?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Food standards control copper in water and several foods by setting ADI values. The metal, an essential element influencing biochemical functions, is involved in oxidative stress resistance. However, high metal concentrations can be toxic, especially in nerve functions. Copper at 1 and 2 mM could toxic generations of Drosophila melanogaster flies by assessing the development, learning, memory, locomotion, activities of catalase and acetylcholinesterase, and the neutralizing DPPH-free radicals. Flies cultured in a copper-containing medium lengthened their maturation period and reduced hatching numbers. The learning ability in larvae and the climbing ability of adult flies also decreased between generations. The antioxidative capacity showed the differences between fly groups. Catalase activity gradually decreased with each generation, while acetylcholinesterase did not indicate profound differences between generations. In conclusion, a daily intake of 1–2 mM copper could affect fruit flies’ behaviors, nervous system functions, and the benchmark dose of copper in food.
REVIEWING EDITOR:
1. Introduction
Drosophila melanogaster (fruit flies) are experimental models commonly used to study the molecular and development processes observed in higher evolutionary animals. Fruit flies’ nervous system is introduced to be simple but has many conserved genes and is homologous to humans (Mirzoyan et al., Citation2019; Yamaguchi & Yoshida, Citation2018). Therefore, they have essential genetic tools to model brain diseases, specifically neurodegenerative ones. Several biochemical and molecular processes have also been found, and gene transfer for modeling in fruit flies has been applied (Mirzoyan et al., Citation2019; Yamaguchi & Yoshida, Citation2018). Given its short lifespan and simple culturing conditions, numerous assays can be performed on flies. These can be used for drug screening or physiological response testing before further being conducted on higher evolutionary animals: rats, rabbits, and chimpanzees (Pandey & Nichols, Citation2011). Still, it must be considered in some aspects, mainly due to human and fruit flies’ different genomics and physiology. The model needs more assessments to improve on this species to be perfect for studying neurodegeneration and doing variant assays, especially behavioral assays. Nevertheless, under the limitation of research or a desire to understand the most fundamental molecular process, the fruit fly model is worthy of consideration due to its features and benefits.
Several scientists and researchers have recently become interested in neurological diseases and their mechanisms. Besides, although the causative mechanism has been identified, many neurological diseases, especially neurodegenerative diseases, have not yet discovered the underlying pathology. Some authors investigated rabbits for the hallmark of Alzheimer’s disease and found some effect of copper on the disease; Copper can induce various neurodegenerative diseases and is currently considered a neurotoxin at specific doses (Desai & Kaler, Citation2008; Eskici & Axelsen, Citation2012; Sparks & Schreurs, Citation2003; Waggoner et al., Citation1999).
The concentration of copper was controlled via the acceptable daily intake (ADI) levels in food and water was 2–3 mg per kg or litter regulated by the World Health Organization (WHO) report and national food standards (5656FR26548, Citation1991; Council Directive, 98/83/EC, Citation1998; QCVN, Citation2009; World Health Organization [WHO], 2004). ADI of a particular compound is calculated via its Benchmark dose (BMD) (Davis et al., Citation2011). The cation has been implicated in developing reactive oxygen species (ROS) in the brain and increased ROS levels, damaging and contributing to aging and cell death. Several studies have examined the effects of copper sulfate (CuSO4) on fruit flies’ development and metabolism (Halmenschelager & da Rocha, Citation2019). However, studies examining copper’s effect on multi-generation fruit flies and their nervous system are limited.
This study aimed to investigate the potential neurotoxicity of copper ions on the nervous system of wild-type fruit flies at varying concentrations and generations. The study aims to contribute data on heavy metal toxicity in flies and the nervous system to create a model of fruit flies for neurodegenerative diseases through foodborne induction. The study also aims to determine if the toxic level of copper affects the acceptable daily intake (ADI) of the metal in food.
2. Materials and methods
2.1. Materials and flies’ culturing and farming
Wild-type Drosophila melanogaster (Yellow-White strain) was obtained from the Bloomington Drosophila Stock Center (Bloomington, IN). The flies were kept on standard food media and conditions instructed by the Center: Indiana University Bloomington. Copper sulfate (CuSO4) was a product of Sigma Co. (St. Louis, MO). The remaining chemicals applied were of analytical grade.
2.2. Effect of Cu on the fertility and development of flies
CuSO4 was added to the food medium with variant concentrations, including 0.15, 1.0, 2.0, 3.0, 4.0, 5.0, 7.5, and 10 mM. Each culturing vial contained 10 male and 10 female fruit flies, and each concentration (treatment) had 3 replications (3 vials). After 7 days of feeding, the quantity of died F0 flies was observed (D7d), then the first apparent time (A1st) and quantity of F1 individual (Ni) at each maturing stage—larvae, pupae, and adult were calculated. For the last two stages, the total number of individuals was calculated after 11 days and 15 days (NT11, NT15), respectively.
After the screening, the concentrations that indicated significant effects on fruit flies’ development, fertility, and motility and did not cause the death of F0 flies were selected for further experiments.
2.3. Behavioral assays
2.3.1. Odor-Taste learning and memory conditioning test
The methods of the Odor-Taste test were performed as previously described (Behzadfar et al., Citation2017; Squitti et al., Citation2002). The two independent training sections were prepared using the odors n-amyl acetate and 1-octanol. One-third of instar larvae from each treatment group were randomly chosen for each training section per each experiment. The control was the flier fed in the negative copper medium. The following equations calculated the Learning Index (LI):
(1)
(1)
(2)
(2)
(3)
(3)
LI score > 0 indicated appetitive learning; if the LI score was ∼ 0, then there was no evidence of learning and memory; if the LI score < 0, aversive learning had occurred.
2.3.2. Negative geotaxis (locomotion test)
The randomly chosen 20 adult flies (10 males and 10 females) were placed in 2 × 10 cm vials for each treatment group and carefully tapped at the bottom to set them up at a common starting point. The proportion of flies that reached the 6 cm mark of the column in 10s was recorded. The control was described in the methods of the Odor-Taste test. The means of the groups represented five independent experiments (each time tapping).
2.4. Biochemical assays
2.4.1. Preparation of fly homogenized sample
Twenty flies (10 males and 10 females) in the treatment group were randomly chosen to be anesthetized and homogenized in 0.5 mL of 0.1 M pH 7.0 sodium phosphate buffer. The mixture was then centrifuged at 10,000 rpm for 20 min at 4 °C (Scilogex SCI24R, USA). The supernatant was divided from the pellet and used for the biochemical assays. A control used the above mixture with the homogenized flies growing in the negative copper medium.
Protein concentrations in the whole-body homogenates were carried out as described by Mukherjee & Mishra using the Bradford assay based on BSA (bovine serum albumin) equivalents (Bradford, Citation1976; Mukherjee & Mishra, Citation2020).
2.4.2. DPPH scavenging activity
The 20 µL of fly homogenized sample was added to 50 µL of methanol in each well, and measured the OD to obtain blanks. Then 125 µL of 0.004% DPPH were added to the solution and incubated in the dark for 30 min at room temperature. The homogenized flies, as described above, were used as controls. The OD was measured at 517 nm by the spectrometer (Multiskan GO, Thermo Fisher Scientific, Finland). A mixture of only methanol (70 µL) and DPPH (125 µL) was used as a control (Bag & Mishra, Citation2020). The activity was calculated by the equation:
(4)
(4)
2.4.3. Catalase activity
Prepare a 30 mM H2O2 solution by mixing 0.34 mL of 30% H2O2 into 100 mL of 50 mM buffer phosphate. The solution needs to be made fresh each time to avoid degradation.
The spectrophotometer with a quartz cuvette was used to estimate the enzyme-specific activity at the wavelength of 240 nm. Phosphate buffer and H2O2 solution containing 100 µL of sample and 900 µL of sodium phosphate buffer were mixed to the cuvette and measured the OD (blank). The control used the homogenized flies as described. 500 µL of H2O2 solution was then subjected to the mixture. Measure OD immediately and every minute for 3 min (Aebi, Citation1984; Bag & Mishra, Citation2020). The activity was calculated by the following equation:
(5)
(5)
Where: ΔA = the difference between the initial and final absorbance, Vt = the total volume of the reaction (3 mL), Ɛ240 = the molar extinction coefficient for H2O2 at OD240 (34.9 mol−1 cm−1), d = the optical path length of the cuvette (1 cm), Vs= the volume of the sample (1 mL), Ct = the total protein concentration in the sample, 0.01 = absorbance change caused by 1 U of enzyme per min at OD240.
2.4.4. Acetylcholinesterase activity
Determination of AChE activity was carried out as described previously (Ben Younes & Sayadi, Citation2011; Riaz et al., Citation2018). The reaction mixture consisted of 10 µL of fly homogenizer, 10 µL of 2.6 mM acetylcholine chloride substrate mixed in methanol, 200 µL of 0.1 mM, pH 7.0 sodium phosphate buffer, and measured OD to get blank. The control used the homogenized flies as described. Then, 80 µL of 0.3% Fast blue B salt was added to the mixture and measured OD every 5 min for 20 min at the wavelength of 405 nm. The results were standardized by the protein concentration of the samples. Enzyme-specific activity (AAChE) was calculated as μmol of acetylthiocholine hydrolyzed per mg protein per minute.
(6)
(6)
Where: AAChE= AChE-specific activity, ΔA = the difference between the original and final absorbance, Vt = the total volume of the reaction (3 mL), Ɛ405 = the molar extinction coefficient (1.36 mol−1 cm−1) (Abdullmajed et al., Citation2011), d = the optical path length of each well in the 96 wells plate (0.84 cm), Vs = the volume of the sample (1 mL), Ct = the total protein concentration in the sample, 0.001 = absorbance change caused by 1 U of enzyme per min at OD240.
2.5. Data analysis
The values are introduced as the average ± amount of variation. Analysis of variance (ANOVA) tool was used to assess the significant differences among multiple groups under various treatments, followed by Tukey’s test using Minitab 16 software (Sydney, NSW, Australia) and GraphPad Prism5.0 software (GraphPad Software LLC). In all the groups, differences were considered statistically significant with p < 0.05. The later software was also used for graphing.
3. Results
3.1. Effect of different copper concentrations on the growth of fruit flies
showed an insignificant difference in the number of dead F0 flies at all concentrations. It indicated that at any copper ion concentration (up to 10 mM), copper did not affect the survival of adult flies. However, at higher concentrations, the fly’s production and development were negatively affected by copper concentration, especially in the fly’s life cycle. In the egg stage, with a concentration of Cu2+ from 4 mM or higher, fly eggs could not be hatched. At 7.5 mM and 10 mM Cu2+ treatments, a few eggs were born and could be observed on the surface of the food medium. In stages of larval, pupal, and adult, the retardation of the fly growing was detected. It could be evident through the required days to reach those metamorphosis stages at the ion concentrations from 2 mM to 3 mM. In particular, at the ion concentration of 3 mM, days of pupation (13 days) and fly emergence (18 days) were longer than those fed in the 2 mM ion, 9 and 13 days, respectively; and the hatching flies were also significantly lower than those fed in the concentration of 0.15 mM, 1 mM, and control treatments.
Table 1. Development of fruit flies at different concentrations of CuSO4.
In general, the supplement of copper negatively affected the flies’ growth and development. The fly’s life cycle did not indicate any difference at the copper concentration of 0.15 mM and 1 mM. The 2 mM Cu2+ did not affect the hatching time but expressed the variant in the total quantity of pupae and final hatched flies. The concentration of 3 mM strongly influenced the development of pupae and adult flies. From the ion concentration of 4 mM or higher, the copper strongly inhibited the fly’s life cycle so that flies could not hatch and grow.
The copper ion concentrations of 1 mM and 2 mM were selected for further experiments to conduct and ensure a sufficient number of adjacent offspring generations by behavioral and biochemical assays affected by copper.
3.2. Copper toxicity caused a decline in flies’ neural functions through behavioral assays
3.2.1. Copper induces learning and memory deficits in Odor-Tase learning assay
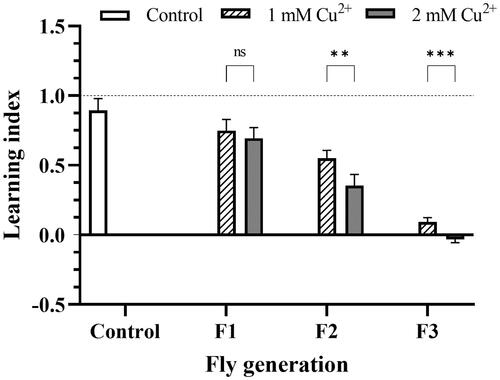
shows the decrease in learning and memory ability of fruit fly larvae grown in copper-containing media via generations since the experiments were used with the 3rd stage larvae and no F0 treatment. The result showed that the distribution decreased significantly between generations and the control; in the F3 generation, flies did not show any learning. Regarding concentration, F1 larvae at both concentrations were relatively similar in LI, but in the following generations, F2 and F3 appeared gradually different in 1 mM and 2 mM copper with p** < 0.01 and p*** < 0.001, respectively.
Figure 1. The decline in learning and memory ability of copper-treated larvae. The F1, F2, and F3 flies were independently trained in two sections, which were prepared using the odors n-amyl acetate, and 1-octanol. The control was the flier fed in the negative copper medium. LI was calculated by the relation of preferable AM or OCT indicated in EquationEquations (1)–(3). LI score > 0 indicated appetitive learning; LI score ∼ 0 indicated no evidence of learning and memory; if the LI score < 0, aversive learning had occurred. The values are introduced as the average ± amount of variation of triplicate experiments, and the difference was measured using t–test (p** <0.01; p***<0.001).
3.2.2. Locomotor impairment in flies after copper exposure
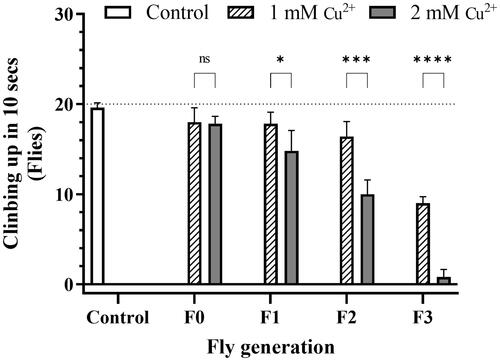
The experiment investigating adult fruit flies’ damaging geotaxis ability showed that, for the F0 generation, the copper concentrations did not significantly affect the moving ability of flies and did not express the difference from the control. The locomotor ability was almost normal. This ability decreased in both concentration and generation manner, and the F3 generation flies almost lost the moving ability in the 2 mM copper ion treatment, with the 0.8 flies (). During the experimental process, some abnormal behaviors of flies were recorded, especially in 2 mM Cu2+ of all generations and in 1 mM Cu2+ of the last one, such as flies falling off the wall when trying to climb, crawling up slowly or wildly slowly, grooming less, and weaker. The effect of decreased AChE enzyme activity on mobility will be further discussed in the results of the AChE experiment.
Figure 2. Copper caused locomotor impairment in flies. Twenty randomly chosen adult flies were placed in the bottom of 2 × 10 cm vials as a common starting point for each treatment group. The control was described in the legend of . The proportion of flies that could reach the 6 cm height column in 10 s was recorded. Data are introduced as the mean ± standard deviation of five independent experiments and difference was measured using the t–test (p*<0.05; p** <0.01; p***<0.001; p****<0.0001).
3.3. Neurological and metabolic disorders of flies after copper toxicity by biochemical assays
3.3.1. Estimation of protein concentration
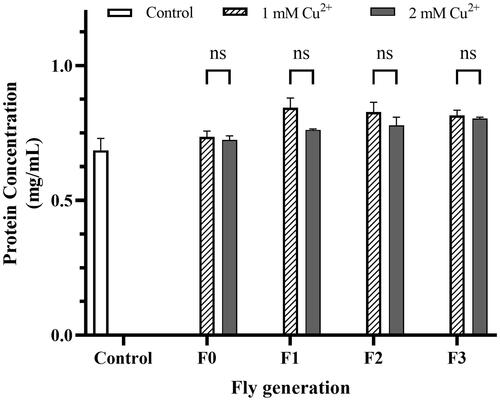
The total protein estimation results did not illustrate any significant difference between the treatments, including the control treatment (). Although it was still possible to see a slight increase in protein concentration over the generations, there was no significant difference (t–test and ANOVA) in fly protein concentration between the indicated concentrations of copper. Protein concentration data were used to furtherly calculate the catalase enzyme activity assay and acetylcholinesterase (AChE) enzyme activity assay.
Figure 3. Protein concentration of the treatments. The flies were prepared by homogenized protein concentration in the different concentrations of CuSO4 (1 mM and 2 mM). The control was described in the legend of . Bradford’s method using BSA as the standard was applied to estimate the treatments. The values are introduced as the average ± amount of variation of triplicate experiments, and the difference was measured using the t–test and 2-way ANOVA.
3.3.2. Decrease in free radical scavenging activity in flies caused by copper toxicity
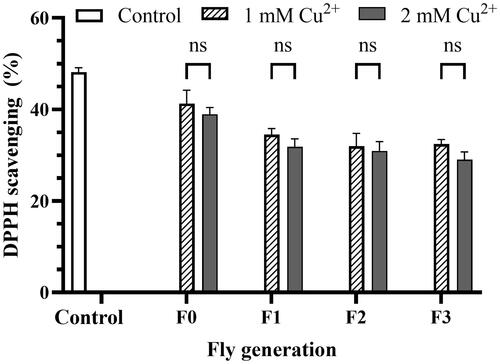
illustrates that the percentage of DPPH free radical scavenging activity decreased from control to generation fed by copper-adding mediums. The activity of the F0, F1, F2, and F3 flies significantly differed from that of the control. However, no significant differences existed between F1, F2, and F3 generations. Moreover, the activities in both 1 mM and 2 mM copper medium in all generations of flies did not significantly differ.
Figure 4. DPPH scavenging percentage reduction by copper toxicity. The mixture containing 20 µL of fly homogenized sample and 50 µL methanol (Blank) added 125 µL of 0.004% DPPH solution was kept warm in the dark for 30 min at room condition and measured OD at the wavelength of 517 nm. The mixture using the homogenized flies grown in the negative copper medium was used as a control. The DPPH scavenging percentage was calculated by EquationEq. (4)(4)
(4) , presented in Materials and Methods. The values are introduced as the average ± amount of variation of triplicate experiments, and the difference was measured using the 2-way ANOVA and the t-test. For 1 mM Cu2+ p = 0.033 F0, p = 0.009 F1, p = 0.010 F2, p = 0.005 F3 vs. Control, respectively; for 2 mM Cu2+ p = 0.023 F0, p = 0.003 F1, p = 0.005 F2, p = 0.000 F3 vs. Control, respectively.
3.3.3. Copper inhibits catalase activity
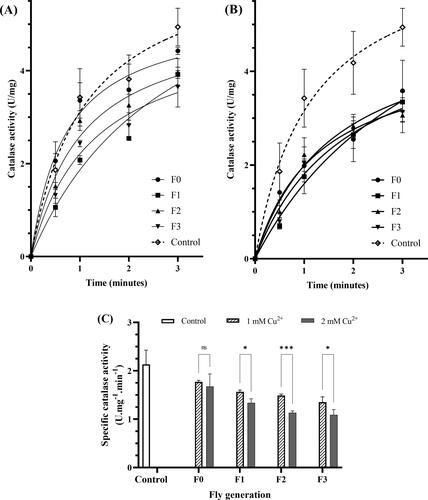
showed the chronological specific catalase activity and that the activities of 1 mM and 2 mM copper-adding mediums significantly differed from those of the control flies. The specific catalase activity per minute in control (2.13 U/mg) was not different from that of F0 flies. However, it was much higher than that of F1, F2, and F3 fliers in both copper concentrations, p* in 1 mM copper ions medium, p* and p** in 2 mM copper ions medium (). In the third generation, catalase-specific activities of F3 flies in the 1 mM and 2 mM Cu2+ mediums were 1.35 U/mg and 1.09 U/mg, respectively. They reached about 50% of the control. Besides, the enzyme showed similar specific activity in both 1 mM and 2 mM copper ions medium in each generation. It means 1 mM copper ion or higher concentration effectively inhibits the enzyme activity.
Figure 5. Copper induces catalase inhibition. The effect of 1 mM (A) and 2 mM (B) Cu2+ on catalase activity of variant fly generation, respectively. (C) the effect of Cu2+ on the specific catalase activity per minute of the flies. The values are introduced as the average ± amount of variation of triplicate experiments, Nonline fit Michaelis-Menten model, and the difference was measured using the 2-way ANOVA and the t-test. The reaction mixture in a cuvette, containing 100 µL of fly homogenized sample and 900 µL of 50 mM sodium phosphate buffer, was measured OD at the wavelength of 240 nm, then adding 500 µL of H2O2 solution and measured OD immediately. The OD value was read every 1 min for 3 min. The 100 mL of 30 mM H2O2 in 50 mM buffer phosphate was used as blank. The control was showed in the legend of . Enzyme activity (p* < 0.05; p** < 0.01; p*** < 0.001; p**** < 0.0001) was calculated by EquationEq. (5)(5)
(5) as described in Materials and Methods. (C) For 1 mM Cu2+: p = 0.030 F1, p = 0.0224 F2, p = 0.0127 F3 vs. Control, respectively; for 2 mM Cu2+: p = 0.010 F1, p = 0.004 F2, p = 0.005 F3 vs. Control, respectively.
3.3.4. Copper inhibits acetylcholinesterase activity
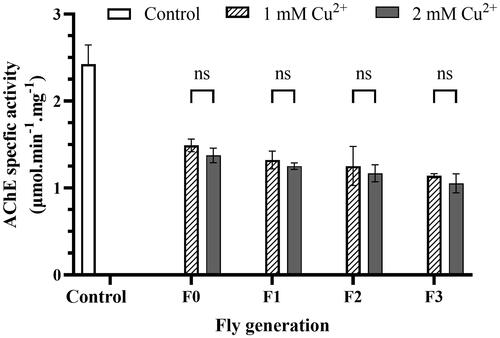
presented that the AChE enzyme activity of flies fed and exposed to copper sharply decreased and reached half compared to that of no copper-exposing mediums. The enzyme-specific activity of the control was 2.43 µmol.min−1.mg−1, and it downed to 1.49 µmol.min−1.mg−1 and 1.38 µmol.min−1.mg−1 of the F0 exposed to 1 mM and 2 mM Cu2+ mediums, respectively. Although there was no significant difference between the F0 generation (exposed only) and the copper-feeding generations F1 and F2, there was still a slight decrease in AChE activity between the later generations, one generation apart, and the difference is statistically significant between F0 and the F3 (last) generations. Similarly, there was a slight difference between the two concentrations (not statistically significant) in a particular generation, and the 2 mM Cu2+ treatment gave lower results (). Thus, it means that the effect of copper on the AChE enzyme activity in flies was not too different between the forms of exposure and concentration, and the effect on the afterlife was insignificant.
Figure 6. Copper induces AChE inhibition. The reaction mixture consisted of 10 µL of fly homogenizer, 10 µL of acetylcholine chloride in 2.6 mM methanol, and 200 µL of 0.1 mM, pH 7.0 sodium phosphate buffer. Measure OD at the wavelength of 405 nm to get blank and add 80 µL of 0.3% Fast blue B salt to the mixture and measure OD at the wavelength every 5 min for 20 min. Protein concentration of the samples helped to standardization of the results. The control was described in the legend of . Enzyme activity was calculated by EquationEq. (6)(6)
(6) , described in Materials and Methods. The values are introduced as the average ± amount of variation of triplicate experiments, and the difference was measured using the 2-way ANOVA and the t-test. For 1 mM Cu2+: p = 0.0022 F0, p = 0.0014 F1, p = 0.0030 F2, p = 0.0006 F3 vs. Control, respectively; for 2 mM Cu2+ p = 0.0015 F0, p = 0.0008 F1, p = 0.0008 F2, p = 0.0006 F3 vs. Control, respectively.
4. Discussion
suggests a potential effect of copper on the pupae and adult flies at a concentration of 3 mM, with more pronounced adverse effects observed at concentrations of 4 mM or higher. Thus, copper concentrations lower than 4 mM were chosen to survey the metal’s effect on the fly’s nervous systems. The 1 mM and 2 mM copper ion concentrations were selected for later experiments.
The negative effect of copper on memory and learning ability shown in was also demonstrated that seahorses and mice showed impaired associative learning after copper exposure (Behzadfar et al., Citation2017; Pilehvar et al., Citation2020) and showed differences in male and female flies (Zamberlan et al., Citation2020). Copper also induces oxidative stress on the hippocampus, which is primarily responsible for converting and forming memories (Lu et al., Citation2022). In humans, the high copper content in the brains of Alzheimer’s patients is associated with poor neural function (learning, memory, intellectual skills) and a shrunken frontal lobe (Squitti et al., Citation2002). One possible explanation for the observed memory deficits could be oxidative stress caused by copper, as suggested by our findings and supported by other studies. The primary mechanism by which copper affected memory function was mainly from the formation or non-neutralization of ROS in mitochondria, causing oxidative damage, membrane swelling, and ATP reduction, which induced apoptosis (Armstrong, Citation2006; Behzadfar et al., Citation2017). Mitochondria play a vital role in neural plasticity and are essential in forming memory and learning ability (Tanaka et al., Citation2008). Copper-induced dysfunction in neuronal mitochondria can be considered a factor of memory and learning impairments due to toxicity.
Furthermore, copper negatively impacts the olfactory nervous system (Pyle & Mirza, Citation2007) due to the accumulation of copper in the olfactory epithelium, impairs the ability to detect scents. At the molecular level, copper interferes with neuronal signaling and inhibits the transmission of signals from the sensory epithelium to the brain. Exposure to copper leads to the down-regulation of several genes, including those responsible for ion channels, G-proteins, and olfactory receptors, within the olfactory epithelium (Tilton et al., Citation2008). Nevertheless, this implies that the observed deficits in associative learning—specifically, the inability of flies to link olfactory cues with rewards such as food—could stem from copper-induced impairment of the olfactory system rather than a direct loss of learning capacity. Future learning conditioning experiments using different approaches should be carried out to confirm the phenomenon.
This study showed differences in locomotor function among control, F0, F1, F2, and F3 groups (). These differences might be attributed to the various pathways of copper exposure, with F0 being exposed through the surrounding environment and subsequent generations through food. Fruit flies mainly received food in the larval stage, were most voracious in the third instar larval stage, and only ate a small amount of food as adults (Wong et al., Citation2009). It might explain the different effects of copper on contact exposure, inhalation (F0), and foodborne exposure (F1, F2, and F3). However, these are preliminary observations and cannot be established as a direct cause-effect pathway. Copper-induced toxicity, which has been observed to disrupt movement in both fruit flies and seahorses (Haverroth et al., Citation2015; Siddique et al., Citation2015; Zhang et al., Citation2015), may be attributed to its detrimental effects on the nervous system. These effects include damage to proteins and DNA, lipid peroxidation, mitochondrial swelling, and ultimately cell death, as documented in various studies (Cristóvão et al., Citation2016; Letelier et al., Citation2010; Sun et al., Citation2016). Such mechanisms could explain the observed impairments in the locomotion of flies, mirroring similar findings in vertebrates.
In the case of Alzheimer’s disease, the Amyloid-β Precursor Protein (APP) could mediate copper-induced neurotoxicity and showed that in the primary neuron cultures, APP containing divalent copper induces cell death (Cristóvão et al., Citation2016), related to catalyzing the divalent to monovalent copper reduction generating ROS (Anthony et al., Citation1999), which lead to increased oxidative stress in neurons, damaging to parts of the brain responsible for locomotor function (similar to the memory deficit mechanism described above). Besides, copper can interact with protein and low-molecular-thiol-containing and disrupt their function, affecting the insert’s locomotor.
In Alzheimer’s disease research, it has been suggested that the Amyloid-β Precursor Protein (APP) may play a role in copper-induced neurotoxicity. Previous studies conducted that APP can bind divalent copper, and this interaction leads to cell death in primary neuron cultures (Cristóvão et al., Citation2016). This process involves the reduction of divalent copper to monovalent copper, which generates reactive oxygen species (ROS). The increase in ROS contributes to oxidative stress in neurons, causing damage to brain regions involved in locomotion and memory, thus potentially contributing to the symptoms observed in Alzheimer’s disease (Anthony et al., Citation1999). Moreover, copper can bind to proteins and compounds containing low-molecular-weight thiols, interfering with their normal functions. This interaction may disrupt the insect’s ability to move (Oliveira et al., Citation2022). Research on the genome of fruit flies under oxidative stress suggested potential genes involved in mobility dysfunction under oxidative stress (Jordan et al., Citation2012), including form3, Lar, Eip63F-1, luna, CG13579, side, and CG3391.
In the measurement of the DPPH free radical scavenging activity (), we found that the antioxidant activity of fruit flies differed from the control group. However, the insignificant differences in antioxidant activity between generations for both 1 mM and 2 mM copper ions suggest that copper’s antioxidative activity in fruit flies was mainly affected by the exposure pathway and did not impact the subsequent generations. Moreover, the similar antioxidant activity in the two concentrations indicates that the metal exposure did not affect the antioxidant activity in fruit flies.
In , we observed a significant reduction in catalase activity in copper-exposed flies suggestinng an impaired defense against oxidative stress. The activity decrease was relatively consistent across different groups, and the significant differences between the F0 treatments and the 3 later generations, including the adjacent F1, suggest that the effect of copper exposure on the enzyme activity was lower than that of copper-feeding treatment. The insignificant difference in enzyme activity between the two concentrations of F0 indicates that concentration exposure did not affect. However, the differences in the effect of metal at varying concentrations were more pronounced in the later copper-feeding generations via the gradual decrease of catalase activity, implying that copper exposure could further affect the flies’ ability to protect against oxidative stress.
The catalase activity decrease observed in this study was similar to that of other authors (Abolaji et al., Citation2020), showing that catalase activity was nearly halved compared with control flies, with a vast difference between the two concentrations, 1 mM and 2 mM (). Copper ions showed a strong inhibitory effect on blood catalase, with the inhibitory capacity proportional to the concentration of the inhibitor (Yordanova, Citation2017). Oxidative stress plays a significant role in the aging of the brain and the progression of neurodegenerative diseases like Alzheimer’s and Parkinson’s, which are characterized by memory loss and difficulties with movement, as seen in behavioral studies (Gemma et al., Citation2007). This stress disrupts the balance of cellular processes and defense mechanisms, damaging mitochondrial DNA and hindering the electron transport chain, leading to accelerated aging and cell death (Haddadi et al., Citation2014). Catalase, an enzyme that helps prevent oxidative damage by neutralizing reactive oxygen species (ROS) and breaking down hydrogen peroxide (H2O2), protects cells. However, copper’s ability to inhibit catalase activity compromises this protective mechanism, resulting in increased oxidative stress, as demonstrated in our DPPH assay experiments.
The effect of copper on catalase activity could be explained as follows: Cu2+ could promote the Fenton reaction (Abolaji et al., Citation2020), which means it generated hydroxyl radicals and other ROS as by-products of Cu2+ reaction with H2O2 (Strausak et al., Citation2001). Besides, CuSO4 acted as a non-competitive inhibitor of catalase (Dounce, Citation1983), thus inhibiting catalase activity and causing H2O2 accumulation and a correlation between impairment of the antioxidant system, learning and memory deficit in rats (Fukui et al., Citation2001). This study surveyed the impairment via the odor-taste, catalase, and DPPH experiments. The differences in antioxidative activity between the control, F0, and later generations in DPPH experiments were correlated with those of catalase, meaning that copper exposure reduced the cell protective ability from free radical damage in flies. The intensity of the influence was likely to affect the following generations.
The results of AChE activity () align with previous studies conducted on flies using 1 mM (Halmenschelager & da Rocha, Citation2019) or 1 mM and 3 mM divalent copper (Klimaczewski et al., Citation2018). In these studies, the AChE activity of the sample and the control was significantly decreased, but insignificantly differed between examined concentrations. The decrease in AChE activity can be attributed to copper-induced protein unfolding and failed aggregation (Frasco et al., Citation2005). Because of the cations binding to functional groups of enzymes (Najimi et al., Citation1997), causing functional group-bound proteins to lose their catalytic properties. Therefore, metal-protein interactions can inhibit the AChE catalytic activity, disrupting the enzyme function. The copper concentrations required to inhibit AChE or ChE were 10–20 mmol/L and 1 μM in vitro and in vivo, respectively (de Lima et al., Citation2013; Halmenschelager & da Rocha, Citation2019). Therefore, the effects of cation exposure in vivo in larvae and adult flies might be indirect. It means that the behavioral and intellectual deficits could be not solely the direct effect of copper on AChE but also from other copper-induced damages such as oxidative stress and cell death.
AChE plays a crucial role in cholinergic neurotransmission, which regulates cognitive and behavioral functions (Nunes, Citation2011). The activity of AChE is essential for cholinergic transmission across synapses, as it catalyzes the hydrolysis of acetylcholine into choline and acetic acid, ultimately leading to the endpoint of neural signaling transmission (Taylor, Citation2017). Chemically inhibited AChE can cause overstimulation of post-synaptic AChE receptors, resulting in various physiological variations in flies, from behavioral impairment to death (Tilton et al., Citation2008). In addition, the cholinergic system is involved in locomotor activity found in vertebrates, suggesting that the copper cation’s effect on AChE reduction in flies was more related to AChE inhibition in the ganglia than in the brain (head) (Halmenschelager & da Rocha, Citation2019; Klimaczewski et al., Citation2018; Pepeu & Giovannini, Citation2004). The ventral ganglia play various functions during initiation, targeting, and maintenance of movement, control larval locomotion, and rely on aminergic and cholinergic transmission. Therefore, a change in AChE may disrupt the motility and other behaviors of larvae and adult flies.
Copper interacts with the ATP7A gene, crucial in regulating copper-dependent enzymes, particularly those involved in nervous system processes (Hwang et al., Citation2014). The activity of these enzymes is influenced by the concentration of the enzyme and the substances it acts on (substrates) and their interactions. However, copper can bind to specific sites on enzymes, known as allosteric sites, which can modify enzyme activity without directly competing with the substrate. This nuanced role of copper suggests its complex influence on biochemical pathways, particularly within the nervous system. Observing variations in enzyme activity across different generations of flies could provide insights into how copper might alter enzyme levels through changes in gene expression. Such findings underscore the importance of further research to understand copper’s broader biological impacts, especially its potential long-term effects across generations.
Regarding food safety, the concentrations of copper in this study ranged from 1 mM to 2 mM and were equal to 0.0635 g/kg and 0.127 g/kg, respectively. Besides, the regulations of the maximal level of total copper in drinking water were set by some developing and developed nations (Viet Nam, United States, and Europe), and WHO guidelines from 1 mg/L to 2 mg/L (5656FR26548, 1991; Council Directive, 98/83/EC, 1998; QCVN, Citation2009; WHO, Citation2004). ADI is standardized as safety factor 100, meaning ADI is 100-fold lower than the BMD (Davis et al., Citation2011; Lee et al., Citation2017). In this study, the concentration of copper, 0.0635 g/kg and 0.127 g/kg, is not 100-fold higher than the ADI of copper in food (no more than 2 or 3 mg) (WHO, Citation2004), meaning that the copper ADI may not adapt to the safety factor. However, it is also crucial to note that the responses to copper observed in fruit flies in our study do not directly correspond to what we might expect in humans. Fruit flies are a valuable model for scientific research, but they have different metabolism and tolerance levels than humans. The copper concentrations that cause changes in fruit fly behavior and physiology are specific to their species and size, and we cannot automatically assume the same for humans. Thus, this serves as a suggestive alarm to the regulation, and further studies need to be carried out on various models, including human, to identify the BMD to recalculate the ADI of copper in water and food (Davis et al., Citation2011; Lee et al., Citation2017), especially organic and inorganic copper compounds that can affect human health.
5. Conclusions
The experiments showed that copper (as CuSO4) adversely affected fruit flies’ development, biochemical processes (), and neurological function that can be passed down through subsequent generations observed in learning and locomotion. Disruptions in Catalase and AChE enzyme activity showed no significance in the following generations, and the converse effects were observed between generations in Protein and DPPH. The copper concentrations affected behavioral assays but did not affect biochemical assays of DPPH, Catalase, AChE, and Protein. Copper exposure in fruit flies can adversely affect the regulation of biochemical processes, particularly the cellular protection mechanism against oxidative stress potentially leading to damage in the nervous system, resulting in a decline in neurological function. The observed decrease in neural function is due to the increased oxidative stress caused by copper exposure, leading to cellular damage and death. Additionally, copper exposure inactivates the AChE enzyme, further interfering with neural signaling transmission. The toxic concentration of copper is not 100-fold higher than the ADI of copper in food. Therefore, the ADI should be paid attention to.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Men Tran Thanh
Men Tran Thanh work in Biology Department, College of Natural Sciences at Can Tho University, Viet Nam. He completed his undergraduate degree in Biotechnology in 2005 at Can Tho University, Master of Molecular Engineering at the Kyoto Institute of Technology, Japan, in 2014. In 2016, he obtained his Ph.D. in Material and Life science at Kyoto Institute of Technology, Kyoto city, Japan. He participated in research on the fruit fly Drosophila melanogaster and the genes involved in the metabolic processes of the fruit flies. Since 2017, he has spent time on various studies on plant species with medicinal plant, weed resistance and insecticidal properties and those with anti-aging activity.
Trang Bui Hoang Thu
Trang Bui Hoang Thu is currently pursuing a Master’s degree in Neuroscience following her graduation with a Bachelor’s in Biotechnology. During the time carrying out this study, she was affiliated with the Institute of Food and Biotechnology. Her primary areas of interest lie in neurodegenerative diseases, especially to explore the intricate fields of neurotoxicity and neuroinflammation. This focus stems from her desire to understand how biological and chemical agents affect nerve tissue, potentially leading to inflammatory responses and neurological damage. Her academic journey and research are driven by the aim to develop targeted therapies that can mitigate these effects and improve overall neurological health.
Chau Tuan Thanh
Chau Tuan Thanh is PhD in Materials and Life Science, Kyoto Institute of Technology (Japan), 2012. Now he works at institute of Food and Biotechnology, Can tho university,Can tho City, Vietnam. His research interests: Recombinant Enzyme, Food Engineering, Food Quality, Freezing Technology, Natural Compounds.
Thao Truong Thị Phuong
Thao Truong Th Phuong now work at Department of Biology, College of Natural Sciences, Can Tho University. Her field of study: Applied Marine Biosciences at Tokyo University of Marine Science and Technology, Japan. Research interest: Isolation and identification of the primary active compound in the plant extract, evaluate the biological activities of the plant extract and purified compounds, mice model disease (Type 2 diabetes, inflamed mice, obesity model…), evaluation the mechanism of drug bioavailability and metabolism.
Khang Do Tan
Khang Do Tan now work at Department of Molecular Biology Institute of Food and Biotechnology, Can Tho University. His ersearch interest: DNA barcodes, gene expression, genetic diversity, bioactive compounds.
Kaeko Kamei
Kaeko Kamei now work as full proffessor at Molecular Chemistry and engineering Dapartment, Kyoto institute of technology, Kyoto, Japan. Her research field: functional biochemistry, structural biochemistry, chemistry and chemical methodology of biomolecules, regulation of bacterial disease using bacteriophage and natural products, functional analysis of lipid metabolism-related gene and screening for physiological active substance using Drosohila, analysis of biological effects of chemicals using Drosophila.
References
- 56FR26548. (1991). 40 CFR Parts 141 and 142, drinking water regulations maximum contaminant level goals and national primary drinking water reguiations for lead and copper. Federal Register. https://archives.federalregister.gov/issue_slice/1991/6/7/26457-26582.pdf#page=92
- Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption, 98/83/EC. (1998). The Council of the European Union 1998.
- Abdullmajed, H., Al-Ahmad, A., & Hussain, K. (2011). The preparation, characterization and the study of the linear optical properties of a new azo compound. Journal of Basrah Researches. (Sciences), 37, 64.
- Abolaji, A. O., Fasae, K. D., Iwezor, C. E., Aschner, M., & Farombi, E. O. (2020). Curcumin attenuates copper-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Toxicology Reports, 7, 261–268. https://doi.org/10.1016/j.toxrep.2020.01.015
- Aebi, H. (1984). [13] Catalase in vitro. In Methods enzymol (Vol. 105, pp. 121–126). Academic Press.
- Anthony, R. W., Gerd, M., Fran, M., Shayne, B., James, C., Hui, Z., Ashley, I. B., Konrad, B., Colin, L. M., & Roberto, C. (1999). The Alzheimer’s disease amyloid precursor protein modulates copper-induced toxicity and oxidative stress in primary neuronal cultures. Journal of Neuroscience. 19(21), 9170–9179. https://doi.org/10.1523/JNEUROSCI.19-21-09170.1999
- Armstrong, J. S. (2006). The role of the mitochondrial permeability transition in cell death. Mitochondrion, 6(5), 225–234. https://doi.org/10.1016/j.mito.2006.07.006
- Bag, J., & Mishra, M. (2020). Biochemical assays to detect the antioxidant level in Drosophila melanogaster. In M. Mishra (Ed.), Fundamental approaches to screen abnormalities in Drosophila (pp. 151–168). Springer US.
- Behzadfar, L., Abdollahi, M., Sabzevari, O., Hosseini, R., Salimi, A., Naserzadeh, P., Sharifzadeh, M., & Pourahmad, J. (2017). Potentiating role of copper on spatial memory deficit induced by beta amyloid and evaluation of mitochondrial function markers in the hippocampus of rats. Metallomics: integrated Biometal Science, 9(7), 969–980. https://doi.org/10.1039/c7mt00075h
- Ben Younes, S., & Sayadi, S. (2011). Purification and characterization of a novel trimeric and thermotolerant laccase produced from the ascomycete Scytalidium thermophilum strain. Journal of Molecular Catalysis B: Enzymatic. 73(1-4), 35–42. https://doi.org/10.1016/j.molcatb.2011.07.014
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254. https://doi.org/10.1016/0003-2697(76)90527-3
- Cristóvão, J. S., Santos, R., & Gomes, C. M. (2016). Metals and neuronal metal binding proteins implicated in Alzheimer’s disease. Oxidative Medicine and Cellular Longevity, 2016, 9812178. https://doi.org/10.1155/2016/9812178
- Davis, J. A., Gift, J. S., & Zhao, Q. J. (2011). Introduction to benchmark dose methods and U.S. EPA’s benchmark dose software (BMDS) version 2.1.1. Toxicology and Applied Pharmacology, 254(2), 181–191. https://doi.org/10.1016/j.taap.2010.10.016
- de Lima, D., Roque, G. M., & de Almeida, E. A. (2013). In vitro and in vivo inhibition of acetylcholinesterase and carboxylesterase by metals in zebrafish (Danio rerio). Marine Environmental Research, 91, 45–51. https://doi.org/10.1016/j.marenvres.2012.11.005
- Desai, V., & Kaler, S. G. (2008). Role of copper in human neurological disorders. The American Journal of Clinical Nutrition, 88(3), 855S–858S. https://doi.org/10.1093/ajcn/88.3.855S
- Dounce, A. L. (1983). A proposed mechanism for the catalatic ation of catalase. Journal of Theoretical Biology, 105(4), 553–567. https://doi.org/10.1016/0022-5193(83)90219-9
- Eskici, G., & Axelsen, P. H. (2012). Copper and oxidative stress in the pathogenesis of Alzheimer’s disease. Biochemistry, 51(32), 6289–6311. https://doi.org/10.1021/bi3006169
- Frasco, M. F., Fournier, D., Carvalho, F., & Guilhermino, L. (2005). Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers: biochemical Indicators of Exposure, Response, and Susceptibility to Chemicals, 10(5), 360–375. https://doi.org/10.1080/13547500500264660
- Fukui, K., Onodera, K., Shinkai, T., Suzuki, S., & Urano, S. (2001). Impairment of learning and memory in rats caused by oxidative stress and aging, and changes in antioxidative defense systems. Annals of the New York Academy of Sciences, 928(1), 168–175. https://doi.org/10.1111/j.1749-6632.2001.tb05646.x
- Gemma, C., Vila, J., Bachstetter, A., & Bickford, P. C. (2007). Frontiers in neuroscience oxidative stress and the aging brain: From theory to prevention. In D. R. Riddle (Ed.), Brain aging: Models, methods, and mechanisms. CRC Press/Taylor & Francis.
- Haddadi, M., Jahromi, S. R., Sagar, B. K. C., Patil, R. K., Shivanandappa, T., & Ramesh, S. R. (2014). Brain aging, memory impairment and oxidative stress: A study in Drosophila melanogaster. Behavioural Brain Research, 259, 60–69. https://doi.org/10.1016/j.bbr.2013.10.036
- Halmenschelager, P. T., & da Rocha, J. B. T. (2019). Biochemical CuSO(4) toxicity in Drosophila melanogaster depends on sex and developmental stage of exposure. Biological Trace Element Research, 189(2), 574–585. https://doi.org/10.1007/s12011-018-1475-y
- Haverroth, G. M. B., Welang, C., Mocelin, R. N., Postay, D., Bertoncello, K. T., Franscescon, F., Rosemberg, D. B., Dal Magro, J., & Dalla Corte, C. L. (2015). Copper acutely impairs behavioral function and muscle acetylcholinesterase activity in zebrafish (Danio rerio). Ecotoxicology and Environmental Safety, 122, 440–447. https://doi.org/10.1016/j.ecoenv.2015.09.012
- Hwang, J. E. C., de Bruyne, M., Warr, C. G., & Burke, R. (2014). Copper overload and deficiency both adversely affect the central nervous system of Drosophila. Metallomics: integrated Biometal Science, 6(12), 2223–2229. https://doi.org/10.1039/C4MT00140K
- Jordan, K. W., Craver, K. L., Magwire, M. M., Cubilla, C. E., Mackay, T. F. C., & Anholt, R. R. H. (2012). Genome-wide association for sensitivity to chronic oxidative stress in Drosophila melanogaster. PloS One, 7(6), e38722. https://doi.org/10.1371/journal.pone.0038722
- Klimaczewski, C. V., Ecker, A., Piccoli, B., Aschner, M., Barbosa, N. V., & Rocha, J. B. T. (2018). Peumus boldus attenuates copper-induced toxicity in Drosophila melanogaster. Biomedicine & Pharmacotherapy = Biomedecine & pharmacotherapie, 97, 1–8. https://doi.org/10.1016/j.biopha.2017.09.130
- Lee, B.-M., Kacew, S., & Kim, H. S. (2017). Lu’s basic toxicology: Fundamentals, target organs, and risk assessment. CRC Press.
- Letelier, M. E., Sánchez-Jofré, S., Peredo-Silva, L., Cortés-Troncoso, J., & Aracena-Parks, P. (2010). Mechanisms underlying iron and copper ions toxicity in biological systems: Pro-oxidant activity and protein-binding effects. Chemico-Biological Interactions, 188(1), 220–227. https://doi.org/10.1016/j.cbi.2010.06.013
- Lu, Q., Zhang, Y., Zhao, C., Zhang, H., Pu, Y., & Yin, L. (2022). Copper induces oxidative stress and apoptosis of hippocampal neuron via pCREB/BDNF/and Nrf2/HO-1/NQO1 pathway. Journal of Applied Toxicology: JAT, 42(4), 694–705. https://doi.org/10.1002/jat.4252
- Mirzoyan, Z., Sollazzo, M., Allocca, M., Valenza, A. M., Grifoni, D., & Bellosta, P. (2019). Drosophila melanogaster: A model organism to study cancer. Frontiers in Genetics, 10, 51. https://doi.org/10.3389/fgene.2019.00051
- Mukherjee, S., & Mishra, M. (2020). Biochemical estimation to detect the metabolic pathways of Drosophila. In M. Mishra (Ed.), Fundamental approaches to screen abnormalities in Drosophila (pp. 135–149). Springer US.
- Najimi, S., Bouhaimi, A., Daubèze, M., Zekhnini, A., Pellerin, J., Narbonne, J. F., & Moukrim, A. (1997). Use of acetylcholinesterase in Perna perna and Mytilus galloprovincialis as a biomarker of pollution in Agadir Marine Bay (South of Morocco). Bulletin of Environmental Contamination and Toxicology, 58(6), 901–908. https://doi.org/10.1007/s001289900419
- Nunes, B. (2011). The use of cholinesterases in ecotoxicology. In D. M. Whitacre (Ed.), Reviews of environmental contamination and toxicology (Vol. 12, pp. 29–59). Springer New York.
- Oliveira, C. S., Nogara, P. A., Lima, L. S., Galiciolli, M. E. A., Souza, J. V., Aschner, M., & Rocha, J. B. T. (2022). Toxic metals that interact with thiol groups and alteration in insect behavior. Current Opinion in Insect Science, 52, 100923. https://doi.org/10.1016/j.cois.2022.100923
- Pandey, U. B., & Nichols, C. D. (2011). Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacological Reviews, 63(2), 411–436. https://doi.org/10.1124/pr.110.003293
- Pepeu, G., & Giovannini, M. G. (2004). Changes in acetylcholine extracellular levels during cognitive processes. Learning & Memory (Cold Spring Harbor, N.Y.), 11(1), 21–27. https://doi.org/10.1101/lm.68104
- Pilehvar, A., Town, R. M., & Blust, R. (2020). The effect of copper on behaviour, memory, and associative learning ability of zebrafish (Danio rerio). Ecotoxicology and Environmental Safety, 188, 109900. https://doi.org/10.1016/j.ecoenv.2019.109900
- Pyle, G. G., & Mirza, R. S. (2007). Copper-impaired chemosensory function and behavior in aquatic animals. Human & Ecological Risk Assessment. 13(3), 492–505. https://doi.org/10.1080/10807030701340995
- QCVN. (2009). National technical regulation on drinking water quality (Vol. QCVN 01:2009/BYT). Ministry of Health of Viet Nam.
- Riaz, B., Zahoor, M. K., Zahoor, M. A., Majeed, H. N., Javed, I., Ahmad, A., Jabeen, F., Zulhussnain, M., & Sultana, K. (2018). Toxicity, phytochemical composition, and enzyme inhibitory activities of some Indigenous weed plant extracts in fruit fly, Drosophila melanogaster. Evidence-Based Complementary and Alternative Medicine: eCAM, 2018, 2325659–2325611. https://doi.org/10.1155/2018/2325659
- Siddique, Y. H., Haidari, M., Khan, W., Fatima, A., Jyoti, S., Khanam, S., Naz, F., Rahul, Ali, F., Singh, B. R., Beg, T., Mohibullah, & Naqvi, A. H. 2015. Toxic potential of copper-doped ZnO nanoparticles in Drosophila melanogaster (Oregon R). Toxicology Mechanisms and Methods, 25(6), 425–432. https://doi.org/10.3109/15376516.2015.1045653
- Sparks, D. L., & Schreurs, B. G. (2003). Trace amounts of copper in water induce β-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America, 100(19), 11065–11069. https://doi.org/10.1073/pnas.1832769100
- Squitti, R., Lupoi, D., Pasqualetti, P., Forno, G. D., Vernieri, F., Chiovenda, P., Rossi, L., Cortesi, M., Cassetta, E., & Rossini, P. M. (2002). Elevation of serum copper levels in Alzheimer’s disease. Neurology, 59(8), 1153–1161. https://doi.org/10.1212/wnl.59.8.1153
- Strausak, D., Mercer, J. F. B., Dieter, H. H., Stremmel, W., & Multhaup, G. (2001). Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases. Brain Research Bulletin, 55(2), 175–185. https://doi.org/10.1016/S0361-9230(01)00454-3
- Sun, Q., Ying, M., Ma, Q., Huang, Z., Zou, L., Liu, J., Zhuang, Z., & Yang, X. (2016). Proteomic analysis of hippocampus in mice following long-term exposure to low levels of copper. Toxicology Research, 5(4), 1130–1139. https://doi.org/10.1039/c5tx00456j
- Tanaka, D., Nakada, K., Takao, K., Ogasawara, E., Kasahara, A., Sato, A., Yonekawa, H., Miyakawa, T., & Hayashi, J.-I. (2008). Normal mitochondrial respiratory function is essential for spatial remote memory in mice. Molecular Brain, 1(1), 21. https://doi.org/10.1186/1756-6606-1-21
- Taylor, P. (2017). Anticholinesterase agents. In L. L. Brunton, R. Hilal-Dandan, & B. C. Knollmann (Eds.), Goodman & Gilman’s: The pharmacological basis of therapeutics (13rd ed.). McGraw-Hill Education.
- Tilton, F., Tilton, S. C., Bammler, T. K., Beyer, R., Farin, F., Stapleton, P. L., & Gallagher, E. P. (2008). Transcriptional biomarkers and mechanisms of copper-induced olfactory injury in zebrafish. Environmental Science & Technology, 42(24), 9404–9411. https://doi.org/10.1021/es801636v
- Waggoner, D. J., Bartnikas, T. B., & Gitlin, J. D. (1999). The role of copper in neurodegenerative disease. Neurobiology of Disease, 6(4), 221–230. https://doi.org/10.1006/nbdi.1999.0250
- Wong, R., Piper, M. D. W., Wertheim, B., & Partridge, L. (2009). Quantification of food intake in Drosophila. PloS One, 4(6), e6063. https://doi.org/10.1371/journal.pone.0006063
- World Health Organization (WHO). (2004). Copper in drinking-water, background document for development of who guidelines for drinking-water quality (Vol. WHO/SDE/WSH/03.04/88). World Health Organization.
- Yamaguchi, M., & Yoshida, H. (2018). Drosophila as a model organism. In Drosophila models for human diseases (pp. 1–10). Elsevier.
- Yordanova, E. (2017). “In vitro” and “in vivo” inhibitory effect of copper sulphate upon the activity of blood catalase. Scripta Scientifica Medica, 11, 97–103. doi:https://doi.org/10.14748/ssm.v11i0.3553
- Zamberlan, D. C., Halmenschelager, P. T., Silva, L. F. O., & da Rocha, J. B. T. (2020). Copper decreases associative learning and memory in Drosophila melanogaster. The Science of the Total Environment, 710, 135306. https://doi.org/10.1016/j.scitotenv.2019.135306
- Zhang, T., Xu, L., Wu, J.-J., Wang, W.-M., Mei, J., Ma, X.-F., & Liu, J.-X. (2015). Transcriptional responses and mechanisms of copper-induced dysfunctional locomotor behavior in zebrafish embryos. Toxicological Sciences: An Official Journal of the Society of Toxicology, 148(1), 299–310. https://doi.org/10.1093/toxsci/kfv184