?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Onion basal rot poses a serious threat to onion production in the Upper East Region of Ghana. A survey was conducted in 2022 in three purposefully sampled districts and a municipality in the region to determine the incidence and severity of the disease and to assess the perception and knowledge of farmers on the spread and control of the disease in the Region. In each district/municipality, three communities were purposefully selected for the study. A questionnaire was administered to 180 onion farmers randomly selected to assess the farmers’ perceptions, knowledge, and agronomic practices. Fifteen onion fields, from each district/municipality, were randomly selected for the assessment of the incidence and severity of the disease. Symptomatic onion plants were collected for pathogen isolation using potato dextrose agar. The causal organism was identified as Fusarium oxysporum f. sp. cepae based on morphological characteristics and confirmed by pathogenicity test. Under irrigated conditions, the incidence of onion bulb rot was highest in Bansi (28.92%) and lowest in Kuka (19.47%). The disease severity index, on the other hand, was highest and lowest at Yalugu (44.25%) and Bugri Corner (30.15%), respectively. The disease incidence was significantly higher in the rainy season than in the dry season.
REVIEWING EDITOR:
1. Introduction
Onion (Allium cepa L.) is an herbaceous biennial plant in the Liliaceae family and is mostly cultivated for its edible bulbs. The plant has a flattened disc stem at its base and upper tubular leaves that form a pseudo-stem. Although most of the common Allium species originated on the Asian continent, they are currently grown on large scales throughout the entire world (Marrelli et al., Citation2018). Globally, onion accounts for about 24% of the total vegetable production (Cramer et al., Citation2021). In the 2019 production year, about 5 192 651 hectares of land were cultivated with onions worldwide, and this translated into a production volume of 99 968 016 tonnes (FAO, Citation2020). On a country-by-country basis, China is the largest onion producer in the world with 24 966 366 tonnes production volume per annum, followed by India and the USA in second and third positions with quantities amounting to 22 819 000 tonnes and 3 170 270 tonnes, respectively.
Onion is among the main vegetable crops cultivated in Ghana. The country produces 143,982 tonnes of onion yearly and is placed 15th in Africa and 57th in the world (AtlasBig.com, Citation2018). With regard to the area of land cultivated, the onion is the third most important vegetable crop in Ghana, after tomatoes and chili pepper. Large-scale onion production is done in the Northern and Upper East Regions of Ghana, particularly around Bawku and Bolgatanga (Honger et al., Citation2015). In southern Ghana, commercial onion production is found in Fanteakwa and Kwahu in the Eastern Region, Mankessim in the Central Region, Akatsi in the Volta Region, Ashaiman in the Greater Accra Region and Berekum in the Bono Region. Onion production in Ghana has even been expanded to other parts of the country, such as the Western Region (Awuah et al., Citation2009). Onion production in Ghana is dominated by males due to the communal land tenure system practiced in most communities (Alidu, Citation2013) or due to the household responsibilities of women (Akrofi et al., Citation2016). The use of onions is as old as human existence and dates back over 4,000 years. It uses antedates written history (Necola, Citation2007). Onion is grown for its strong or mild flavours and constitutes an important component of most people’s meals. Onion is widely used as food, salad, medicine and for the enhancement of the flavour of food (Kankam et al., Citation2019; Shin et al., Citation2023).
Onions are easily infected by a wide range of fungal diseases. Some of these diseases include pink root, Fusarium basal rot, Stemphyllium leaf blight, purple blotch, Botrytis leaf blight, black mold and white rot (Cramer et al., Citation2021). Fusarium basal rot (FBR) poses a serious threat to onion cultivation all over the world (Shin et al., Citation2023). Pathogenic Fusarium oxysporum is documented to be among the most devastating plant pathogens in the world (Kalman et al., Citation2020). It is a known soil-borne pathogen and has a very high degree of host specificity, with more than 120 different formae speciales (Summerell et al., Citation2011). The economic damage to onion plants due to Fusarium oxysporum f. sp. cepae can be severe, with recorded yield losses reported to be more than 50% (Kalman et al., Citation2020). It produces three main asexual spores: macroconidia, microconidia, and chlamydospores (Cramer, Citation2000; Schwartz et al., Citation2007). Chlamydospores, a relatively thick-walled spore, are produced by the older mycelia and enable Fusarium oxysporum f. sp. cepae to persist in the soil for a very long time during unfavourable conditions (Galván et al., Citation2008).
Onion basal rot disease is a global canker. It has been reported to have caused serious devastation in onion fields worldwide (Dauda et al., Citation2018; Le et al., Citation2021). Globally, it is the second-most destructive soil-borne disease that affects the onion plant (Cramer, Citation2000). The disease can be caused by just one Fusarium species (Yağmur et al., Citation2024) or a complex of different Fusarium species (Ghanbarzadeh et al., Citation2014). This makes identification and management of the disease very complicated (Le et al., Citation2021). Le et al. (Citation2021) further opined that the composition of the Fusarium species complex responsible for onion basal rot disease seems to differ from one geographic region to another. For example, Awuah et al. (Citation2009) and Alidu (Citation2013) reported onion basal rot disease in Ghana to be caused by Fusarium oxysporum, f. sp. cepae, while Haapalainen et al. (Citation2016) and Shin et al. (Citation2023) found a complex of Fusarium species responsible for the disease in Finland and South Korea, respectively.
According to the Ministry of Food and Agriculture (MoFA) officials in the Upper East Region of Ghana, onion basal rot is ranked first among the diseases that affect crops in the Bawku traditional area, which is made up of five districts and a municipality. The MoFA staff and farmers in the area have limited knowledge about the pathogen that causes the disease and how it can be controlled. This resulted in huge losses, and some farmers abandoned onion production. Although onion bulb rot has a huge economic impact on onion farmers in Ghana, especially in the Bawku area, limited research has been conducted to determine the fungal genera or species that cause the disease and how to effectively manage it.
The objectives of this study were therefore aimed at isolating and identifying the causal agent of onion basal rot disease, investigate its incidence and severity as well as, assess the farmers’ agronomic practices that might have contributed to the prevalence of the disease.
2. Materials and methods
2.1. Ethical approval
Ethical approval was obtained from Department of Horticulture and Crop Production Research Ethics Committee (DHCP-ref 106) of the university of energy and natural resources and MoFA before starting the questionnaire administration for the study.
2.2. The study area
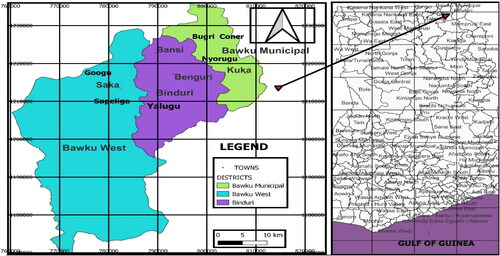
The study was conducted in the Bawku Municipality, the Bawku West District, and the Binduri District of Ghana, where onion is intensively cultivated ().
2.3. Informed consent
Consents of the respondents/participants were verbally agreed before the questionnaire was administered.
2.4. Farmers’ knowledge, perceptions and disease control practices
The locations were sampled based on the high prevalence of the disease. In each district or municipality, three onion-cultivating communities were chosen after consultations with the Ministry of Food and Agriculture’s Extension Agents (AEAs) for the administration of the questionnaire and field survey. A questionnaire comprising structured and unstructured questions was used to collect data on onion farmers’ perceptions and knowledge of the prevalence, mode of transmission, and management of onion basal rot. The questionnaires were administered in Benguri, Bansi, and Yalugu in the Binduri District. For the Bawku Municipality, it was administered in Bugri Corner, Nyorigu, and Kuka, while in the Bawku West District, it was administered at Googu, Saka, and Sapeliga. A total of 180 farmers, that is, 20 farmers from each of the nine (9) selected communities, were randomly selected for questionnaire administration (). The questionnaire was principally focused on the profile of the farmer, the farmer’s knowledge and perception about the causal agent of the disease, mode of transmission, control measures, as well as its negative impact on the farmers’ livelihoods.
Table 1. Distribution of the communities selected and number of farmers interviewed.
2.5. Incidence and severity of disease
A total of 45 onion farms, consisting of five farms from each of the nine communities across the two districts and the municipality, were randomly selected for the field survey on the incidence as well as the severity of the onion basal rot disease ().
The field survey was carried out in both the dry season and the rainy season. The dry season survey was conducted from January to March 2022, while the rainy season survey was from July to August 2022. GPS coordinates for all the communities sampled were determined and recorded. On each farm, a measuring tape, pegs, and a rope were used to mark out a 1 m × 1 m area in each of the four corners of the almost rectangular fields and in the center, and both healthy and diseased plants in the demarcated areas were counted and recorded. The incidence of onion basal rot suspected to be caused by Fusarium oxysporum on onion plants was assessed and expressed in percentages using the formula:
The severity of the disease on onion bulbs observed in the various study locations was calculated and expressed in percentages using Asare-Bediako et al.'s (Citation2015) modified formula and measured using a 5-point score ().
where S = severity score; N = number of infected plants having the same score; T = total number of plants observed; M = maximum severity score.
Table 2. Disease severity measurement using a 5-point score.
2.6. Pathogen isolation
Onion plants showing symptoms such as curling of leaves, yellowing with tip dieback, rotting of stem, bulb rot, and water-soaked appearance, as well as discoloration, were randomly selected together with their roots, soil, and debris and packed into brown envelopes and sent to the plant pathology laboratory of the CSIR-Savanna Agricultural Research Institute (SARI) for pathogen isolation. The isolation of the casual pathogen was done in accordance with the modified protocol described by Muntala et al. (Citation2020).
The causative organism was isolated from diseased plant samples in vitro. The diseased parts, together with the adjacent healthy portions, were cut into small pieces (about 3 mm) using a flamed scalpel and forceps. The cut segments were surface-sterilized by placing them in 70% ethanol for a minute, and then thoroughly rinsed thrice using sterile double-distilled water. The sterilized cut segments were carefully placed on sterile, solidified Potato Dextrose Agar (PDA) after they were dried on blotter paper. The PDA was amended with chloramphenicol, a broad-spectrum antibiotic, to prevent bacterial growth. The cut segments were placed at an equal distance and replicated three times in each Petri dish, wrapped in parafilm, and incubated at 28 ± 1 °C in the dark for seven days. After the fungus’s growth, the hyphal tip sub-culture technique was employed to develop monogenic cultures, which were later used for pathogen identification as well as pathogenicity testing.
2.7. Pathogen identification
The purified culture of the putative pathogen was prepared and assessed for morphological and cultural features using parameters such as growth rate, colour, shape, and size of mycelium and reproductive structures such as chlamydospores, macroconidia, and microconidia. Observation for the above morphological and cultural characteristics was done under a light microscope (Novex Holland) using both dry and wet mount methods. With the dry mount method, the fungal mycelium was picked with a transparent cellotape by gently placing the cellotape on the fungal outgrowth in the Petri dish. The cellotape containing the fungal mycelium was placed on the slide and then placed on the stage of the microscope for examination (40x). For the wet mount technique, a 5-microliter pipette was used to repeatedly pipette 5 ul of double-distilled water onto the fungal outgrowth in the Petri dish. This resulted in the release of fungal spores into the water in the pipette, which was then carefully dropped onto the microscope’s slide and placed on the stage of the microscope for observation. The pathogen was identified by comparing the features observed to those described in universally accepted fungi identification keys (Kalman et al., Citation2020; Mandal et al., Citation2018; Shamyuktha et al., Citation2020).
2.8. Onion bulb pathogenicity assay
To complete Koch’s postulate, the onion bulb pathogenicity test was carried out in a sterile environment within a laminar hood. For the pathogenicity test, the stored purified culture was re-cultured on a prepared potato dextrose agar and kept at 28 ± 2 °C for 3 days for sporulation. The outer scales of healthy onion bulbs were removed, and the bulbs were sterilized in 70% ethanol and blotted dry with tissue paper. Before inoculation of the onion bulbs, a flamed 5 mm diameter cork borer was used to create deep wells on the onion. Mycelial plugs were then excised from a 3-day-old culture of the isolate and used to inoculate the onion bulbs by filling up the wells with the mycelial plugs. The removed outer scales were then replaced, and each onion bulb was covered with tissue paper and wrapped with parafilm to maintain a relatively higher humidity and prevent unwanted contamination, as described by Kalman et al. (Citation2020). The control was set up using the same procedure, except that the bulbs were inoculated with sterilized PDA. The bulbs were kept in the dark at 28 ± 2 °C for 14 days to allow the inoculum to incubate. The experiment was replicated three times and observed regularly for symptoms of the disease. Inoculated onion bulbs were cut, and disease symptoms were assessed on both the outer and inner portions of the bulbs. The causative organism was re-isolated from symptomatic inoculated bulbs and identified to affirm Koch’s postulates.
2.9. Data analysis
The data from the administered questionnaire was analyzed with the Statistical Product and Service Solutions (SPSS) programme, version 26, and the results were presented in the form of tables and charts. The data on disease incidences and severity indices were first arcsine transformed before analysis of variance (ANOVA) with GenStat, 12th edition (VSN International). The treatment means were separated by the LSD method at a 5% level of probability. A student’s t-test was used to compare mean disease incidence and disease severity for the dry season and the rainy season.
3. Results
3.1. Background information of respondents
Results from the study revealed that the majority (73.3%) of the farmers were males, while 26.7% were females. On age, most (40.6%) of the respondents were within the age range of 40–49 years. This was followed by those within 30–39 years (28.9%), 50–59 years (15.6%), 20–29 years (8.9%), and 6.1% of them were aged 60 years and above. This shows that 93.9% of the farmers interviewed were in the age range of 20–59 years, implying that onion farming in the region is dominated by the youth and the middle-aged (). Furthermore, the majority (49.4%) of the farmers had no formal education, and only 8.9% of them had tertiary education. Farmers who had primary, JHS/middle school, and SHS education were 22.2%, 12.2%, and 7.2%, respectively ().
Table 3. Background information of respondents.
3.2. Farmers’ knowledge and perceptions about onion basal rot
Most (49.4%) of the respondents regarded onion basal rot as the most prevalent among the onion diseases in the study area. The others were damping off (26.7%), leaf twisting (11.1%), and leaf blight (8.3%). 4.4% of the respondents, however, considered other onion diseases such as pink root rot, downy mildew, and purple blotch, among others, as major setbacks to onion production (). The majority (21.7%) of the farmers are of the opinion that the disease is caused by too much water, whereas 19.4% and 18.3% attributed the cause of the disease to high temperatures and insect pests, respectively. Also, 15% of the farmers thought the disease was caused by soil organisms, and 20% admitted having no knowledge of the exact cause of the disease ().
Table 4. Farmers’ knowledge and perceptions about onion basal rot.
Most (41.7%) of the farmers opined that the transmission of the disease within farms and from farm to farm is through water (rain and irrigated water); 22.2% attributed it to insect pests; and 13.9% had no idea as to what is actually responsible for the transmission of onion basal rot. About 11.1% and 10.0% of the farmers opined that the disease is transmitted through planting materials (seeds and seedlings) and air, respectively, while 1.1% attributed it to other factors such as farm implements, weeds, and soil microbes (). The study also revealed that about 61.7% of the respondents thought the disease could not be controlled, while 38.3% believed it could be controlled using the right agrochemicals (). According to the majority of the farmers who said the disease could not be controlled, they have used different agrochemicals such as furadan, karate, ridomil gold, Sulphur 80, commando, magic powder, and so on in an attempt to control the disease, but these measures proved ineffective in eradicating the disease. Most of the farmers (65.0%) in the two districts and municipality were of the view that onion basal rot is prevalent in the rainy season, while 35.0% think the incidence of the disease is higher during the dry season ().
3.3. Onion farmers’ agronomic practices
Among the farmers interviewed, only 22.8% of the farmers in the study area used certified seeds, whiles 77.2% of them used farmer-saved seeds in their onion production (). Again, only 3.9% of them treat their onion seeds, and as high as 96.1% revealed they do not treat their seeds (). Some of the chemicals used by the farmers in treating their seeds include seedrex, seed power, lambda super 2.5 EC, magic powder, and wood ash. With regard to seedbed treatment, the results strongly suggested that most (68.9%) of the farmers do not treat their seedbeds, whiles 17.2% employ the burning of stubble (residues from the previous cropping season) and 13.9% treat their seedbeds with chemicals in order to prevent the buildup of the inoculum of disease-causing organisms (). Some of the chemical treatment practices were: broadcasting magic powder, wood ash, and Furadan on seedbeds prior to sowing or transplanting; spraying seedbeds with Ridomil Gold, and Lambda Super 2.5 EC.
Table 5. Onion farmers’ agronomic practices.
It is evident from the study that a very high percentage (93.9%) of the farmers were into monocropping, and only 6.1% practiced mixed cropping (). Also, most (76.1%) of farmers in the region contact their colleague farmers whenever their onion plants are diseased, while 23.9% said they usually contact Agriculture Extension Agents (AEAs) when challenged with diseases. None of the farmers contacted a specialist plant pathologist during a disease infestation ().
Results from the study revealed that 54.4% of the farmers are of the opinion that the disease could be controlled using chemicals, whiles 11.1% and 7.2% adopt crop rotation and farm hygiene, respectively. Furthermore, 9% of the farmers think a combination of two or more preventive measures is the most effective means of managing onion basal rot, whereas 22.5% of the farmers have either no idea how to manage the disease or do not adopt any preventive or control measures (). Only 2.2% of the respondents amended their farmlands with organic manure, 65.0% used inorganic fertilizer, and 32.8% of them combined the usage of both organic and inorganic fertilizers ().
3.4. Symptoms observed on onion plants
During the survey in the region, various symptoms suspected to be associated with onion basal rot were examined in the various communities and recorded. Some symptoms were observed on onion leaves, while others were on the onion bulbs. The first visible symptom observed was the yellowing of leaves, which starts from the tip and progresses towards the base. Wilting of entire onion plants and curling of the leaves were observed in some of the fields, as well as the rotting of both mature and immature onion bulbs, which turned red-brown in colour. Other symptoms observed were the rotting of roots leading to root abscission, in which bulbs were easily uprooted from the soil. Bulb tissues were also brown and water-soaked when cut, and some produced some sort of foul smell. White fungal mycelia were visible on some bulbs, especially those at the advanced stage of disease development. The developed symptoms are shown in .
3.5. Fungi associated with onion basal rot
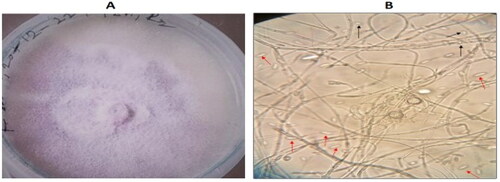
Fusarium oxysporum f. sp. cepae was constantly isolated from diseased onion bulbs collected from different locations in the three Districts/Municipality in the Upper East Region of Ghana. Most of the isolates covered a 9-cm Petri dish in 9 days. Fusarium oxysporum f. sp. cepae produced whitish cottony mycelium on the surface with a violet pigmentation on the backside of the plate (). Microscopic observation revealed that the isolates produced macroconidia, which were hyaline and boat-shaped with at least three septa, while microconidia were without septa and oval to kidney-shaped ().
3.6. Pathogenicity assay
The symptoms educed by Fusarium oxysporum on the inoculated onion bulbs during the pathogenicity test were recorded. The pathogen successfully infected the inoculated onion bulbs and produced symptoms including rotting of the bulbs, bulbs turning red-brown, water-soaked, and some produced foul smells (). The control, which was inoculated with sterile PDA, remained undisturbed and symptomless from the beginning to the end of the experiment (). Fusarium oxysporum was consistently re-isolated from rot bulbs, and this confirmed it as the causative organism of onion bulb rot.
3.7. Mean incidence and severity of onion basal rot in the locations surveyed
The onion basal rot disease was prevalent in all the locations surveyed in the Upper East Region of Ghana under both rainy and dry season onion production conditions (). Analysis of variance showed significant differences (P < 0.05) for incidence and severity of the disease among the various fields.
Table 6. Incidence and severity of onion basal rot disease in the Upper East Region of Ghana.
Under irrigated conditions (dry season), the incidence of onion basal rot was highest in Bansi (28.92%) and lowest in Kuka (19.47%). Disease incidence at Bansi was significantly (P < 0.05) higher than all the other locations except Sapeliga (26.97%), Saka (24.63%), and Nyorigu (25.18%). The disease severity index, on the other hand, was highest and lowest at Yalugu (44.25%) and Bugri Corner (30.15%), respectively. The disease severity of Bugri Corner did not vary significantly from those observed at Saka (34.10%), Benguri (31.15%), and Bansi (38.00%), but varied significantly from the other communities ().
Under rain-fed conditions, the mean disease incidence of the onion bulb rot was between 25.45% and 35.63% (). Disease incidence was highest at Sapeliga (35.63%); however, this did not vary significantly (P < 0.05) from Kuka (33.21%), Nyorigu (34.71%), Saka (34.17%), and Yalugu (31.45%), but was significantly different from the remaining communities (). The severity of the disease for the same period showed significant variation (P < 0.05) among the farms surveyed, with a maximum and minimum severity of 43.35% and 33.90%, respectively. The highest disease severity of 43.35% was recorded at Sapeliga, and it differed significantly from the other communities except Bugri Corner (39.95%), Nyorigu (43.28%), and Googu (39.98%). Benguri (33.90%) registered the lowest disease severity, and this was significantly different from the other communities except Bansi (35.37%) and Saka (36.39%) ().
Among the three districts and a municipality studied, the incidence and severity of onion basal rot under rain-fed conditions varied significantly (P < 0.05) from district to district. Bawku West District recorded the highest disease incidence of 30.25%, and this did not differ significantly from Bawku Municipality (29.37%), but varied significantly from the Binduri District (22.69%), which had the minimum incidence. Disease severity for the same period was highest at the Bawku Municipality (42.18%) and lowest at the Binduri District (34.97%). The disease severity of the Bawku Municipality was significantly (P < 0.05) higher than the Binduri District (34.97%), but not the Bawku West District (41.25%) ().
Table 7. Incidence and severity of onion basal rot disease among the three districts/municipality.
Although diseases incidence for the dry season was highest in the Bawku West District (24.60%), it was however not significantly different (p < 0.05) from the Bawku Municipality (21.74%) and the Binduri District (23.36%). Similarly, disease severity for the same period was not statistically significant among the three districts/municipality ().
3.8. Effect of season on incidence and severity of onion basal rot
To determine the effect of season on the incidence () and severity () of onion bulb rot, an independent sample t-test was conducted. revealed that disease incidence for the rainy season (M = 27.43, SD = 7.11) was significantly higher than that of the dry season (M = 23.23, SD = 5.10), t (88) =3.22, p =.0018. These findings suggest that rain-fed onion production conditions are more likely to favour the incidence of onion basal rot.
Table 8. Mean disease incidence for dry season and rainy season.
Table 9. Mean disease severity for dry season and rainy season.
Results from showed that there was no significant effect of season on disease severity, t (88) = 0.63, p = 0.5277, despite the fact that the rainy season (M = 39.46, SD = 6.28) attained a higher disease severity than the dry season (M = 38.47, SD = 6.20).
4. Discussion
It is evident from the results that onion production in the region is dominated by male farmers. A similar finding was previously reported by Alidu (Citation2013) in the Eastern Region of Ghana. This development could be due to the communal land tenure system practiced in the area, which makes land ownership by women very difficult compared to their male counterparts. It could also be due to the labour-intensive nature of onion farming in our part of the world, since manual farming with its associated drudgery is a common practice. The domestic responsibilities of women in the studied area could also limit the time available to them and, as such, may have prevented most of them from participating in onion production. Most of the farmers interviewed were within the ages of 20 and 59, implying that onion farming in the region is dominated by the youth and the middle-aged. This can also be attributed to the labour-intensive nature of onion farming. Most of the farmers had no formal education, and this could be responsible for the adoption of poor agronomic practices in the studied communities. This finding is corroborated by Asare-Bediako et al. (Citation2015), who opined that there was a positive correlation between low levels of education and the adoption of practices that promote the spread of diseases. The low educational level of most of the farmers in the region could negatively impact the adoption of improved disease management practices such as the destruction of stubble as well as the removal of living plants that harbour pathogens (Asare-Bediako et al., Citation2015; Lewis and Miller, Citation2004).
Upon interacting with the farmers during the study, it came to light that diseases and pests are a major threat to onion production in the surveyed communities. The farmers named onion bulb rot disease as one of the most important onion diseases that infect onion plants in the various locations. Other diseases identified were damping off, leaf twister, leaf blight, and downy mildew. These diseases have also been identified on onion fields in the Eastern Region of Ghana by Awuah et al. (Citation2009) and Alidu (Citation2013), thus confirming the prevalence of these diseases on onion farms in Ghana. This finding could be partially responsible for the high losses recorded in most onion farms in the country, and hence affirming the fact that diseases are a major setback to onion production in Ghana. Fusarium basal rot disease alone had been reported to account for 90% seedling loss and 50% reduction in yields of susceptible onion cultivars (Akrofi et al., Citation2016; Mishra et al., Citation2014).
Most of the farmers thought the disease was caused by too much water. They intimated that continuous downpours in the wet season and over-irrigation during the dry season are responsible for the occurrence of the disease. This perception among the farmers in the study area may not totally be wrong because, though water is not the causative agent of the disease, it promotes the development of the disease, as corroborated by Mishra et al. (Citation2014) and Sowley et al. (Citation2018). Moisture is important for the germination of fungal spores and the penetration of the host by the germ tube (Agrios, Citation2005). It also aids in the activation of fungal pathogens prior to infecting the host plant, as well as the distribution and spread of inoculum on the same plant and from plant to plant (Agrios, Citation2005; Mishra et al., Citation2014; Sowley et al., Citation2018). It also emerged that some farmers attributed the disease to insect pests and, as such, adopted the application of insecticides with the hope of controlling the disease. Some of the formally educated farmers revealed that the disease is caused by soil microorganisms such as bacteria and fungi.
The disease incidence and severity scores have proved that the disease is indeed prevalent in the region. Awuah et al. (Citation2009) and Alidu (Citation2013) observed similar findings when they reported that the disease was prevalent in the Eastern Region of Ghana. This ought to be an important database to guide farmers and other stakeholders in the onion value chain to put in place the right preventive measures in order to mitigate the effects of the disease on crop yields. The incidence and severity of the disease for both dry and rainy seasons varied significantly from community to community. Disease incidence and severity were also significantly different from one district to another, except for severity during the dry season. The findings of this study are in consonance with Gutierrez and Cramer (Citation2005), who recorded like findings in La Cruces, New Mexico.
The disease was widespread in the study area and was recorded on all the farms surveyed. This phenomenon could be due to the adoption of poor agronomic practices by the farmers. This is in agreement with Asare-Bediako et al. (Citation2015), who opined that the high incidence of fungal diseases of chili pepper in the Ashanti, Brong-Ahafo, Central, and Volta Regions was a result of the adoption of poor agronomic practices by farmers in these regions. The use of farmer-saved seeds, for example, can lead to the spread of seed-borne fungal diseases such as onion bulb rot disease. Similarly, the majority of the farmers in the two districts and the municipality were practicing monocropping, and this might have contributed positively to the prevalence of the disease. Monocropping provides a safe haven for plant pathogenic pathogens by ensuring the continued availability of a source of inoculum, especially in fields used for both dry-season and rainy-season onion cultivation. Monocropping is also characterized by dense populations with genetic homogeneity, and as a result, once a disease becomes established, it can rapidly spread to epidemic proportions (Arya, Citation2002; Obeng-Ofori et al., Citation2007). This finding is again backed by Le et al. (Citation2021), who are of the opinion that successive cultivation of susceptible Allium species and varieties in fields with an infection history makes the control of the disease very difficult. Although the severity of the disease was moderate, it still calls for concern because, once the disease becomes established on the field, its development continues even after harvest and can lead to serious postharvest losses.
On the effect of season on the incidence and severity of onion basal rot, it came to light that the incidence of the disease was significantly higher under rain-fed onion production conditions. The high incidence recorded in the rainy season might be ascribed to the favourable environmental conditions, particularly high soil moisture and humidity. High moisture and humidity have been reported by several researchers to have a positive correlation with the incidence and severity of fungal diseases (Agrios, Citation2005; Mishra et al., Citation2014; Muntala et al., Citation2020; Sowley et al., Citation2018).
For effective management of the disease, accurate identification of the causative agent is paramount. To this end, the causative organism of onion basal rot was successfully isolated and identified morphologically as Fusarium oxysporum f. sp. cepae using standard fungi identification keys described in various literatures. Fusarium oxysporum f. sp. cepae produced whitish cottony mycelium on the surface with a violet pigmentation on the backside of the plate. Microscopic examination revealed the production of both microconidia and macroconidia. Macroconidia were hyaline and boat-shaped with at least three septa, while microconidia were without septa and oval to kidney-shaped. These observations were consistent with those described by (Kalman et al., Citation2020; Mandal et al., Citation2018; Shamyuktha et al., Citation2020; Yağmur et al., Citation2024). Also backing our finding are Awuah et al. (Citation2009) and Alidu (Citation2013), who equally identified the same fungus as responsible for onion basal rot in the Eastern Region of Ghana. This assertion was again corroborated by the pathogenicity of Fusarium oxysporum f. sp. cepae on mature onion bulbs during the pathogenicity test. The pathogen successfully infected the inoculated onion bulbs and produced symptoms including rotting of the bulbs, turning red-brown, being water-soaked, and some producing foul smells. These symptoms were similar to those observed and described by (Cramer, Citation2000; Cramer et al., Citation2021; Le et al., Citation2021).
Poor farm sanitation was observed as one of the factors responsible for the wide spread of onion basal rot in the communities surveyed. For example, some of the farms with a high incidence and severity of the disease were noted to have had a lot of crop residues all over the farms. It was a common practice for farmers to uproot diseased plants or weeds and leave them among the crops. The crop residues that are usually not properly disposed of could serve as a source of inoculum for Fusarium sp. since the pathogen can survive as a saprophyte in the crop residues. In agreement with these findings, Cramer (Citation2000) reported that the causative pathogen for onion basal rot disease can survive either as chlamydospores in soil or as saprophytes in crop residues. Weeds are also implicated in contributing to the survival, propagation, and transmission of this pathogen (Haapalainen et al., Citation2016). However, some farmers adopt seedbed treatment mainly through the burning of crop residues, which has a sterilizing effect on the soil, and this can help in reducing the inoculum level of Fusarium, thereby decreasing the incidence of onion bulb rot (Le et al., Citation2021).
It was observed from the study that most farmers start to implement control measures only after visible symptoms have developed. This finding is in consonance with Opoku (Citation2012), when he reported that farmers begin to put in place disease preventive measures at the wrong time, mostly at the time that colossal economic damage has already been caused. The continued use of insecticides could result in insecticide resistance among insect pests that are equally destructive to the onion plant. The inefficacy of the available synthetic fungicides to effectively control the disease, as claimed by some of the farmers, might be a result of the wrong application time, mode of application, and poor dosage of fungicide application. This is corroborated by Afari-Sefa et al. (Citation2015), who reported that farmers’ perceptions of the efficacy of some agrochemicals based on advice from agrochemical input dealers could have accounted for the misuse of fungicides and pesticides. The widespread availability of Fusarium in the soil as well as the production of resting structures such as chlamydospores renders the use of synthetic fungicides less effective in controlling the pathogen (Akrofi et al., Citation2016; Jessica et al., Citation2006).
Another worrying observation made when it comes to disease management was the fact that the majority of the farmers consulted their colleague farmers, while a few contacted MoFA officers when their crops were diseased. Most of these ‘farmer consultants’ are indeed not knowledgeable enough in plant disease management. They most often recommend pesticides for controlling plant diseases. Again, most of the MoFA officers are not specialist plant pathologists and, as such, are not always in the right position to help these helpless farmers effectively manage onion bulb rot disease.
It was observed from the survey that most farmers in the districts and municipality used seeds from their previous season’s harvest or seeds or seedlings from other colleague farmers. These farmer-saved seeds are usually not given any seed treatment, and this could be responsible for the high incidence of seed-borne fungi in the districts and municipality. Similar findings were reported among tomato and chili pepper farmers in Ghana by Opoku (Citation2012) and Asare-Bediako et al. (Citation2015), respectively.
To effectively manage the disease, farmers ought to adopt a four- or longer-year crop rotation scheme. Effective crop rotation of at least a 4-year cycle has been found to reduce the inoculum of Fusarium in the soil and, as well, reduce bulb rot (Cramer, Citation2000; Wright et al., Citation2015). Farmers could also adopt an Integrated Pest and Vector Management (IPVM) program in order to minimize the overreliance on synthetic chemicals and their associated harmful impacts on farmers and the environment. The use of onion varieties resistant to bulb rot disease is crucial if farmers in the study area are to minimize losses. There is, however, no known onion variety that is resistant to onion basal rot disease (Saxena and Cramer, Citation2009). Plant breeders in the country must therefore make concerted efforts to produce onion varieties that are resistant to onion bulb rot disease.
5. Conclusion
The study revealed that the disease is widespread in all locations surveyed. The incidence and severity of the diseases were both significantly varied among locations, and the incidence of the disease was significantly higher during the rainy season. The causative pathogen of the disease was morphologically identified as Fusarium oxysporum f. sp. cepae. The prevalence of the disease was a result of the poor agronomic practices adopted by farmers. Farmers are therefore encouraged to adopt farm management practices that have the potential to minimize the occurrence and spread of the disease.
Authors’ contributions
AO: conceptualization, original draft; methodology, research, formal analysis; AM: conceptualization, evaluation of original draft; methodology, formal analysis, validation, supervision; review and editing; final approval of the version to be submitted; SL: evaluation of original draft; methodology, supervision, review and editing; final approval of the version to be submitted; KGS: evaluation of original draft; formal analysis; review and editing; validation.
Disclosure statement
The authors have no competing interests.
Data availability statement
All data are available within the manuscript and there is no additional data to be reported.
Additional information
Notes on contributors
Aminu Osman
Aminu Osman research interest is in fungal diseases of vegetables.
Muntala Abdulai
Muntala Abdulai research interest is in molecular plant pathology.
Salim Lamini
Salim Lamini research interest is in plant diseases and host resistance.
Kwadwo Gyasi Santo
Kwadwo Gyasi Santo research interest is in agronomy and sustainable agriculture.
References
- Afari-Sefa, V., Asare-Bediako, E., Kenyon, L., & Micah, J. A. (2015). Pesticide use practices and perceptions of vegetable farmers in the cocoa belts of the Ashanti and Western Regions of Ghana. Advances in Crop Science and Technology, 3(3), 174. https://doi.org/10.4172/2329-8863.1000174
- Agrios, G. N. (2005). Plant pathology (5th ed., pp. 922). Elsevier Academic Press.
- Akrofi, S., Kotey, D. A., Ahiatsi, E. N., & Larbi-Koranteng, S. (2016). Onion farming practices in Eastern Region of Ghana: Implications for research. Asian Journal of Agriculture and Food Sciences, 4(4), 179–190.
- Alidu, A. I. (2013). Etiology and control of bulb rot disease of onion (Allium cepae L.) in the Eastern Region of Ghana [MPhil Thesis]. University of Ghana. Legon.
- Arya, P. S. (2002). A textbook of vegetable culture. Kalyani Publishers.
- Asare-Bediako, E., Addo-Quaye, A., Boakye, B., Sarbah, J. M., Asante, P., & Dorm, E. (2015). Incidence and severity of viral and fungal diseases of Chili Pepper (Capsicum frutescens) in some districts in Ghana. International Journal of Plant & Soil Science, 7(3), 147–159. https://doi.org/10.9734/IJPSS/2015/16830
- AtlasBig.com. (2018). World’s top onion producing countries. www.atlasbig.com/en-us/countries-onion-production
- Awuah, R. T., Kwoseh, C., Kuranteng, S. L., Okpala, R. O. C., & Amoako-Attah, I. (2009). Appearance of Fusarium basal rot disease of onion in the Kwahu South District of Ghana. Ghana Journal of Horticulture, 7, 84–88.
- Cramer, C. S. (2000). Breeding and genetics of Fusarium basal rot resistance in onion. Euphytica, 115(3), 159–166. https://doi.org/10.1023/A:1004071907642
- Cramer, C. S., Mandal, S., Sharma, S., Nourbakhsh, S. S., Goldman, I., & Guzman, I. (2021). Recent advances in onion genetic improvement. Agronomy, 11(3), 482. https://doi.org/10.3390/agronomy11030482
- Dauda, W., Alao, S., Zarafi, A., & Alabi, O. (2018). First report of die-back disease of onion (Allium cepa L.) induced by Fusarium equiseti (Mart) Sacc in Nigeria. International Journal of Plant & Soil Science, 21(3), 1–8. https://doi.org/10.9734/IJPSS/2018/38339
- FAO. (2020). Food and Agriculture Organisation of the United Nations. Statistical Year Book 2020. http://www.fao.org/faostat/en/#data/QC
- Galván, G. A., Koning-Boucoiran, C. F. S., Koopman, W. J. M., Burger-Meijer, K., González, P. H., Waalwijk, C., Kik, C., & Scholten, O. E. (2008). Genetic variation among Fusarium isolates from onion, and resistance to Fusarium basal rot in related Allium species. European Journal of Plant Pathology, 121(4), 499–512. https://doi.org/10.1007/s10658-008-9270-9
- Ghanbarzadeh, B., Mohammadi Goltapeh, E., & Safaie, N. (2014). Identification of Fusarium species causing basal rot of onion in East Azerbaijan province, Iran and evaluation of their virulence on onion bulbs and seedlings. Archives of Phytopathology and Plant Protection, 47(9), 1050–1062. https://doi.org/10.1080/03235408.2013.829628
- Gutierrez, J. A., & Cramer, C. S. (2005). Screening short-day onion cultivars for resistance to Fusarium basal rot. HortScience, 40(1), 157–160. https://doi.org/10.21273/HORTSCI.40.1.157
- Haapalainen, M., Latvala, S., Kuivainen, E., Qiu, Y., Segerstedt, M., & Hannukkala, A. O. (2016). Fusarium oxysporum, F. proliferatum and F. redolens associated with basal rot of onion in Finland. Plant Pathology, 65(8), 1310–1320. https://doi.org/10.1111/ppa.12521
- Honger, J. O., Gyempeh, N., Offei, S. K., & Cornelius, E. W. (2015). Importance of the onion leaf twister disease in Ghana and the effect of Trichoderma asperellium on the mycelial growth and sporulation of the causal agent. Ghana Journal of Science, 55, 51–65.
- Jessica, A., Gutierrez, R. M., & Christopher, C. S. (2006). Screening winter sown, intermediate-day onion cultivars for resistance to Fusarium basal rot. HortTechnology, 16(1), 177–181. https://doi.org/10.21273/HORTTECH.16.1.0177
- Kalman, B., Abraham, D., Graph, S., Perl-Treves, R., Harel, Y. M., & Degani, O. (2020). Isolation and identification of Fusarium spp., the causal agents of onion (Allium cepa) basal rot in Northeastern Israel. Biology, 9(4), 69. https://doi.org/10.3390/biology9040069
- Kankam, F., Alidu, M. R., & Sowley, E. N. K. (2019). Fungi associated with onion (Allium cepa L.) bulb rot and the impact of storage containers on the occurrence of the fungi in market centers, Tamale. Ghana Journal of Horticulture, 14(1), 38–47.
- Le, D., Audenaert, K., & Haesaert, G. (2021). Fusarium basal rot: Profile of an increasingly important disease in Allium spp. Tropical Plant Pathology, 46(3), 241–253. https://doi.org/10.1007/s40858-021-00421-9
- Lewis, I. L. M., & Miller, A. S. (2004). Anthracnose fruit rot of pepper. Extension Fact sheet, Plant Pathology. The Ohio State University.
- Mandal, M., Rana, D., & Misra, D. K. (2018). Studies on cultural characteristics of Fusarium isolates causing panama wilt of banana under West Bengal. Journal of Pharmacognosy and Phytochemistry, 7(3), 833–840.
- Marrelli, M., Amodeo, V., Statti, G., & Conforti, F. (2018). Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities. Molecules (Basel, Switzerland), 24(1), 119. https://doi.org/10.3390/molecules24010119
- Mishra, R. K., Jaiswal, R. K., Kumar, D., Saabale, P. R., & Singh, A. (2014). Management of major diseases and insect pests of onion and garlic: A comprehensive review. Journal of Plant Breeding and Crop Science, 6(11), 160–170. https://doi.org/10.5897/JPBCS2014.0467
- Muntala, A., Norshie, P. M., Santo, K. G., & Saba, C. K. S. (2020). Colletotrichum gloeosporioides species complex: Pathogen causing anthracnose, gummosis and die-back diseases of cashew (Anacardium Occidentale L.) in Ghana. European Journal of Agriculture and Food Sciences, 2(6), 1–10. https://doi.org/10.24018/ejfood.2020.2.6.146
- Necola, S. (2007). Allium cepa variety evaluation for organic farming in the 5th International Symposium on Edible Allianceae (pp. 45). ISHS.
- Obeng-Ofori, D., Yirenkyi-Danquah, E., Ofosu-Anim, J., & Ofori, K. (2007). Vegetable and spice crop production in West Africa (pp. 162). City Publishers.
- Opoku, B. A. (2012). Incidence and severity of major fungal diseases of tomato (Solanum lycopersicum l.) in three districts within forest and forest-savannah agro-ecological zones of Ghana [MSc. Thesis]. KNUST.
- Saxena, A., & Cramer, C. S. (2009). Screening of onion seedlings for resistance against New Mexico isolates of Fusarium oxysporum f. sp. cepae. Journal of Plant Pathology, 91(1), 199–202.
- Schwartz, F. H., Gent, H. D., & Bartolo, E. M. (2007). Onion Fusarium basal plate rot identification and life cycle.
- Shamyuktha, J., Sheela, J., Rajinimala, N., Jeberlinprabina, B., & Ravindran, C. (2020). Survey on onion basal rot disease incidence and evaluation of Aggregatum Onion (Allium cepa L. Var. Aggregatum Don.) genotypes against Fusarium oxysporum f. sp. cepae. International Journal of Current Microbiology and Applied Sciences, 9(7), 529–536. https://doi.org/10.20546/ijcmas.2020.907.058
- Shin, J. H., Lee, H. K., Back, C. G., Kang, S. H., Han, J. W., Lee, S. C., & Han, Y. K. (2023). Identification of fusarium basal rot pathogens of onion and evaluation of fungicides against the pathogens. Mycobiology, 51(4), 264–272. https://doi.org/10.1080/12298093.2023.224359
- Sowley, E. N. K., Kankam, F., & Tawiah, E. (2018). Preliminary study on aflatoxin contamination of maize (Zea mays) grains in two districts of Northern Region of Ghana. Asian Plant Research Journal, 12(1), 1–7. https://doi.org/10.9734/aprj/2018/v1i226269
- Summerell, B. A., Leslie, J. F., Liew, E. C., Laurence, M. H., Bullock, S., Petrovic, T., Bentley, A. R., Howard, C. G., Peterson, S. A., Walsh, J. L., & Burgess, L. W. (2011). Fusarium species associated with plants in Australia. Fungal Diversity, 46(1), 1–27. https://doi.org/10.1007/s13225-010-0075-8
- Wright, P., Falloon, R., & Hedderley, D. (2015). Different vegetable crop rotations affect soil microbial communities and soil borne diseases of potato and onion: Literature review and a long-term field evaluation. New Zealand Journal of Crop and Horticultural Science, 43(2), 85–110. https://doi.org/10.1080/01140671.2014.979839
- Yağmur, A., Demir, S., Canpolat, S., Rezaee Danesh, Y., Farda, B., Djebaili, R., Pace, L., & Pellegrini, M. (2024). Onion Fusarium basal rot disease control by arbuscular mycorrhizal fungi and Trichoderma harzianum. Plants (Basel, Switzerland), 13(3), 386. https://doi.org/10.3390/plants13030386