown color (Žilić et al., Citation2016). However, this study was carried out to determine flavonoids compounds content in re-sprouting, fresh, mature and overmature corn silk, as well as to determine the maturity stage in which Stigma maydis can be most efficiently used to as tea or food ingredient rich in bioactive compounds. According to our results, Stigma maydis at silking stage are much more suitable for use as a source of flavonoids compounds than silks at the milky stage and dough stage. As shown in , rutin, luteolin, quercetin, apigenin, kaempferol and epicatechin were found in the highest amounts of 0.113, 4.595, 3.119, 1.785, 1.902 and 1.044 μg/100 g in silking stage, silking stage, silking stage, dough stage, silking stage and dough stage, respectively. Similar variations in flavonoid content have been documented with silking stage or dough stage SM having as much as 2–4 fold higher content of flavonoids than milky stage, dough stage or silking stage Stigma maydis.
3.5. Total phenolic and phenolic acid contents
Total phenolic content ranging from 1.044 to 1.714 mg/g of dry samples was observed among four maturity stages from silking stage to dough stage (). The increase in total phenolic content at the silking stage—milky stage stages was associated with biochemical changes occurring at different growth stages and differences in plant responses to stress. Changes in the biosynthetic pathways or phenolic compound partitioning of Stigma maydis during the growth stages resulted in changes in phenolic content (Farhadi et al., Citation2020). During silk aging (milky stage and dough stage), phenolic compounds undergo enzymatic oxidation resulting in the production of quinones which condense with themselves or proteins to produce brown-colored complexes (Sundar et al., Citation2023). For total phenolic content, milky stage had the highest total phenolic content (1.714 mg/g), followed by blister stage (1.686 mg/g), dough stage (1.346 mg/g) and physiological silking stage (1.044 mg/g), respectively ().
Table 5. The total phenolic and phenolic acid contents of Stigma maydis.
The silk of different corn maturity stages greatly in composition and content of its total phenolic, phenolic acids (gallic acid, vanillic acid, caffeic acid and chlorogenic acid) of silking stage, blister stage, milky stage and dough stage corn silk is shown in . As shown in result, chlorogenic acid was major phenolic acid found in silking stage corn silk followed by vanillic acid, caffeic acid and gallic acid. Chlorogenic acids (CAs) exhibit many biological properties and have been associated with a series of health benefits. The breakdown of chlorogenic acid is very important to the flavor and color of corn silk tea (Corrigan et al., Citation2023). By comparison, the content of vanillic acid increased sharply from 184.889 μg/100 g in silking stage to 1428.82 μg/100 g in dough stage. Vanillic acid (VA) is an aromatic phenolic acid, which is a slightly yellow crystal or powder in appearance with a molecular weight of 168.14 g/mol (Hu et al., Citation2021). Due to broad-spectrum pharmacological actions of vanillic acid (inflammatory diseases, metabolic diseases, bone loss, diabetes mellitus, and neurodegenerative disease treatments), VA gained the attention of research scientists (Kaur et al., Citation2021a). The content of gallic acid in silk at the dough stage was higher by 82.7% in relation to that in fresh silk. In contrast, caffeic acid was more stable.
3.6. Hypoglycemic activity
Diabetes is a kind of metabolic disease that result in high blood sugar levels over a prolonged period. Prolonged hyperglycemia in diabetic patients triggers diabetic complications, such as cardiovascular and atherosclerosis disease (Kaewnarin et al., Citation2016; Wang et al., Citation2018). Moreover, type 2 diabetes is normally attributed to numerous unhealthy lifestyle, such as physical inactivity, smoking, obesity and poor diet, and have to managed by taking drugs to prevent or delay the absorption of glucose from meals (Boath et al., Citation2012). The key digestive enzymes (α-amylase and α-glucosidase) feature in the breakdown of carbohydrates into glucose before their consequent uptake into the bloodstream. Nowadays, many therapeutical drugs of the enzymes inhibition have been developed, such as acarbose, voglibose and miglitol. However, they have been shown to pose adverse side-effects, such as flatulence, diarrhea and abdominal discomfit, which reduce treatment effectiveness and patient compliance (Pantidos et al., Citation2014).
The extraction conditions were the key factors that influenced the composition of the Stigma maydis extract using the hypoglycemic activity as the evaluation criterion. shows the yields of aqueous and ethanolic extracts with four SM maturity stages (silking stage, blister stage, milky stage and dough stage). The highest amount of SM extracts from silking stage, with a value of 13.14% for ethanol extract and 7.39% for the aqueous extract. This was much higher than those obtained for the other three maturity stages. In comparison with four maturity stages SM extracted by water, yields of ethanolic extracts almost more than twice for each maturity stage. This might be due to the more polar solvent system they used, it is reported that extraction yield positively correlated to solvent polarity when extracting bioactive compounds from ingredients (Yuan et al., Citation2018).
Table 6. The yields of SM aqueous and ethanolic extracts.
The inhibitory effects of ethanolic and aqueous extracts from four maturity stages SM on α-amylase activity are shown in . It is indicated that the inhibition rate of α-amylase was dependent on the concentration of extracts, and there was a dose-effect relationship. Stigma maydis of all ethanolic extracts exhibited better inhibitory α-amylase activity than aqueous extracts. It can be seen that ethanolic extracts from SM (milky stage) showed 92.03 ± 1.07% inhibitory α-amylase activity at 10 mg/mL, followed by extracts from blister stage (91.68 ± 0.60%), silking stage (89.74 ± 1.41%) and dough stage (55.35 ± 1.40%), while extracts by water showed weaker inhibitory effects of α-amylase for each maturity stage at 10 mg/mL. Moreover, there were no inhibitory effects when the concentration of aqueous extracts from 1 to 4 mg/mL in milky stage and dough stage.
Figure 4. The inhibition ratio of extract isolated from Stigma maydis on (a) α-amylase, aqueous extract, (b) α-amylase, alcohol extract, (c) α-glucosidase, aqueous extract, (d) α-glucosidase, alcohol extract, (e) pancreatic lipase, aqueous extract, (f) pancreatic lipase, alcohol extract with four maturity stages.
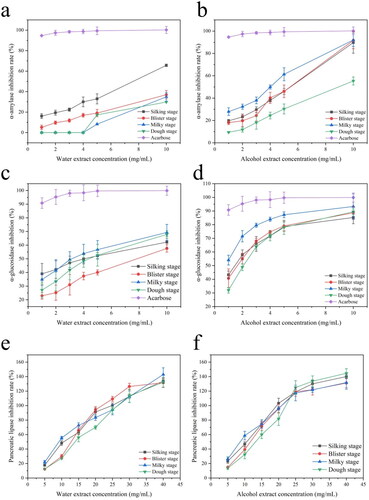
displays the inhibitory effects of aqueous and ethanolic extracts from four maturity stages of Stigma maydis on the α-glucosidase activity. The trend line illustrated that the inhibitory activities against α-glucosidase were enhanced with the concentration of extracts. The highest inhibitory performance was achieved by the extract of Stigma maydis, with a value of 93.29 ± 0.44% observed for ethanolic extract of the milky stage and 69.33 ± 0.88% for the aqueous extract of the milky stage. And this was much higher than the other three maturation stages obtained. Compared with positive control acarbose which showed an IC50 value of 0.13 mg/mL, the SM extract showed less inhibition of α-glucosidase. Thus, there is a need for further separation and purification of the crude extracts. On the other hand, Studies have shown that the solubility of total flavonoids is higher in ethanol than in aqueous (Tian et al., Citation2018). Therefore, the flavonoid content of the ethanol extract was higher than that of the aqueous extract. And according to , the flavonoid content of Stigma maydis increased with the increase in maturation time. Flavonoids themselves had activities capable of hypoglycemic activity (Wang et al., Citation2022). Therefore, the strong inhibitory activity of ethanolic extracts against α-amylase and glucosidase was related to the flavonoid content.
Obesity is a key risk factor for certain metabolic syndromes, for example, diabetes, cardiovascular disease, hypertension and so on (Zhang et al., Citation2018). Inhibition of lipase activity has been recognized as an effective treatment for obesity. In addition, one of the most recent research hotspots is the investigation of natural ingredients that fight obesity. Pancreatic lipase is a critical enzyme, which responsible for digestion and absorption of 50–70% dietary triacylglycerol (TG) in the intestinal lumen of human (Patil et al., Citation2017). The inhibitory effects of aqueous and ethanolic extracts from four maturity stages of Stigma maydis on pancreatic lipase activity are shown in . At four maturity stages, the inhibition rate of 25 mg/ml ethanol extract exceeded 100% and the Stigma maydis showed the highest inhibitory activity. In comparison with the positive control orlistat, a commercial pancreatic lipase inhibitor which showed an IC50 value of 0.58 mg/mL, the inhibition of aqueous and ethanolic SM extracts was relatively less effective. The IC50 of four maturity stages SM on inhibition of pancreatic lipase activity were 8.24 mg/mL, 9.51 mg/mL, 11.03 mg/mL and 12.46 mg/mL in milky stage, silking stage, blister stage and dough stage ethanolic extracts, respectively, which revealed that milky stage of SM had better inhibitory activity on pancreatic lipase than three others. Furthermore, the inhibition effect of aqueous extracts on pancreatic lipase competed well in a dose-related manner with a higher dosage (15 mg/mL) exhibiting the potent effect (more than 50% inhibition ratio).
4. Conclusion
A series of active ingredients changes occur in the composition of Stigma maydis during the Shenke 601 waxy corn maturation process, which affects its nutritional value. Although the content of most compounds showed similar variations, there were some differences in the maximum content of different active compounds with respect to Stigma maydis growth. As Stigma maydis grew, polysaccharide and crude fat content decreased, crude protein and ash content increased, saponin content decreased significantly, and total phenolic content and total flavonoid content increased. Eight different active compounds (polysaccharides, crude fat, saponins, rutin, lignans, quercetin, kaempferol and chlorogenic acid) developed ideally during the silking stage. Except for the mentioned active compounds, most the other compounds were higher in the dough stage. In addition, Stigma maydis extracts showed good inhibition of α-amylase, α-glucosidase and pancreatic lipase, especially the ethanol extract of SM showed better inhibition than the aqueous extract and there was a dose effect relationship. As a nutritious food and functional ingredient, Stigma maydis is a natural resource that provides a theoretical basis for the utilization of corn processing by-products.
Disclosure Statement
The authors declare that there are no conflicts of interest.
Additional information
Funding
Notes on contributors
Yi Zhang
Yi Zhang Doctor, Associate Researcher of Shanghai Academy of Agricultural Sciences, with research interests in agricultural product processing.
Jinxin Pang
Jinxin Pang He is a master’s degree student at Shanghai Academy of Agricultural Sciences (SAAS), with research interests in the processing of agricultural products and the study of specialty functional factors.
Lianmou Yao
Lianmou Yao Master’s degree, Shanghai Academy of Agricultural Sciences, with research interests in agricultural product processing.
Chunfang Wang
Chunfang Wang Doctor, assistant researcher of Shanghai Academy of Agricultural Sciences, research interests in agricultural products preservation and processing.
Bingjie Chen
Bingjie Chen Doctor, Assistant Researcher, Shanghai Academy of Agricultural Sciences, research interests in agricultural products processing.
Qi Zheng
Qi Zheng Doctor, Assistant Researcher, Shanghai Shuneng Irradiation Technology Co., Ltd, engaged in research, promotion and development services in the preservation and processing of agricultural products.
Yingxiong Hu
Yingxiong Hu Doctor, Associate Researcher, Shanghai Academy of Agricultural Sciences, engaged in research related to specialty corn.
Yongjin Qiao
Yongjin Qiao Doctor, researcher, director of shanghai agricultural products preservation and processing engineering technology research center, mainly engaged in the research of agricultural products preservation and processing.
References
- Adhikary, S., & Dasgupta, N. (2023). Role of secondary metabolites in plant homeostasis during biotic stress. Biocatalysis and Agricultural Biotechnology, 50, 1. https://doi.org/10.1016/j.bcab.2023.102712
- Andrade, J. K. S., Barros, R. G. C., Rezende, Y. R. R. S., Nogueira, J. P., de Oliveira, C. S., Gualberto, N. C., & Narain, N. (2021). Evaluation of bioactive compounds, phytochemicals profile and antioxidant potential of the aqueous and ethanolic extracts of some traditional fruit tree leaves used in Brazilian folk medicine. Food Research International (Ottawa, Ont.), 143, 110282. https://doi.org/10.1016/j.foodres.2021.110282
- Boath, A. S., Stewart, D., & McDougall, G. J. (2012). Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chemistry, 135(3), 929–14. https://doi.org/10.1016/j.foodchem.2012.06.065
- Chaiittianan, R., Chayopas, P., Rattanathongkom, A., Tippayawat, P., & Sutthanut, K. (2016). Anti-obesity potential of corn silks: Relationships of phytochemicals and antioxidation, anti-pre-adipocyte proliferation, anti-adipogenesis, and lipolysis induction. Journal of Functional Foods, 23, 497–510. https://doi.org/10.1016/j.jff.2016.03.010
- CNIPA. State Intellectual Property Office of The People’s Republic of China. China patent announcement. http://epub.sipo.gov.cn/index.action
- Corrigan, H., Dunne, A., Purcell, N., Guo, Y., Wang, K., Xuan, H., & Granato, D. (2023). Conceptual functional-by-design optimisation of the antioxidant capacity of trans-resveratrol, quercetin, and chlorogenic acid: Application in a functional tea. Food Chemistry, 428, 136764. https://doi.org/10.1016/j.foodchem.2023.136764
- Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. J. A. C. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356. https://doi.org/10.1021/ac60111a017
- Farhadi, N., Babaei, K., Farsaraei, S., Moghaddam, M., & Ghasemi Pirbalouti, A. (2020). Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Industrial Crops and Products, 152, 112570. https://doi.org/10.1016/j.indcrop.2020.112570
- Gulati, A., Singh, J., Rasane, P., Kaur, S., Kaur, J., & Nanda, V. (2023). Anti-cancerous effect of corn silk: a critical review on its mechanism of action and safety evaluation. 3 Biotech, 13(7), 246. https://doi.org/10.1007/s13205-023-03673-1
- Guo, Q., Chen, Z., Santhanam, R. K., Xu, L., Gao, X., Ma, Q., Xue, Z., & Chen, H. (2019). Hypoglycemic effects of polysaccharides from corn silk (Stigma maydis) and their beneficial roles via regulating the PI3K/Akt signaling pathway in L6 skeletal muscle myotubes. International Journal of Biological Macromolecules, 121, 981–988. https://doi.org/10.1016/j.ijbiomac.2018.10.100
- Guo, Q., Ma, Q., Xue, Z., Gao, X., & Chen, H. (2018). Studies on the binding characteristics of three polysaccharides with different molecular weight and flavonoids from corn silk (Stigma maydis). Carbohydrate Polymers, 198, 581–588. https://doi.org/10.1016/j.carbpol.2018.06.120
- Hasanudin, K., Hashim, P., & Mustafa, S. (2012). Corn Silk (Stigma maydis) in healthcare: A phytochemical and pharmacological review. Molecules (Basel, Switzerland), 17(8), 9697–9715. in: https://doi.org/10.3390/molecules17089697
- Hu, R., Wu, S., Li, B., Tan, J., Yan, J., Wang, Y., Tang, Z., Liu, M., Fu, C., Zhang, H., & He, J.-H. (2021). Dietary ferulic acid and vanillic acid on inflammation, gut barrier function and growth performance in lipopolysaccharide-challenged piglets. Animal Nutrition, 8(1), 144–152. https://doi.org/10.1016/j.aninu.2021.6.009
- Jaegle, B., Uroic, K., Holtkotte, X., Lucas, C., Termath, A., Schmalz, H., Bucher, M., Hoecker, U., Hülskamp, M., & Schrader, A. (2016). A fast and simple LC-MS-based characterization of the flavonoid biosynthesis pathway for few seed(ling)s. BMC Plant Biology, 16(1), 190. https://doi.org/10.1186/s12870-016-0880-7
- Kaewnarin, K., Suwannarach, N., Kumla, J., & Lumyong, S. (2016). Phenolic profile of various wild edible mushroom extracts from Thailand and their antioxidant properties, anti-tyrosinase and hyperglycaemic inhibitory activities. Journal of Functional Foods, 27, 352–364. https://doi.org/10.1016/j.jff.2016.09.008
- Kaur, J., Gulati, M., Gowthamarajan, K., Vishwas, S., Kumar Chellappan, D., Gupta, G., Dua, K., Pandey, N. K., Kumar, B., & Singh, S. K. J. M. h (2021a). Combination therapy of vanillic acid and oxaliplatin co-loaded in polysaccharide based functionalized polymeric micelles could offer effective treatment for colon cancer: A hypothesis. Medical Hypotheses, 156, 110679. https://doi.org/10.1016/j.mehy.2021.110679
- Kaur, P., Singh, J., Kaur, M., Rasane, P., Kaur, S., Kaur, J., Nanda, V., Mehta, C. M., & Sowdhanya, D. (2023). Corn silk as an agricultural waste: A comprehensive review on its nutritional composition and bioactive potential. Waste and Biomass Valorization, 14(5), 1413–1432. https://doi.org/10.1007/s12649-022-02016-0
- Kaur, N., Singh, B., Kaur, A., Yadav, M. P., Singh, N., Ahlawat, A. K., & Singh, A. M. (2021b). Effect of growing conditions on proximate, mineral, amino acid, phenolic composition and antioxidant properties of wheatgrass from different wheat (Triticum aestivum L.) varieties. Food Chemistry, 341(Pt 1), 128201. https://doi.org/10.1016/j.foodchem.2020.128201
- Kilci, A., & Gocmen, D. (2014). Phenolic acid composition, antioxidant activity and phenolic content of tarhana supplemented with oat flour. Food Chemistry, 151, 547–553. https://doi.org/10.1016/j.foodchem.2013.11.038
- Li, Y., Tan, B., Cen, Z., Fu, Y., Zhu, X., He, H., Kong, D., & Wu, H. (2021a). The variation in essential oils composition, phenolic acids and flavonoids is correlated with changes in antioxidant activity during Cinnamomum loureirii bark growth. Arabian Journal of Chemistry, 14(8), 103249. https://doi.org/10.1016/j.arabjc.2021.103249
- Li, Y., Zhao, C., Lu, C., Zhou, S., Tian, G., He, L., Bao, Y., Fauconnier, M. L., Xiao, H., & Zheng, J. (2021b). Simultaneous determination of 14 bioactive citrus flavonoids using thin-layer chromatography combined with surface enhanced Raman spectroscopy. Food Chemistry, 338, 128115. https://doi.org/10.1016/j.foodchem.2020.128115
- Lu, X., Fei, L., Li, Y., Du, J., Ma, W., Huang, H., & Wang, J. (2023). Effect of different plant growth regulators on callus and adventitious shoots induction, polysaccharides accumulation and antioxidant activity of Rhodiola dumulosa. Chinese Herbal Medicines, 15(2), 271–277. https://doi.org/10.1016/j.chmed.2022.07.005
- Pantidos, N., Boath, A., Lund, V., Conner, S., & McDougall, G. J. (2014). Phenolic-rich extracts from the edible seaweed, ascophyllum nodosum, inhibit α-amylase and α-glucosidase: Potential anti-hyperglycemic effects. Journal of Functional Foods, 10, 201–209. https://doi.org/10.1016/j.jff.2014.06.018
- Patil, M., Patil, R., Bhadane, B., Mohammad, S., & Maheshwari, V. (2017). Pancreatic lipase inhibitory activity of phenolic inhibitor from endophytic Diaporthe arengae. Biocatalysis and Agricultural Biotechnology, 10, 234–238. https://doi.org/10.1016/j.bcab.2017.03.013
- Sabiu, S., O’Neill, F. H., & Ashafa, A. O. T. (2016). Kinetics of α-amylase and α-glucosidase inhibitory potential of Zea mays Linnaeus (Poaceae), Stigma maydis aqueous extract: An in vitro assessment. Journal of Ethnopharmacology, 183, 1–8. https://doi.org/10.1016/j.jep.2016.02.024
- Sarepoua, E., Tangwongchai, R., Suriharn, B., & Lertrat, K. (2015). Influence of variety and harvest maturity on phytochemical content in corn silk. Food Chemistry, 169, 424–429. https://doi.org/10.1016/j.foodchem.2014.07.136
- Sarepoua, Tangwongchai, R., Suriharn, K., & Lertrat, K. (2013). Relationships between phytochemicals and antioxidant activity in corn silk. International Food Research Journal, 20, 2073–2079.
- Singh, J., Inbaraj, B. S., Kaur, S., Rasane, P., & Nanda, V. (2022a). Phytochemical Analysis and Characterization of Corn Silk (Zea mays, G5417) in. Agronomy, 12(4), 777. https://doi.org/10.3390/agronomy12040777
- Singh, J., Kaur, S., Rasane, P., Kumar, V., & Nanda, V. (2023a). Effect of particle size on physical, techno-functional and antioxidant properties of corn silk powder. International Journal of Food Science & Technology, 58(5), 2679–2685. https://doi.org/10.1111/ijfs.15988
- Singh, J., Rasane, P., Kaur, S., & Nanda, V. (2022b). Comparative analysis of antioxidant potential and techno-functional properties of selected corn silk varieties at different developmental stages. Journal of Food Measurement and Characterization, 16(4), 2685–2698. https://doi.org/10.1007/s11694-022-01382-6
- Singh, J., Rasane, P., Nanda, V., & Kaur, S. (2023b). Bioactive compounds of corn silk and their role in management of glycaemic response. Journal of Food Science and Technology, 60(6), 1695–1710. https://doi.org/10.1007/s13197-022-05442-z
- Sundar, S., Singh, B., & Kaur, A. (2023). Influence of hot-air and infra-red pretreatments on oxidative stability, physicochemical properties, phenolic and fatty acid profile of white and black chia seed (Salvia hispanica L.) oil. Journal of Food Composition and Analysis, 123, 105556. https://doi.org/10.1016/j.jfca.2023.105556
- Tian, X., Liu, Y., Feng, X., Khaskheli, A. A., Xiang, Y., & Huang, W. (2018). The effects of alcohol fermentation on the extraction of antioxidant compounds and flavonoids of pomelo peel. LWT, 89, 763–769. https://doi.org/10.1016/j.lwt.2017.11.049
- Vidal-Gutiérrez, M., Robles-Zepeda, R. E., Vilegas, W., Gonzalez-Aguilar, G. A., Torres-Moreno, H., & López-Romero, J. C. (2020). Phenolic composition and antioxidant activity of Bursera microphylla A. Gray. Industrial Crops and Products, 152, 112412. https://doi.org/10.1016/j.indcrop.2020.112412
- Wang, J., Kan, L., Nie, S., Chen, H., Cui, S. W., Phillips, A. O., Phillips, G. O., Li, Y., & Xie, M. (2015). A comparison of chemical composition, bioactive components and antioxidant activity of natural and cultured Cordyceps sinensis. LWT - Food Science and Technology, 63(1), 2–7. https://doi.org/10.1016/j.lwt.2015.03.109
- Wang, C., Li, W., Chen, Z., Gao, X., Yuan, G., Pan, Y., & Chen, H. (2018). Effects of simulated gastrointestinal digestion in vitro on the chemical properties, antioxidant activity, α-amylase and α-glucosidase inhibitory activity of polysaccharides from Inonotus obliquus. Food Research International (Ottawa, Ont.), 103, 280–288. https://doi.org/10.1016/j.foodres.2017.10.058
- Wang, C., Yin, Y., Cao, X., & Li, X. (2016). Effects of Stigma maydis polysaccharide on the intestinal microflora in type-2 diabetes. Pharmaceutical Biology, 54(12), 3086–3092. https://doi.org/10.1080/13880209.2016.1211153
- Wang, Y., Zhang, Y., Cheng, J., Zhao, J., Shi, R., He, L., Li, Q., & Chen, Y. (2022). Efficient purification of flavonoids from bamboo shoot residues of Phyllostachys edulis by macroporous resin and their hypoglycemic activity. Food Chemistry: X, 16, 100505. https://doi.org/10.1016/j.fochx.2022.100505
- Worsztynowicz, P., Napierała, M., Białas, W., Grajek, W., & Olkowicz, M. (2014). Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L.). Process Biochemistry, 49(9), 1457–1463. https://doi.org/10.1016/j.procbio.2014.06.002
- Yuan, Y., Zhang, J., Fan, J., Clark, J., Shen, P., Li, Y., & Zhang, C. (2018). Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Research International (Ottawa, Ont.), 113, 288–297. https://doi.org/10.1016/j.foodres.2018.07.021
- Zhang, C., Ma, Y., Gao, F., Zhao, Y., Cai, S., & Pang, M. (2018). The free, esterified, and insoluble-bound phenolic profiles of Rhus chinensis Mill. fruits and their pancreatic lipase inhibitory activities with molecular docking analysis. Journal of Functional Foods, 40, 729–735. https://doi.org/10.1016/j.jff.2017.12.019
- Zhang, Y., Wang, C., Liu, C., Wang, X., Chen, B., Yao, L., Qiao, Y., & Zheng, H. (2020b). Recent developments in Stigma maydis polysaccharides: Isolation, structural characteristics, biological activities and industrial application. International Journal of Biological Macromolecules, 150, 246–252. https://doi.org/10.1016/j.ijbiomac.2020.01.294
- Zhang, H. L., Wu, Q. X., Wei, X., & Qin, X. M. (2020a). Pancreatic lipase and cholesterol esterase inhibitory effect of Camellia nitidissima Chi flower extracts in vitro and in vivo. Food Bioscience, 37, 100682. https://doi.org/10.1016/j.fbio.2020.100682
- Zhou, W. Y., Lin, B., Hou, Z. L., Shi, S. C., Wang, Y. X., Huang, X. X., & Song, S. J. (2020). Isolation of macrocarpene-type sesquiterpenes from Stigma maydis with neuroprotective activities. Fitoterapia, 141, 104448. https://doi.org/10.1016/j.fitote.2019.104448
- Žilić, S., Janković, M., Basić, Z., Vančetović, J., & Maksimović, V. (2016). Antioxidant activity, phenolic profile, chlorophyll and mineral matter content of corn silk (Zea mays L): Comparison with medicinal herbs. Journal of Cereal Science, 69, 363–370. https://doi.org/10.1016/j.jcs.2016.05.003
- Znidarcic, D. (2012). Performance and characterization of five sweet corn cultivars as influenced by soil properties. Journal of Food, Agriculture and Environment, 10, 495–500.