Abstract
Using diluted seawater for irrigation presents a potential solution to tackle water scarcity and optimize water usage in regions where there is a shortage of freshwater resources. Therefore, the objective of current study was to assess the efficacy of proline and zinc nanoparticles (ZnO-NPs), either alone or in combination, in alleviating adverse impacts of diluted seawater irrigation [2.5% (EC, 1.6) and 5% (EC, 2,3)] on the growth and productivity of pea plants (cv. Master B) during the winter seasons of 2022/2023 and 2023/2024. The results indicated that irrigation with diluted seawater had negative effects on several growth parameters of peas, including plant height, leaf area, plant dry weight, chlorophyll pigment (Ch a, Ch b, and total Ch) content, and seed chemical composition. In contrast, foliar application of ZnO-NPs, proline, or their combination improved plant growth, productivity, oxidative enzyme activities, net photosynthesis, and phenolic compound content under salinity stress. Furthermore, these treatments positively influenced the content of essential nutrients (NPK), vitamin C, carbohydrate percentage, and crude protein in pea seeds. The combination of ZnO-NPs and proline yielded the highest values for most parameters during the experimental seasons. Overall, the interaction between ZnO-NPs and proline showed promise in enhancing pea plant growth and productivity, especially in environments characterized by salt stress.
REVIEWING EDITOR:
1. Introduction
Pea (Pisum sativum L.) is one of the most important legume crops. It is cultivated and consumed worldwide. Pea is a valuable source of plant-based protein, dietary fiber, vitamins (such as vitamins C, K, and several B vitamins), minerals (including iron, potassium, and magnesium), and phytonutrients. It is typically grown for its edible seeds and is used in various culinary preparations, including soups, salads, stir-fries, and side dishes. Peas are also commonly used in frozen or canned forms. Its high nutritional value, versatility, and ease of cultivation make peas an important crop in many agricultural systems (El-Saied & Elsayed, Citation2022).
Limiting irrigation water availability is a major problem in many regions, particularly in areas facing water scarcity or competing demand for water resources. This can have a detrimental impact on agricultural productivity and food production (Begna, Citation2020). The scarcity of freshwater and changing climate pose significant challenges to agricultural irrigation through reduction in quantity and quality of irrigation water (Amiri Forotaghe et al., Citation2022; Ebrahimi et al., Citation2021).
Seawater is abundantly available, and using it directly for crop irrigation is not without challenges and limitations (El-Beltagi et al., Citation2022a,Citation2022b; Javeed et al., Citation2021). Egypt faces significant challenges in terms of water scarcity owing to its arid and desert climate, rapid population growth, and limited freshwater resources. Diluted seawater in agriculture (seawater intrusion) has been explored as a potential solution to address freshwater scarcity in certain regions. Diluting seawater with freshwater or using a blend of seawater and freshwater can help reduce salinity levels and make it more suitable for irrigation (Mansouri & Kheloufi, Citation2017). The use of diluted seawater for irrigation is becoming increasingly necessary to sustain economically viable crop production, particularly in regions that face freshwater scarcity. Diluted seawater has been explored and evaluated as a potential alternative irrigation source, with research indicating its capacity to improve plant growth under specific conditions (Kheloufi et al., Citation2016).
Recently, there has been a growing focus on utilizing nanoparticles (NPs) to bolster plant growth and productivity under saline conditions. The application of NPs, such as ZnO-NPs, has emerged as a promising approach to boost plant resilience to salinity stress (Mahawar et al., Citation2023). ZnO-NPs demonstrate antioxidant characteristics and can neutralize reactive oxygen species (ROS), thereby alleviating oxidative stress in plants. This antioxidant activity plays a crucial role in protecting plant cells from damage and maintaining their normal physiological functions (Priyanka et al., Citation2019). The application Zn also serves to shield the photosynthetic system from oxidative stress caused by salinity, while enhancing stomatal function, chlorophyll production, carbon assimilation, and the accumulation of osmolytes and hormones. Furthermore, Zn application fosters the production of secondary metabolites and boosts the expression of genes responsive to stress, thereby activating antioxidant defenses to combat the harmful effects of salt stress (Alprol et al., Citation2023; Shao et al., Citation2023). Furthermore, Zn serves as a cofactor for numerous enzymes that regulate essential metabolic pathways and promote cellular functions in plants (Rai et al., Citation2021). ZnO-NPs have garnered considerable attention in agriculture and other fields owing to their advantageous properties. These include high conductivity, non-toxicity, ready availability, eco-friendliness, and cost-effectiveness compared with other types of nanoparticles (Al Jabri et al., Citation2022; Priyanka et al., Citation2019).
Proline is a well-recognized adaptive free amino acid in plants that accumulate under stress conditions including salinity. As an osmolyte, proline plays a crucial role in regulating osmotic balance and protecting cellular structures from the detrimental effects of elevated salt concentrations (Ismail & Halmy, Citation2018). Proline acts as a scavenger of ROS, helping mitigate oxidative damage and maintain cellular redox homeostasis (Okuma et al., Citation2008). Proline has been implicated in the regulation of numerous physiological processes associated with salt-stress tolerance. It exhibits the ability to modulate ion transport mechanisms, such as the regulation of ion channels and transporters, thereby assisting in maintaining the ion balance under salt stress conditions. Additionally, proline can affect the functionality of enzymes involved in antioxidant defenses, stress signaling pathways, and hormone biosynthesis and signaling (Ismail & Halmy, Citation2018; Naliwajski & Skłodowska, Citation2021).
This study sought to explore how irrigating with saline water affects the growth and productivity of pea. It aimed to mitigate the adverse impacts of saline irrigation water by examining the individual and combined effects of zinc nanoparticles and proline on the growth, biochemical composition, and physiological responses of the pea cultivar Master B when exposed to diluted seawater irrigation.
2. Materials and methods
Two pot experiments were conducted in a greenhouse at the Agricultural Botany Department, Faculty of Agriculture, Kafrelsheikh University, Kafr El-Sheikh, Egypt, during two successive winter seasons, 2022/2023 and 2023/2024. The experiments were conducted using a completely randomized design with three replicates. Treatments consisted of all possible combinations of two levels of seawater dilution (2.5% and 5%) and two exogenous treatments: application of zinc nanoparticles (ZnO-NPs) with 30 nm in diameter, prepared according to El-Shafai et al. (Citation2024) at concentration of 20 mg/L, and proline at 300 mg/L and their combination. These treatments aimed to investigate their effects on the growth, biochemical, physiological, and yield characteristics of the pea cultivar Master B. Foliar applications of ZnO-NPs and proline amino acids at rates specified in the study were carried out twice during the experiment. These applications commenced 21 d after sowing, with an interval of two weeks between each application.
2.1. Soil sampling
Before commencing the experiment, soil was collected from the surface layer of a designated farm in Sakha, Kafr El-Sheikh Governorate, Egypt. Soil analysis was conducted following the method outlined by Dane & Topp (Citation2020) and Sparks et al. (Citation2020), and the results are presented in .
Table 1. The physical and chemical characteristics of experimental soil surface (0–30 cm) evaluated during the 2022/2023 and 2023/2024 seasons.
The pea seeds utilized were sourced from the Agricultural Research Center (ARC) and sown on November 7th and 9th of November in both 2022 and 2023, respectively. The seeds were sown in polyethylene pots measuring 30 cm in diameter and 30 cm in depth. Each pot was filled with 15 kg air-dried soil. Fifteen days after sowing, the plants were thinned and only the three most uniform plants per pot were retained. Harvesting was performed on January 25th in the first and 22nd in the second season. Adherence to the Ministry of Agriculture and Soil Reclamation (MASR), Egypt, and recommendations were maintained for all agricultural practices. Calcium superphosphate (15.5% P2O5) was added at 3.3 g per pot. The nitrogen dose (effective dose) in ammonium sulfate form (20.5% N) was applied at 0.7 g a pot of planting. Potassium sulfate (48% K2O) was added at 1.6 g per pot. The recommended fertilizer doses were divided into two applications. The first dose was applied 15 days after sowing, followed by a second dose two weeks later. The pots were irrigated with diluted seawater in equal volumes tailored to the specific needs of the plants according to the proportions studied. In contrast, the control treatment involved irrigation with freshwater alone.
2.2. Vegetative traits
Growth parameters of pea plants were estimated using three samples collected 50 days after sowing after applying all treatments with proline, ZnO-NPs, and their interaction to measure the plant height (cm) and leaf area (cm2), which were measured using a portable laser leaf area meter (CI-02; CID Bio-Science). The plant samples were dried in an oven at 70 °C until a constant weight was achieved. After cooling in a desiccator, the dried samples were reweighed to determine their final dry weights.
2.3. Chlorophyll pigments
Fifty days after sowing, the third leaf from the plant tip was harvested and extracted in 5 ml of dimethylformamide to estimate the concentrations of chlorophyll (chl. a, b, or total) pigments in µg/g fresh weight according to Moran (Citation1982).
2.4. Antioxidants enzymes
To determine the activities of antioxidant enzymes, lipid peroxides, _-tocopherol and protein level, fresh leaves (500 mg) were homogenized at 4 _C in 5 cm3 of 100 mM sodium phosphate buffer (pH 7.5) containing 1 mM ascorbic acid, 1 mM EDTA, 0.5 M NaCl, 1%. The homogenate was centrifuged at 20,000_ g for 20 min at 4 _C. The supernatant was used to determine enzyme activity as well as lipid peroxides and protein content. Crude extracts were used for _-tocopherol level determination.
For assays of antioxidant enzyme activity, 50 days after seed sowing, the third leaf from the plant tip was harvested and finely ground using liquid nitrogen. Ground tissue was then extracted with ice-cold 0.1 M Tris-HCl buffer (pH 7.5) containing 5% sucrose and 0.1% 2-mercaptoethanol, with a buffer volume to fresh weight ratio of 3:1. Following homogenization, the resulting mixture was centrifuged at 10,000 × g for 20 min at 4 °C. The supernatant obtained after centrifugation was used to determine the enzyme activity and protein content. It is worth noting that all steps of the enzyme extraction and assay were performed at 4 °C to maintain enzyme stability. Catalase (CAT, EC 1.11.1.6) activity was determined by measuring the decomposition of hydrogen peroxide (H2O2) at 240 nm, in accordance with the method described by Aebi (Citation1984). The enzyme’s activity was denoted as U per milligram of protein, which corresponds to mM of H2O2 produced per minute per milligram of protein. POD (EC 1.11.1.7) activity was determined following the protocol outlined by Polle et al. (Citation1994). The enzyme’s activity was indicated as U per milligram of protein, equivalent to µmol of ascorbate consumed per minute per milligram of protein.
Polyphenol oxidase (PPO) (EC 1.14.18.1) activity was assessed following the protocol outlined by Oktay et al. (Citation1995). The reaction mixture comprised 100 μl of crude enzyme, 600 μl of catechol, and 2.3 ml of phosphate buffer (0.1 M, pH 6.5). Absorbance readings at 420 nm were taken initially and after 1 minute. One unit of PPO activity was defined as the enzyme quantity causing a 0.001 increase in absorbance per minute at 420 nm. Enzyme activity was expressed as units per milligram of protein. Protein concentrations were determined following the method outlined by Bradford (Citation1976), utilizing bovine serum albumin (BSA) as a reference standard.
2.4.1. Endogenous proline content, membrane stability and total phenols content
Fifty days after sowing, fresh leaves were used to estimate the proline content (µmol g−1FW) using a spectrophotometer (Shimadzu UV 1601) at a wavelength of 520 nm, following the method described by Bates et al. (Citation1973). Additionally, electrolyte leakage was estimated based on the protocol outlined by Lutts et al. (Citation1996) and total phenolic compounds were determined according to the methodology described by Bessada et al. (Citation2016).
2.5. Transpiration rate, stomata conductance, leaf CO2 and net photosynthesis
Fifty days after sowing, assessing the carbon (C) and water content of a plant entails evaluating its stomatal characteristics, diffusive conductance, and the rates of CO2 or water vapor exchange. To accomplish this, a portable porometer referred to as the ‘steady-state porometer, LICOR, LI-1600, Lincoln, NE, USA.’ This device utilizes an open gas exchange system to measure the concentrations of CO2 and H2O entering and leaving a cuvette positioned on or near leaves (Sicher & Barnaby, Citation2012). Stomatal conductance (cm−2s−1) was assessed in fully expanded leaves, while the net photosynthetic rate (NPR, A) (g m−2) utilized in stomatal conductance (gs) models was calculated using the following formula: A = Amax × f (PAR). Here, photosynthetically active radiation (PAR) is acquired from on-site measurements conducted in the morning, around noon, and afternoon. Following the calculation of the CO2 concentration, the leaf transpiration rate (LTR) (μg cm−2 s−1) was directly assessed on the same leaf, encompassing the combined rates of both the upper and lower leaf surfaces. Additionally, this equipment allows for the analysis of CO2 response curves in the field, with the evaluation of adaxial or abaxial diffusive resistance conducted on upper fully expanded leaves during the estimation process using a steady-state porometer (Gaballah et al., Citation2023).
2.5.1. Leaf H2O2 content
The extract for determining the concentration of hydrogen peroxide (H2O2) in pea leaves (50 days after sowing) was prepared as follows: 0.1 g of fresh sample was ground with liquid nitrogen and then diluted with 4 mL of 0.1% trichloroacetic acid (TCA). After centrifugation for 10 minutes at 3000 rpm, the supernatant was collected for further analysis. For H2O2 measurements in plant leaves, 500 µL of the supernatant was mixed with 1 mL of 1 M potassium iodide (KI). The absorbance of the mixture was measured at 390 nm using a spectrophotometer (Velikova et al., Citation2000). The H2O2 content was expressed as the fresh weight (µmol g−1 FW).
2.6. Mineral element nutrients
Dried pea seeds were subjected to wet digestion with a mixture of sulfuric and perchloric acid to estimate nitrogen (N), phosphorus (P), and potassium (K) levels. Nitrogen levels were determined using the Kjeldahl method and phosphorus levels were measured using a spectrophotometric method. Additionally, the flame photometer method was used to determine potassium levels, following the procedures outlined by Walinga et al. (Citation2013).
2.7. Yield and yield components
At the full green maturity stage (70 d after sowing), the yield and its components were measured and expressed as fresh pod weight (g), pod number per plant, and yield per plant (g/plant). Additionally, the total carbohydrate and vitamin C concentrations in fresh green pea seeds were estimated following the methods outlined in AOAC (Citation1990). The dry weight (g/seed) of the pea seeds was determined by drying them at 70 °C in an electric oven until a constant weight was achieved. Furthermore, crude protein content in dried pea seeds was estimated according to AOAC (Citation2000).
2.7.1. Statistical analysis
Data analysis was conducted using CoStat version 6.400 (1998–2008) software following the statistical technique outlined in the methodology based on Gomez & Gomez (Citation1984). The significance level for the least significant difference (LSD) test was set at α ≤ 0.05. Duncan’s multiple range test was used to compare the treatment means, as described by Duncan (Citation1955). Additionally, a heatmap was created to summarize the findings related to agronomic and biochemical aspects using the online tool ClustVis (https://biit.cs.ut.ee/clustvis/).
3. Results and discussion
3.1. Vegetative traits
Data presented in indicate significant effects on growth traits, including plant height, dry weight, and leaf area per plant, when pea plants were irrigated with diluted seawater (DSW) compared to the control group, which was irrigated with freshwater, across both seasons. Spraying pea plants with ZnO-NPs, proline, or their combination significantly increased growth traits under well irrigation with fresh water. Applying the ZnO-NPs, proline, and their combination mitigated the harmful effects on these growth traits under irrigation with diluted seawater (DSW1 and 2) in both seasons. The highest growth parameters were recorded for the combination of ZnO NPs and proline (). Diluting seawater (DSW1 and 2) with freshwater can help reduce salinity levels and make it more suitable for irrigation (Mansouri & Kheloufi, Citation2017). Salinity refers to the concentration of dissolved salts in water, and high salinity levels can be detrimental to plant growth. The adverse effects of saline water on plants can be mainly attributed to various factors, including the low osmotic potential of the soil solution, nutritional imbalances, and specific ion effects. These factors impede plant growth and development of plants (Ahmed et al., Citation2019).
Table 2. Effect of ZnO-NPs, proline and their combination on plant height (cm), dry weight (g)/plant and leaf area (cm2)/plant of pea plants irrigated with diluted seawater levels (2.5 and 5%) after 50 days from sowing during 2022/2023 and 2023/2024 seasons.
Applying ZnO-NPs significantly alleviated the adverse effects of salinity stress on the growth parameters of pea plants subjected to irrigation with diluted seawater. This improvement may be attributed to enhancements in various biochemical and physiological factors, including essential mineral uptake, expansion of leaf photosynthetic area, augmentation of the photosynthetic electron transport rate, enhancement of photosystem II (PSII) quantum yield, and modulation of proton conductance under NaCl stress (Mahawar et al., Citation2024). Zinc (Zn) serves as a crucial micronutrient for plants, fulfilling essential roles in range of physiological processes. These include facilitating cell division and expansion, aiding chlorophyll synthesis, promoting protein and auxin production, and contributing to nucleic acid and carbohydrate metabolism of carbohydrates (Hafeez et al., Citation2013).
Increases in plant height and leaf area are associated with high rates of cell division and enlargement. Similarly, an increase in plant dry weight is associated with various metabolic processes that contribute to the synthesis and accumulation of important biomolecules such as carbohydrates, proteins, and nucleic acids. In addition, Zn can readily gain or lose electrons, making it a versatile cofactor for numerous enzymes involved in regulating important biochemical reactions (Rai et al., Citation2021). Nanoparticles of zinc, including zinc nanoparticles or nano-fertilizers, have demonstrated substantial potential in improving plant growth and increasing resilience to environmental stress. The unique properties of nanoparticles, including their small size and high surface area, contribute to their effectiveness as nanofertilizers (Naseer et al., Citation2023). ZnO significantly increased indole-3-acetic acid (IAA) concentration, which increased the density of the lateral roots. This finding supports the hypothesis that bulk ZnO particles may increase lateral root development by increasing IAA concentration of IAA (Castiglione et al., Citation2023). An increase in the density of the root system, including the lateral roots, can positively impact plant growth, including in pea plants. An elevated density of roots leads to a greater total surface area available for absorption of nutrients and water from the soil.
Application of proline via foliar spraying significantly mitigated the negative impact of salinity stress on pea plant growth parameters. Its role in osmotic regulation helps plants tolerate water stress by maintaining cell turgor and osmotic balance, thus contributing to their ability to survive and adapt to adverse saline stress. In response to these conditions, plants can accumulate proline as an osmo-protectant (Naliwajski & Skłodowska, Citation2021). Moreover, proline plays a protective role in various membrane structures within plant cells, including phospholipids, plasmalemma, mitochondria, and plastid membranes (Ashraf & Harris, Citation2004). Its ability to enhance membrane stability, reduce lipid peroxidation, and scavenge ROS allows it to preserve membrane integrity and function, contributing to the ability of plants to withstand and adapt to environmental stress (Signorelli et al., Citation2014; Naliwajski & Skłodowska, Citation2021). Notably, the combination treatment of ZnO-NPs and proline, particularly under diluted seawater irrigation (DSW1 and 2), resulted in the greatest values for pea plant height, leaf area, and dry weight per plant.
3.2. Chlorophyll content
Chlorophyll (Chl) plays a pivotal role in photosynthesis. Measuring the level of chlorophyll in a plant can serve as an indicator of its growth and photosynthetic activity. It was noted that chlorophyll pigment content (a, b, or total chlorophyll) was significantly reduced in pea plants under diluted seawater irrigation compared to that in well-irrigated plants (control). Additionally, the greatest decrease in chlorophyll content was recorded in pea plants irrigated with the highest seawater level (). Salinity stress can adversely affect the chlorophyll content in radish plants (Mahawar et al., Citation2024). Salinity stress may lead to degradation of chlorophyll molecules in plants. High salt levels may disrupt the structure and function of chloroplasts, leading to the breakdown of chlorophyll molecules and reduced chlorophyll pigment content. This degradation can be attributed to oxidative stress caused by salt-induced ROS production. Due to salt stress, the chlorophyll content decreased, probably because of low antioxidant enzyme activity (Nasrudin et al., Citation2022; Yasar et al., Citation2008). Elevated salt concentrations in the soil or irrigation water can create an osmotic imbalance that affects the water and mineral uptake of plants. This disruption in water and mineral uptake can lead to physiological disturbances in plants, including degradation of chlorophyll content in the leaves (Li et al., Citation2017).
Figure 1. Effect of ZnO-NPs, proline and their combination on chlorophyll content (chl. a, b and total chl.) of pea plants irrigated with diluted seawater levels (DSW) after 50 days from sowing during 2022/2023 and 2023/2024 seasons. Values sharing at least one identical letter were not significantly various at the P < 0.05 level.
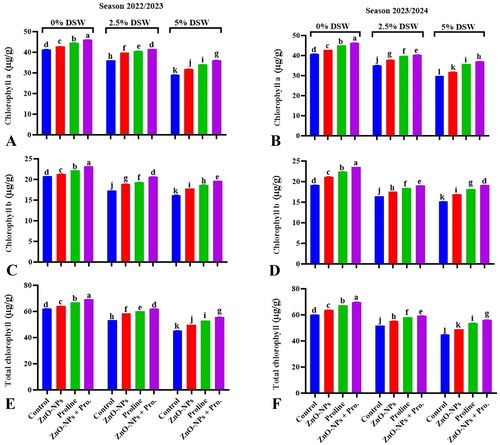
Utilizing ZnO-NPs through foliar spraying effectively reduced the adverse effects of salinity stress. on the chlorophyll content in pea plants compared to those irrigated with fresh water (control). Zinc (Zn) plays a significant role in mitigating the detrimental effects of various abiotic stresses, including salinity (Zafar et al., Citation2017). Zinc (Zn) supplementation has been noted to improve plant growth and alleviate the negative impacts of salinity stress through several mechanisms. These include promoting chlorophyll synthesis and activating the antioxidant defence system (Al-Zahrani et al., Citation2021). Nevertheless, the association between salinity, chlorophyllase activity, and chlorophyll degradation is intricate and can be affected by various factors, including the presence of additional elements, such as zinc (Zn). Zinc is an essential micronutrient in plants and plays a role in various physiological processes, including chlorophyll synthesis and stability. Zn has been shown to reduce the activity of chlorophyllase enzymes, thereby inhibiting chlorophyll degradation. Zinc accumulation in plants has been noted to have a beneficial effect on chlorophyll and carotenoid synthesis, both of which are crucial pigments for photosynthesis. (Srivastav et al., Citation2021). Moreover, Zn application has been found to have positive effects on chlorophyll fluorescence and the ultrastructure of chloroplasts, which are both important aspects of plant photosynthesis (Shao et al., Citation2023).
The advantageous influence of exogenous proline on plant growth under salt stress is commonly attributed to changes in chlorophyll content (El Moukhtari et al., Citation2020). Proline supplements have been found to have positive effects on the chlorophyll pigment content of two-year-old Olea europaea plants exposed to high salt concentrations (Ben Ahmed et al., Citation2011). The enhanced efficiency of photosynthetic pigments in plants treated with exogenous proline under salt stress can be attributed to several mechanisms. These mechanisms include stimulating chlorophyll biosynthesis, inhibiting degradation, and enhancing ROS scavenging of ROS (Ibrahim et al., Citation2019). The synergistic effect of ZnO-NPs and proline treatment, particularly under diluted seawater irrigation (DSW1 and DSW2), seems to have increased chlorophyll content. Hence, the increased levels of chlorophyll observed in this combination indicate enhanced photosynthetic efficiency, potentially resulting in enhanced plant growth or heightened stress tolerance under diluted seawater conditions.
3.3. Antioxidant enzyme activity
Antioxidant enzymes serve as a crucial defense mechanism within cells, helping neutralize the damaging effects of ROS. They are produced as natural byproducts of cellular metabolism and are generated under certain stress conditions, such as salinity stress. It is worth noting that the activity of antioxidant enzymes, including CAT, POX, and PPO, increased in pea plants irrigated with diluted seawater compared to plants irrigated with fresh water during both seasons (). Higher enzyme activity values were recorded in plants irrigated with higher seawater dilutions, indicating an adaptive response to increased salinity stress.
Figure 2. Effect of ZnO-NPs, Proline and their combination on antioxidants enzymes (CAT, POD, and PPO) activity of pea plants irrigated with diluted seawater after 50 days from sowing during 2022/2023 and 2023/2024 seasons. Values sharing at least one identical letter were not significantly various at the P < 0.05 level.
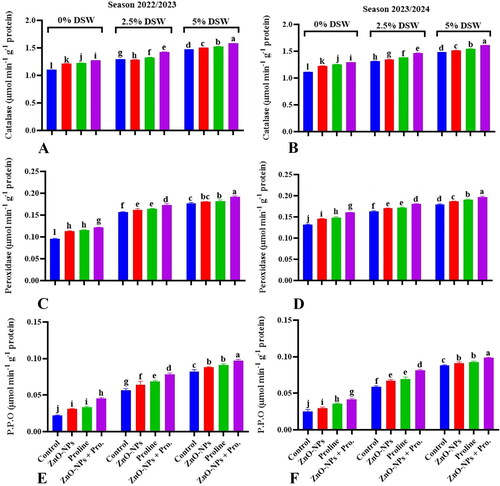
Plants activate antioxidant defense mechanisms to mitigate the harmful effects of ROS and maintain cellular homeostasis under these challenging conditions. ROS are highly reactive molecules known for their ability to inflict damage to different cellular components, including proteins, DNA, lipids, or cellular membranes (Jiang et al., Citation2021). Antioxidant enzymes, such as CAT, POX, and PPO, play vital roles in neutralizing ROS and preserving cellular redox balance (Hasanuzzaman et al., Citation2021).
Using ZnO-NPs through foliar spraying successfully mitigated the negative impacts of salinity stress on antioxidant enzyme activity in pea plants, even when exposed to various levels of seawater dilution.
Zinc has been found to decrease the activity of NADPH oxidase enzymes bound to the cell membrane. NADPH oxidase is an enzyme complex that is responsible for generating ROS in response to various stimuli. Zinc can decrease ROS production by inhibiting NADPH oxidase activity, which helps alleviate oxidative stress (Shao et al., Citation2023). Studies have indicated that foliar application of proline can improve the activity of antioxidant enzymes in plants, including CAT, POX, and PPO. These antioxidant enzymes scavenge ROS and shield plant cells from oxidative damage. It is crucial to note that the effects of proline on salt tolerance may vary depending on the plant species, concentration and timing of proline application, and severity and duration of salt stress (El Moukhtari et al., Citation2020). Proline supplementation, whether provided exogenously or accumulated endogenously, can increase the activity of CAT, SOD, and POX. This augmentation of antioxidant enzyme activity further facilitates ROS scavenging and alleviates salt-induced oxidative stress (Ben Rejeb et al., Citation2014). The combination of ZnO-NPs and proline has been shown to enhance antioxidant enzyme activity in plants cultivated under seawater irrigation levels. This augmentation likely facilitated ROS scavenging and protected plant cells from oxidative damage.
3.4. Endogenous proline content, leaf membrane stability index and phenols content
Under salinity stress, endogenous proline content, leaf membrane stability (LMS), and phenol content are three important factors that play a role in a plant’s response to stress. There was a positive correlation between endogenous proline content, leaf electrolyte leakage, and phenolic compound content with increasing seawater irrigation levels compared to those grown in non-saline irrigation water during both seasons. Specifically, the highest values were observed in pea plants irrigated at the second seawater level (). Proline accumulation plays a role in osmotic adjustment and osmoprotection under salinity stress. It helps to maintain cellular water potential, stabilizes cell membranes, and protects against oxidative damage (Hasanuzzaman et al., Citation2021; Sarker & Oba, Citation2020). Therefore, higher proline content in pea plants irrigated with higher seawater levels indicates a stronger adaptive response to salinity stress. During environmental stress, plant membranes undergo alterations, often leading to increased permeability and compromised integrity. The capacity of cell membranes to regulate ion movement across cell boundaries is a notable indicator of stress or injury in diverse plant tissues.
Figure 3. Effect of ZnO-NPs, proline and their combination on endogenous proline (A,B), leaf membrane stability index (C,D) or phenols content (E,F) of pea plants irrigated with seawater diluted after 50 days from sowing during 2022/2023 and 2023/2024 seasons. Values sharing at least one identical letter were not significantly various at the P < 0.05 level.
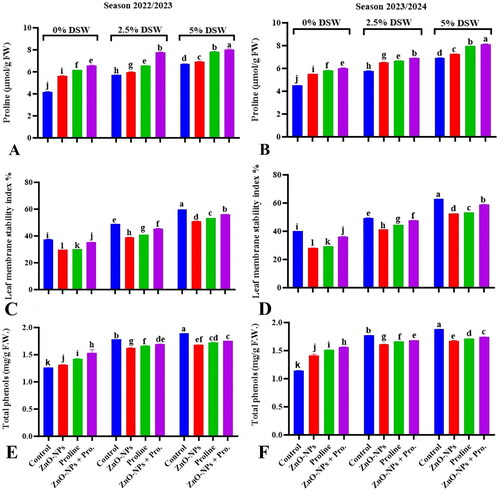
The regulation of ion movement is crucial for maintaining cellular homeostasis and proper physiological functioning (Blokhina et al., Citation2003; Farkhondeh et al., Citation2012). Phenolic compounds constitute a varied group of secondary metabolites that naturally occur in plants. They play crucial roles in plant defence mechanisms against various stresses, including salinity (Miranda et al., Citation2014). The phenolic compound content was significantly higher in salt-stressed pea plants than in unstressed plants (). The data suggest that the total phenolic compound content in pea plants increased when exposed to diluted seawater or saline conditions.
The concurrent increase in phenolic compound content under saline conditions suggests the significance of these compounds in enhancing stress resistance in pea plants. Phenolic compounds exhibit antioxidant activity, which may contribute to ROS detoxification and increase resistance to salinity stress (Ghanem et al., Citation2021). They can directly inactivate free lipid radicals and prevent lipid peroxidation, which causes oxidative damage to lipids in cell membranes. By inhibiting lipid peroxidation, phenolic compounds help to maintain cell membrane integrity and functionality under salinity stress (Castillo et al., Citation2022).
The application of ZnO-NPs led to a notable increase in endogenous proline content in pea leaves across various levels of seawater irrigation during both seasons (). Mahawar et al. (Citation2024) noted that ZnO-NPs enhance the accumulation of endogenous proline under salinity stress in radish plants (Raphanus sativus L.). Indeed, the role of ZnO-NPs in mitigating salt stress in plants can be confirmed by assessing various physiological parameters, including proline concentration. Proline serves multiple functions during stress conditions, including acting as a compatible osmolyte to maintain cell water potential, scavenging reactive oxygen species (ROS) to mitigate oxidative damage, and stabilizing proteins and membranes to maintain cellular integrity (Boscaiu et al., Citation2013). Applying zinc can substantially increase the content of plant phenols and flavonoids, which can contribute to enhanced antioxidant capacity, stress tolerance, and defense responses (Ahmad et al., Citation2017). Zn plays a critical role in preserving membrane stability and regulating the production of phenolic compounds in plants under salinity stress. Optimal levels of zinc can assist in alleviating the negative effects of salinity by protecting cell membranes from oxidative harm and bolstering the plant’s antioxidant defense mechanisms via the production of phenolic compounds (Shao et al., Citation2023).
The exogenous application of proline increased the endogenous proline content in pea plants under seawater irrigation levels during both seasons. This finding suggests that external proline application positively affects endogenous proline accumulation in plants. Proline is a common endogenous osmolyte that accumulates in plants in response to various abiotic stresses, including salinity. Supplying proline exogenously can augment osmoprotectant levels in plants, thereby bolstering their ability to withstand elevated salt concentrations in irrigation water (Heuer, Citation2010). Furthermore, salinity stress triggered the accumulation of endogenous proline in pea plants. Miranda et al. (Citation2014) observed that salinity stress resulted in increased accumulation of endogenous proline in Physalis peruviana L. plants.
The highest endogenous proline accumulation was recorded in the interaction between ZnO-NPs and proline application under both seawater irrigation levels during both seasons. The higher membrane stability resulting from the interaction between ZnO-NPs and proline compared to ZnO-NPs or proline alone suggests a synergistic or additive effect in improving membrane stability under salinity stress. This finding indicates that the combined application of ZnO-NPs and proline has a greater impact on preserving the membrane integrity than when these components are applied individually. Generally, proline accumulation in plants under salinity stress contributes to membrane stability and phenolic compound synthesis. Both processes, the accumulation of endogenous proline and production of phenolic compounds, play crucial roles in enabling plants to adapt to and tolerate saline environments (Hosseinifard et al., Citation2022).
3.5. Stomatal conductance and transpiration rate
Both stomatal conductance and transpiration rate are essential for regulating the water status of plants, uptake of nutrients, and overall plant growth and development.
Under salinity stress, pea plants responded by reducing stomatal conductance and transpiration rates due to water deficit and osmotic stress caused by the saline environment (). Rapid absorption of ions, particularly sodium (Na+) or chloride (Cl-) ions, by plant roots can lead to the accumulation of these ions in plant cells. This accumulation can have several adverse effects on plant-water relations, including osmotic imbalance, decreased water potential, reduced turgor pressure, and impaired water transport (Balasubramaniam et al., Citation2023; Betzen et al., Citation2019). In response to salinity-induced water stress, plants may exhibit stomatal closure as a mechanism to conserve water. When stomata are closed, transpiration rates decrease, thereby reducing water loss from the plant. However, stomatal closure can also limit the carbon dioxide (CO2) uptake required for photosynthesis, potentially compromising overall plant growth and productivity (Mirfattahi et al., Citation2017). Salinity stress induces changes in Abscisic acid (ABA) content and signaling within plants, leading to stomatal regulation (Zhao et al., Citation2021).
Figure 4. Effect of ZnO-NPs, proline and their combination on leaf CO2 (A), leaf H2O2 content (B), stomatal conductance (C), transpiration rate (D) and net photosynthesis (E) leaf membrane stability index (C,D) or phenols content (E and F) of pea plants irrigated with seawater diluted after 50 days from sowing during 2023/2024 season. Values sharing at least one identical letter were not significantly various at the P < 0.05 level.
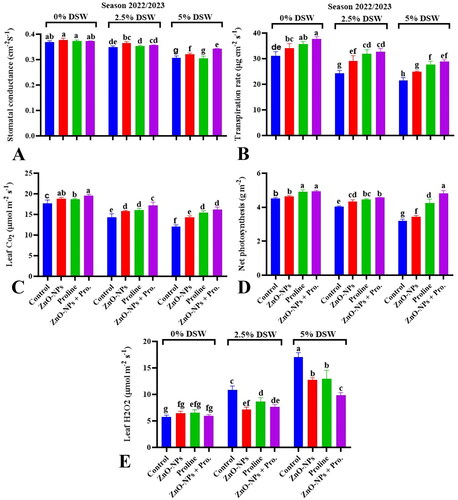
The application of ZnO-NPs via foliage is a promising strategy for alleviating the negative effects of salinity on pea plants. Nevertheless, zinc may indirectly affect transpiration because of its involvement in plant growth, stomatal regulation, and water uptake (Mahawar et al., Citation2024). Applying zinc (Zn) has been shown to influence the levels of phytohormones such as ABA in plants under salt stress (Chattha et al., Citation2022). Overall, although proline primarily functions in osmotic adjustment and stress tolerance, its effects on transpiration rate and stomatal conductance are mediated indirectly through its roles in osmotic regulation, stomatal regulation, antioxidant activity, and overall plant physiology (Ashraf & Foolad, Citation2007). The combination of ZnO-NPs and proline, resulting in increased stomatal conductance and transpiration rate under diluted seawater irrigation, suggests a potential synergistic effect between these factors in mitigating the negative impacts of salinity stress on plant water relations.
3.6. CO2 (fixed or around leaves) and photosynthesis rate
Salinity stress significantly affects CO2 fixed or around leaves and photosynthesis rate in plants due to various physiological changes induced by stress. Data in () indicated that the CO2 fixed or around leaves and photosynthesis rate values were reduced in pea plants irrigated with diluted seawater. The highest reduction occurred with the highest level of diluted seawater, and this trend was recorded in both seasons. Salinity stress typically results in a reduction in the CO2 fixed or around leaves or photosynthesis rate in plants. This is primarily due to stomatal closure, disruptions in nutrient uptake, and direct effects on photosynthetic processes (Van Zelm et al., Citation2020). Although stomatal closure helps reduce water loss by transpiration and minimizes the risk of dehydration, it can also limit the uptake of CO2 required for photosynthesis. This reduction in CO2 availability can compromise the dark reaction phase (Mirfattahi et al., Citation2017). Salt stress affects various aspects of plant physiology including photosynthesis. A notable effect is on the light-harvesting complex (LHC) and state transitions of photosynthesis (Chen & Hoehenwarter, Citation2015). Under salt stress conditions, various metabolic processes within plants, including those related to photosynthesis, are affected. Salt stress can affect ribulose-1,5- bisphosphate carboxylase/oxygenase (RuBisCO) activity and protein stability through various mechanisms. Additionally, it can alter sugar signaling pathways and disrupt plant sugar metabolism (Shumilina et al., Citation2019).
The application of ZnO-NPs mitigated the negative effects of salinity on the CO2 fixed or around leaves and photosynthesis rate in pea plants irrigated with diluted seawater during both seasons. This suggests that ZnO-NPs may have a protective effect on plants experiencing salt stress, potentially by improving water uptake and stomatal conductance (Mahawar et al., Citation2024) and by reducing ABA levels (Chattham et al., 2022). Foliar application of proline had similar effects on alleviating the negative effects of salt stress on CO2 fixed or around leaves and net photosynthesis during both seasons. Proline is widely recognized as an osmoprotectant (Naliwajski & Skłodowska, Citation2021) and has a protective role in various membrane structures, including plastid membranes (Ashraf & Harris, Citation2004). The concurrent application of ZnO-NPs and proline showed promising results in alleviating salt stress in pea plants compared to individual treatments during both seasons. This suggests that the synergistic effects of ZnO-NPs and proline might enhance plant tolerance to salt stress more effectively than either treatment alone.
3.6.1. Leaf H2O2 content
Salinity stress leads to the accumulation of (ROS), including hydrogen peroxide (H2O2), in plant tissues. The increase in leaf hydrogen peroxide (H2O2) content correlated with elevated levels of diluted seawater (). The increase in leaf H2O2 content indicated that pea plants experienced oxidative stress in response to saline irrigation. Oxidative stress can damage biomolecules, such as lipids, proteins, and nucleic acids, leading to impaired physiological functions, reduced growth, and productivity (Balasubramaniam et al., Citation2023; Salama et al., Citation2009).
Interestingly, both ZnO-NPs and proline, when applied individually or in combination, reduced leaf CO2 content in pea plants irrigated with diluted seawater during both the seasons. The highest reduction in leaf CO2 content was found with the ZnO-NP treatment. This result suggests that zinc and proline can reduce the production of ROS, which in turn helps alleviate oxidative stress (Ben Rejeb et al., Citation2014; Shao et al., Citation2023).
3.7. Yield and yield components
3.7.1. Number of pods, green pods weight per plant and seed dry weight (g/seed)
Data in shows a reduction in the number and pod weight per plant and average dry seed weight under irrigation with diluted seawater compared to the control during both seasons. This indicates that saline irrigation negatively affects the reproductive performance and yield of pea plants. The reproductive stage of plants is often particularly sensitive to the adverse effects of salinity stress (Balasubramaniam et al., Citation2023). Several factors contribute to the vulnerability at this stage, including water and nutrient uptake, ion toxicity, osmotic stress, and oxidative stress (Zhao et al., Citation2021).
Figure 5. Effect of ZnO-NPs, proline and their combination on number of pods/plant (A,B), green pods weight/plant (C,D) and an average of seed dry weight (E,F), of pea plants irrigated with seawater diluted after 70 days from sowing during 2023/2024 season. Values sharing at least one identical letter were not significantly various at the P < 0.05 level.
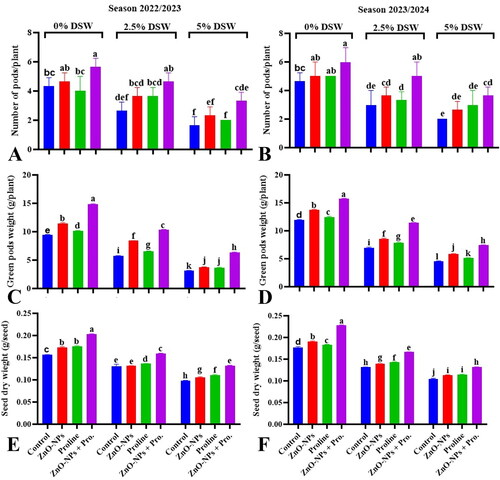
Application of ZnO-NPs and proline, both individually and in combination, mitigated the reduction in the productivity traits of pea plants irrigated with diluted seawater during both seasons. Zinc nanoparticles (ZnO-NPs) may contribute to improved vegetative pea plant growth parameters () and productivity by enhancing nutrient uptake, regulating physiological processes i.e. net photosynthesis, and mitigating oxidative stress induced by salinity (Hafeez et al., Citation2013; Mahawar et al., Citation2024). Proline accumulation is a common adaptive response of plants to salinity stress, and it serves multiple protective roles including osmotic adjustment, antioxidant defense, stabilize proteins and membranes as well as ion balance (Ashraf & Harris, Citation2004; Naliwajski & Skłodowska, Citation2021; Signorelli et al., Citation2014).
3.8. Seed chemical analysis
3.8.1. Total carbohydrates percentage, vitamin C and crude protein percentage
Irrigation with diluted seawater (DSW 1 and 2) led to dynamic changes in the total carbohydrate percentage, vitamin C content, and crude protein content in pea seeds, with a decrease in carbohydrates, vitamin C, and crude protein content during both seasons (). Under salinity stress, plants often experience reduced growth and physiological changes due to osmotic stress and ion toxicity. One common response to salinity stress is the alteration of carbohydrate metabolism in plants (Ahmad et al., Citation2017). Decreasing carbohydrate percentage can contribute to a reduction in leaf area and chlorophyll content in pea plants. These combined effects can lead to a reduction in photosynthesis. The excessive presence of soluble salts in the soil, can improve the uptake of Na+, Cl-, or SO42-. This induces ion toxicity, disrupts nutrient balance, imposes osmotic stress, and interferes with metabolic processes (Gupta & Huang, Citation2014). Salinity can have various effects on plants, including altered growth and metabolism (Abdel-Aziz et al., Citation2020). Most vitamins are involved in the enzymatic systems. The vitamin content of plants is also known to exhibit altered metabolism under the influence of salinity. Applying vitamin C (Ascorbic Acid) may alleviate the negative effects of salt stress on plant growth (Azzedine et al., Citation2011). Ascorbic Acid (AsA) is a potent antioxidant that helps neutralize (ROS) generated under salt stress. The antioxidant activity of AsA protects plant cells and maintains their functionality under salt stress (Mittal et al., Citation2018). Salinity stress can markedly affect the growth and physiology of plants, as well as their protein composition. When plants encounter high levels of salt in the soil, they undergo multiple biochemical and physiological alterations to cope with the stress. One of these changes involves alterations in protein expression, which can lead to changes in crude protein levels in plants (Balasubramaniam et al., Citation2023; El-Beltagi et al., Citation2023).
Figure 6. Effect of proline and zinc nanoparticles (ZnO-NPs) and their combination on total carbohydrates% (A,B), vitamin C (C,D) or crude protein percentage (E,F) contents in seeds of pea plants irrigated with seawater diluted after 70 days from sowing during 2022/2023 and 2023/2024 seasons. Values sharing at least one identical letter were not significantly various at the P < 0.05 level.
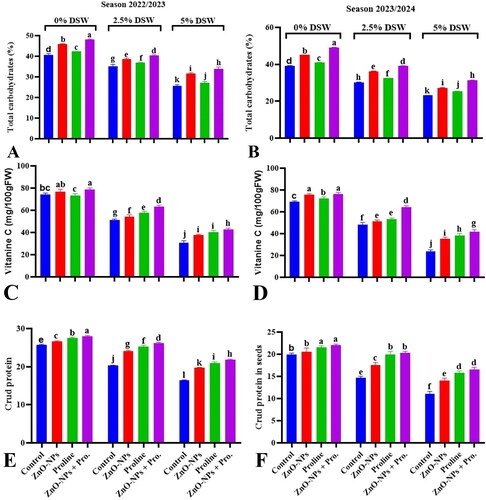
Foliar application of ZnO-NPs significantly mitigated the adverse effects of salinity stress on carbohydrate percentage and the content of vitamin C and total crude protein in seeds of pea plants exposed to seawater dilutions. Zn serves as an essential micronutrient for plants and plays a pivotal role in alleviating the adverse effects of various abiotic stresses, including salinity stress (Zafar et al., Citation2017). Zn supplementation has been shown to improve plant growth and mitigate the adverse effects of salinity stress by enhancing various physiological processes, such as growth, activation of antioxidant defense systems, and chlorophyll synthesis (Al-Zahrani et al., Citation2021; Mohamed et al., Citation2023). Zn is an essential micronutrient necessary for the synthesis of proteins in plants. It acts as a cofactor for various enzymes that participate in protein synthesis, including those responsible for transcription, translation, and post-translational modifications. Therefore, adequate Zn availability is necessary for efficient protein synthesis in plants, including under stress conditions (Shao et al., Citation2023).
Proline plays a crucial role in the physiological adaptation of many plant species to stress, particularly abiotic stresses such as salinity. One notable feature of proline is its capacity to accumulate to high levels in the cytosol of plant cells under stressful conditions (Hosseinifard et al., Citation2022). Additionally, proline acts as a stabilizer of cellular structures and macromolecules. They can directly interact with proteins, nucleic acids, and membranes, protecting them from denaturation or damage caused by stress-induced ROS and other harmful molecules. The ability of proline to scavenge and neutralize ROS contributes to its cytoprotective role (Machado & Serralheiro, Citation2017). Therefore, proline accumulation in plants under salt stress can positively affect carbohydrate percentage, vitamin C, and total crude protein content in pea seeds. Overall, although proline itself does not directly participate in protein synthesis, its accumulation under salinity stress can have several indirect effects that support and enhance protein synthesis. These effects include osmotic adjustment, protection against oxidative stress, stabilization of the protein structure, regulation of gene expression, and reduction of energy requirements. Proline plays a crucial role in helping plants withstand salinity stress and maintaining cellular functions, including protein synthesis (Hosseinifard et al., Citation2022).
3.9. Nitrogen, phosphorus and potassium (NPK) percentage
The data in indicate that irrigation with diluted seawater significantly reduced the essential nutrient content of pea seeds, including nitrogen (N), phosphorus (P), and potassium (K), compared with the control during both seasons. The correlation between salt stress and essential mineral nutrients (N, P, and K) is complex, however. In saline environments, the increased uptake and accumulation of chloride ions (Cl-) may interfere with the uptake and assimilation of nitrate ions (NO3-) by plants, leading to a phenomenon known as Cl-/NO3- antagonism. Salt stress can reduce phosphorus uptake in plants through competitive ion uptake, changes in soil ionic strength, and alterations in mineral solubility. Excessive concentrations of chloride (Cl-) and sulfate (SO42-) ions in the soil can compete with phosphate (PO43-) ions for uptake by plant roots. The reduction in potassium (K+) uptake in plants caused by sodium (Na+) is a primarily competitive process, regardless of the specific anions (such as chloride or sulfate) present in the soil solution (Balasubramaniam et al., Citation2023). The decrease in NPK uptake under salt stress can have detrimental effects on the NPK content of pea seeds, ultimately leading to decreased seed quality.
Figure 7. Effect of proline and zinc nanoparticles (ZnO-NPs) and their combination on percentage of nitrogen (A,B), potassium (C,B), and phosphorus (E,F) percentage in seeds of pea plants irrigated with diluted seawater after 70 days from sowing during 2022/2023 and 2023/2024 seasons. Values sharing at least one identical letter were not significantly various at the P < 0.05 level.
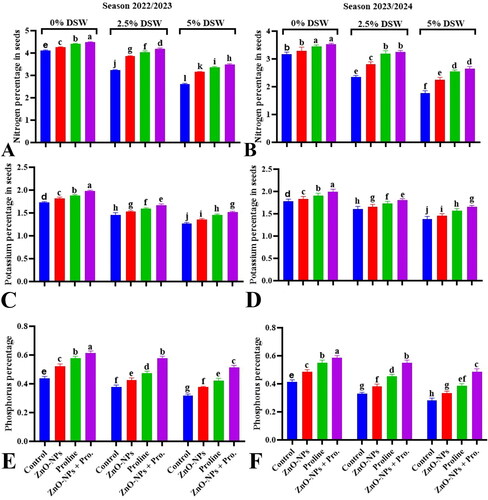
The application of ZnO-NPs and proline, whether applied individually or in combination, mitigated the reduction in the NPK content of pea seeds under irrigation with diluted seawater during both seasons. The peak values were achieved by the combination treatment of ZnO-NPs, and proline underscores the potential synergistic effects of these treatments in mitigating the reduction in the NPK content of pea seeds under diluted seawater irrigation. This suggests that these treatments may confer protection against the adverse effects of salinity on NPK uptake and accumulation in the pea seeds. Zinc (Zn) supplementation has been observed to maintain better relative water content (RWC) and improve nutrient uptake under salt stress conditions by maintaining membrane stability (Rossi et al., Citation2019) and regulating the uptake and transportation of water in plants (Shao et al., Citation2023). Simultaneously, the overproduction and accumulation of proline in salt-stressed plants plays a multifaceted role in enhancing plant tolerance to salt stress. This includes mitigating oxidative stress, preserving osmotic balance, stabilizing cellular structures, and regulating gene expression (Molinari et al., Citation2007).
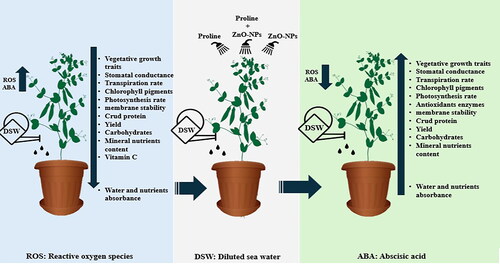
summarizes the detrimental impacts of irrigating pea plants with diluted seawater (salinity stress), affecting various parameters such as vegetative growth, stomatal conductance, transpiration rate, chlorophyll pigment content, net photosynthesis, membrane stability, crude protein, yield, yield components, as well as water and nutrient uptake. Simultaneously, salinity stress induced an increase in reactive oxygen species (ROS) and abscisic acid (ABA) content. However, the application of proline and zinc oxide nanoparticles (ZnO-NPs), either alone or in combination via foliar spray, ameliorated the negative effects of salinity stress on all examined parameters and reduced ROS and ABA levels.
Figure 8. A graphical abstract outlining the adverse effects of irrigating pea plants with diluted seawater (salinity stress) and the mitigation of these negative impacts through the application of proline and zinc oxide nanoparticles (ZnO-NPs), whether applied individually or in combination via foliar spray.
3.10. Comparison of responses across treatments
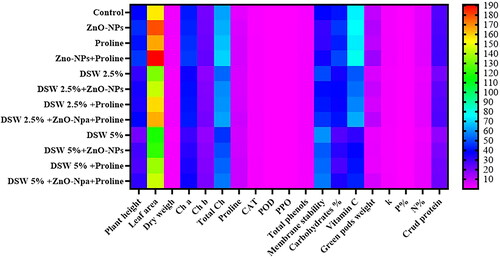
A comparative heatmap analysis was conducted to examine the parameters measured in this study (), including plant height, leaf area, dry weight, chlorophyll pigments (Ch a, Ch b, and total Ch), endogenous proline, antioxidant enzymes (CAT, POD, and PPO), leaf total phenols, membrane stability, seed biochemical analysis (carbohydrates, Vitamin C, and crude protein), seed nutrient element content (NPK), and green pod weight per plant. The analysis revealed a distinct categorization of plant growth and physiological and biochemical responses among salinity-stressed plants, with and without treatment with either ZnO-NPs or proline alone, or in combination. Notably, plants treated with the combination of ZnO-NPs + proline exhibited significantly higher alleviation of salinity stress.
Figure 9. Heatmap analysis of the growth, physiological or biochemical attributes in pea plants unstressed and stressed (irrigated with diluted seawater: DSW 2.5% and DSW 5%) treated with ZnO-NPs, proline and their interaction, chlorophyll a (Ch a), chlorophyll b (Ch b), total chlorophyll (Total Ch), catalase (CAT), peroxidase (POD), polyphenoloxidase (PPO), potassium percentage (K%), phosphorus percentage (P%) and nitrogen percentage (N%).
4. Conclusion
Irrigating pea plants with diluted seawater (2.5% and 5%) adversely affects their growth and productivity. However, the foliar application of ZnO-NPs and proline, either individually or in combination, effectively alleviated the negative effects of salt stress. These treatments resulted in increased vegetative and productivity growth parameters, higher levels of chlorophyll pigments, enhanced activity of antioxidant enzymes, elevated phenolic compound content, and improved net photosynthesis. Consequently, there are enhancements in the pea seed content of NPK, vitamin C, carbohydrates, and crude protein, underscoring the potential of these approaches as environmentally sustainable and eco-friendly for boosting crop yield and quality in saline environments.
Authors’ contributions
Conceptualization, H.S.E.-B., K.A.E.-N., M.I.A.-D., A.A.E.-A. and W.F.S.; methodology, M.F.E.-N., A.M.I., W.F.E., A.A.R., and M.M.S.M.; software, K.A.E.-N., M.I.A.-D., W.F.E., and M.M.S.M.; formal analysis, K.A.E.-N., M.I.A.-D., W.F.E., and M.M.S.M.; investigation, H.S.E.-B. and M.F.E.-N.; resources: K.A.E.-N., M.I.A.-D., and M.M.E.-A. and W.F.S. Writing—original draft preparation: M.F.E.-N., A.M.I., W.F.E., A.A.R., and M.M.S.M.; writing—review and editing: H.S.E.-B., K.A.E.-N., M.I.A.-D., M.M.E.-A. and W.F.S.; funding: H.S.E.-B. All authors provided critical feedback and helped shape the research, analysis, and the manuscript. All authors have discussed the results and contributed to the final manuscript. All authors have read and approved the final manuscript.
Ethical approval statement
This study does not involve experiments involving human or animal.
Consent to publish
All the authors consented to the publication of this study.
Consent to participate
All the authors have consented for participation in this submission.
Acknowledgments
We thank the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (GrantA006), for supporting this research work.
Disclosure statement
Authors declare that they have no competing interests.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
Notes on contributors
Hossam S. El-Beltagi
Hossam S. El-Beltagi is a professor in Agricultural Biotechnology Department, College of Agriculture and Food Sciences, King Faisal University his research interest in plant abiotic and biotic stress; plant secondary metabolites and plant biotechnology.
Kholoud Ahmed El-Naqma
Kholoud Ahmed El-Naqma is a PhD holder in Soil & Water and Environment Research Institute, Agriculture Research Center her research interest in plant, water and soil.
Mohammed I. Al-Daej
Mohammed I. Al-Daej is a professor in Agricultural Biotechnology Department, College of Agriculture and Food Sciences, King Faisal University his research interest in plant crops abiotic stress and plant biotechnology.
Mohamed Mabrouk El-Afry
Mohamed Mabrouk El-Afry is an associate professor in Agricultural Botany Department, Faculty of Agriculture, Kafrelsheikh University his research interest in plant abiotic stress and agriculture.
Wael F. Shehata
Wael F. Shehata is an associate professor in Agricultural Biotechnology Department, College of Agriculture and Food Sciences, King Faisal University his research interest in plant horticulture crops and plant tissue culture.
Mohamed Fathi El-Nady
Mohamed Fathi El-Nady is a professor in Agricultural Botany Department, Faculty of Agriculture, Kafrelsheikh University his research interest in plant abiotic stress and agriculture.
Ahmed Mahmoud Ismail
Ahmed Mahmoud Ismail is an associate professor in Arid Land Agriculture Department, College of Agriculture and Food Sciences, King Faisal University his research interest in plant biotic stress.
Wafaa Fawzy Eltonoby
Wafaa Fawzy Eltonoby is assistant professor in Agricultural Botany Department, Faculty of Agriculture, Kafrelsheikh University his research interest in plant abiotic stress and agriculture.
Adel A. Rezk
Adel A. Rezk is a professor in Agricultural Biotechnology Department, College of Agriculture and Food Sciences, King Faisal University his research interest in plant biotic stress; plant and plant biotechnology.
Metwaly Mahfouz Salem Metwaly
Metwaly Mahfouz Salem Metwaly is an associate professor in Agricultural Botany Department, Faculty of Agriculture, Kafrelsheikh University his research interest in plant abiotic stress and agriculture.
References
- Abdel-Aziz, N. G., Mazher, A. A., Mahgub, M. H., Darwish, M. A., Nassar, R. M. A., & Abdel-Aal1, A. S. (2020). Effect of salinity stress on growth, chemical constituents and stem anatomy of Duranta erecta L. plants. Middle East Journal of Agriculture Research, 9(4), 1–22.
- Aebi, H. (1984). Catalase in vitro. Methods in Enzymology, 105, 121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
- Ahmad, P., Ahanger, M. A., Alyemeni, M. N., Wijaya, L., Egamberdieva, D., Bhardwaj, R., & Ashraf, M. (2017). Zinc application mitigates the adverse effects of NaCl stress on mustard [Brassica juncea (L.) Czern & Coss] through modulating compatible organic solutes, antioxidant enzymes, and flavonoid content. Journal of Plant Interactions, 12(1), 429–437. https://doi.org/10.1080/17429145.2017.1385867
- Ahmed, S., Ahmed, S., Roy, S. K., Woo, S. H., Sonawane, K. D., & Shohael, A. M. (2019). Effect of salinity on the morphological, physiological and biochemical properties of lettuce (Lactuca sativa L.) in Bangladesh. Open Agriculture, 4(1), 361–373. https://doi.org/10.1515/opag-2019-0033
- Al Jabri, H., Saleem, M. H., Rizwan, M., Hussain, I., Usman, K., & Alsafran, M. (2022). Zinc oxide nanoparticles and their biosynthesis: Overview. Life, 12(4), 594. https://doi.org/10.3390/life12040594
- Alprol, A. E., Mansour, A. T., El-Beltagi, H. S., & Ashour, M. (2023). Algal extracts for green synthesis of zinc oxide nanoparticles: Promising approach for algae bioremediation. Materials, 16(7), 2819. https://doi.org/10.3390/ma16072819
- Al-Zahrani, H. S., Alharby, H. F., Hakeem, K. R., & Rehman, R. U. (2021). Exogenous application of zinc to mitigate the salt stress in Vigna radiata (L.) wilczek—Evaluation of physiological and biochemical processes. Plants, 10(5), 1005. https://doi.org/10.3390/plants10051005
- Amiri Forotaghe, Z., Souri, M. K., Ghanbari Jahromi, M., & Mohammadi Torkashvand, A. (2022). Influence of humic acid application on onion growth characteristics on the water deficit conditions. Journal of Plant Nutrition. 45(7), 1030–1040. https://doi.org/10.1080/01904167.2021.1994604
- AOAC. (1990). Official methods of analysis (15th ed.). Association of Official Analytical Chemist.
- AOAC. (2000). Official methods of analysis (18th ed.). Association of Official Analytical Chemists, Inc.
- Ashraf, M., & Foolad, M. R. (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environmental and Experimental Botany, 59(2), 206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
- Ashraf, M., & Harris, P. J. C. (2004). Potential biochemical indicators of salinity tolerance in plants. Plant Science, 166(1), 3–16. https://doi.org/10.1016/j.plantsci.2003.10.024
- Azzedine, F., Gherroucha, H., & Baka, M. (2011). Improvement of salt tolerance in durum wheat by ascorbic acid application improvement of salt tolerance in durum. Journal of Stress Physiology and Biochemistry, 7(1), 27–37.
- Balasubramaniam, T., Shen, G., Esmaeili, N., & Zhang, H. 1. (2023). Plants’ Response Mechanisms to Salinity Stress. Plants, 12(12), 2253. https://doi.org/10.3390/plants12122253
- Bates, L. S., Waldren, R. P., & Teare, I. D. (1973). Rapid determination of free proline for water stress studies. Plant and Soil, 39(1), 205–207. https://doi.org/10.1007/BF00018060
- Begna, T. (2020). Effects of drought stress on crop production and productivity. International Journal of Research Studies in Agricultural Sciences (IJRSAS), 6(9), 34–43.
- Ben Ahmed, C., Magdich, S., Ben Rouina, B., Sensoy, S., Boukhris, M., & Ben Abdullah, F. (2011). Exogenous proline effects on water relations and ions contents in leaves and roots of young olive. Amino Acids, 40(2), 565–573. https://doi.org/10.1007/s00726-010-0677-1
- Ben Rejeb, K., Abdelly, C., & Savouré, A. (2014). How reactive oxygen species and proline face stress together. Plant Physiology and Biochemistry: PPB, 80, 278–284. https://doi.org/10.1016/J.PLAPHY.2014.04.007
- Bessada, S. M., Barreira, J. C., Barros, L., Ferreira, I. C., & Oliveira, M. B. P. (2016). Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.F.: An underexploited and highly disseminated species. Industrial Crops and Products, 89, 45–51. https://doi.org/10.1016/j.indcrop.2016.04.065
- Betzen, B. M., Smart, C. M., Maricle, K. L., & MariCle, B. R. (2019). Effects of increasing salinity on photosynthesis and plant water potential in Kansas salt marsh species. Transactions of the Kansas Academy of Science, 122(1-2), 49–58. https://doi.org/10.1660/062.122.0105
- Blokhina, O., Virolainen, E., & Fagerstedt, K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress. a review. Annals of Botany, 91 Spec No(2), 179–194. https://doi.org/10.1093/aob/mcf118
- Boscaiu, M., Lull, C., Llinares, J., Vicente, O., & Boira, H. (2013). Proline as a biochemical marker in relation to the ecology of two halophytic Juncus species. Journal of Plant Ecology, 6(2), 177–186. https://doi.org/10.1093/jpe/rts017
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1-2), 248–254. https://doi.org/10.1006/abio.1976.9999
- Castiglione, M. R., Bottega, S., Sorce, C., & Spanò, C. (2023). Effects of zinc oxide particles with different sizes on root development in Oryza sativa. Rice Science, 30(5), 449–458. https://doi.org/10.1016/j.rsci.2023.03.016
- Castillo, J. M., Mancilla-Leytón, J. M., Martins-Noguerol, R., Moreira, X., Moreno-Pérez, A. J., Muñoz-Vallés, S., Pedroche, J. J., Figueroa, M. E., García-González, A., Salas, J. J., Millán-Linares, M. C., Francisco, M., & Cambrollé, J. (2022). Interactive effects between salinity and nutrient deficiency on biomass production and bio-active compounds accumulation in the halophyte Crithmum maritimum. Scientia Horticulturae, 301, 111136. https://doi.org/10.1016/j.scienta.2022.111136
- Chattha, M. U., Amjad, T., Khan, I., Nawaz, M., Ali, M., Chattha, M. B., Ali, H. M., Ghareeb, R. Y., Abdelsalam, N. R., Azmat, S., Barbanti, L., & Hassan, M. U. (2022). Mulberry based zinc nano-particles mitigate salinity induced toxic effects and improve the grain yield and zinc bio-fortification of wheat by improving antioxidant activities, photosynthetic performance, and accumulation of osmolytes and hormones. Frontiers in Plant Science, 13, 920570. https://doi.org/10.3389/fpls.2022.920570
- Chen, Y., & Hoehenwarter, W. (2015). Changes in the phosphosproteome and metabolome link early signaling events to rearrangement of photosynthesis and central metabolism in salinity and oxidative stress response in Arabidopsis. Plant Physiology, 169(4), 3021–3033. https://doi.org/10.1104/pp.15.01486
- Dane, J. H., and Topp, C. G. (Eds.) (2020). Methods of soil analysis, Part 4: Physical methods (vol. 20). John Wiley & Sons. Soil Science Society of America.
- Duncan, D. B. (1955). Multiple range and multiple F-test. Biometrics, 11(1), 1–42. https://doi.org/10.2307/3001478
- Ebrahimi, M., Souri, M. K., Mousavi, A., & Sahebani, N. (2021). Biochar and vermicompost improve growth and physiological traits of eggplant (Solanum melongena L.) under deficit irrigation. Chemical and Biological Technologies in Agriculture, 8(1), 1–14. https://doi.org/10.1186/s40538-021-00216-9
- El Moukhtari, A., Cabassa-Hourton, C., Farissi, M., & Savouré, A. (2020). How does proline treatment promote salt stress tolerance during crop plant development? Frontiers in Plant Science, 11, 1127: 1–16. https://doi.org/10.3389/fpls.2020.01127
- El-Beltagi, H. S., Ahmad, I., Basit, A., Abd El-Lateef, H. M., Yasir, M., Tanveer, S. S., Ullah, I., Elsayed, M. M. M., Ali, I., Ali, F., Ali, S., Aziz, I., Kandeel, M., & Ikram, M. Z. (2022a). Effect of Azospirillum and Azotobacter species on the performance of cherry tomato under different salinity levels. Gesunde Pflanzen, 74(2), 487–499. https://doi.org/10.1007/s10343-022-00625-2
- El-Beltagi, H. S., Ahmad, I., Basit, A., Shehata, W. F., Hassan, U., Shah, S. T., Haleema, B., Jalal, A., Amin, R., Khalid, M. A., Noor, F., & Mohamed, H. I. (2022b). Ascorbic acid enhances growth and yield of sweet peppers (Capsicum annum) by mitigating salinity stress. Gesunde Pflanzen, 74(2), 423–433. https://doi.org/10.1007/s10343-021-00619-6
- El-Beltagi, H. S., Al-Otaibi, H. H., Parmar, A., Ramadan, K. M. A., Lobato, A., & El-Mogy, M. M. (2023). Application of potassium humate and salicylic acid to mitigate salinity stress of common bean. Life (Basel, Switzerland), 13(2), 448. https://doi.org/10.3390/life13020448
- El-Saied, R. M., & Elsayed, M. S. (2022). Effect of different concentrations from zinc oxide nanoparticles prepared in date pits extract on pea (Pisum sativum L.) plant. Egyptian Journal of Chemistry, 65(8), 575–582. https://doi.org/10.21608/EJCHEM.2022.121988.5465
- El-Shafai, N. M., Mostafa, Y. S., Ramadan, M. S., & M El-Mehasseb, I. (2024). Enhancement efficiency delivery of antiviral Molnupiravir-drug via the loading with self-assembly nanoparticles of pycnogenol and cellulose which are decorated by zinc oxide nanoparticles for COVID-19 therapy. Bioorganic Chemistry, 143, 107028. https://doi.org/10.1016/j.bioorg.2023.107028
- Farkhondeh, R., Nabizadeh, E., & Jalilnezhad, N. (2012). Effect of salinity stress on proline content, membrane stability and water relations in two sugar beet cultivars. International Journal of AgriScience, 2(5), 385–392.
- Gaballah, M. M., El-Agoury, R. Y., Abo-Marzoka, E. A., Hamad, H. S., Abu Elezz, A. F., Shehab, M. M., Al-Ashkar, I., Aamir Iqbal, M., Liyun, L., & El Sabagh, A. (2023). Genetic analysis of rice genotypes with contrasting response to aerobic conditions. Pakistan Journal of Botany, 55(5), 1–16. https://doi.org/10.30848/PJB2023-5(26)
- Ghanem, A. E. M. F. M., Mohamed, E., Kasem, A. M. M. A., & El-Ghamery, A. A. (2021). Differential salt tolerance strategies in three halophytes from the same ecological habitat: Augmentation of antioxidant enzymes and compounds. Plants (Basel, Switzerland), 10(6), 1100. https://doi.org/10.3390/plants10061100
- Gomez, K. A., & Gomez, A. A. (1984). Statistical procedures for agricultural research (2nd ed., pp. 680). John Wiley and Sons.
- Gupta, B., & Huang, B. (2014). Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. International Journal of Genomics, 2014, 701518–701596. https://doi.org/10.1155/2014/701596
- Hafeez, B. M. K. Y., Khanif, Y. M., & Saleem, M. (2013). Role of zinc in plant nutrition-a review. American Journal of Experimental Agriculture, 3(2), 374–391. https://doi.org/10.9734/AJEA/2013/2746
- Hasanuzzaman, M., Raihan, M. R. H., Masud, A. A. C., Rahman, K., Nowroz, F., Rahman, M., Nahar, K., & Fujita, M. (2021). Regulation of reactive oxygen species and antioxidant defense in plants under salinity. International Journal of Molecular Sciences, 22(17), 9326. https://doi.org/10.3390/ijms22179326
- Heuer, B. (2010). Role of proline in plant response to drought and salinity. In A. Pessarakli (Eds.), Handbook of plant and crop stress (3rd ed., pp. 213–238). CRC Press.
- Hosseinifard, M., Stefaniak, S., Javid, M. G., Soltani, E., Łukasz Wojtyla, L., & Garnczarska, M. (2022). Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. International Journal of Molecular Sciences, 23(9), 5186. https://doi.org/10.3390/ijms23095186
- Ibrahim, A. M. M., Awad, A. E., Gendy, A. S. H., & Abdelkader, M. A. I. (2019). Effect of proline foliar spray on growth and productivity of sweet basil (Ocimum basilicum L.) plant under salinity stress conditions. Zagazig Journal of Agricultural Research, 46(6), 1877–1889. https://doi.org/10.21608/zjar.2019.51896
- Ismail, E., & Halmy, M. (2018). Effect of proline and potassium humate on growth, yield and quality of broad bean under saline soil conditions. Journal of Plant Production, 9(12), 1141–1145. https://doi.org/10.21608/jpp.2018.36641
- Javeed, H. M. R., Wang, X., Ali, M., Nawaz, F., Qamar, R., Ur Rehman, A., Shehzad, M., Mubeen, M., Shabbir, R., Javed, T., Branca, F., Ahmar, S., & Ismail, I. A. (2021). Potential utilization of diluted seawater for the cultivation of some summer vegetable crops: Physiological and nutritional implications. Agronomy, 11(9), 1826, 1–14. https://doi.org/10.3390/agronomy11091826
- Jiang, D., Lu, B., Liu, L., Duan, W., Meng, Y., Li, J., Zhang, K., Sun, H., Zhang, Y., Dong, H., Bai, Z., & Li, C. (2021). Exogenous melatonin improves the salt tolerance of cotton by removing active oxygen and protecting photosynthetic organs. BMC Plant Biology, 21(1), 331. https://doi.org/10.1186/s12870-021-03082-7
- Kheloufi, A., Chorfi, A., & Mansouri, L. M. (2016). The Mediterranean seawater: The impact on the germination and the seedlings emergence in three Acacia species. Journal of Biodiversity and Environmental Sciences, 8(6):, 238–249.
- Li, Y., Cui, L., Yao, X., Ding, X., Pan, X., Zhang, M., Li, W., & Kang, X. (2017). Trade-off between leaf chlorophyll and betacyanins in Suaeda salsa in the Liaohe estuary wetland in northeast China. Journal of Plant Ecology, 11, 569–575. https://doi.org/10.1093/jpe/rtx025
- Lutts, S., Kinet, J. M., & Bouharmont, J. (1996). NaCl-induced senescence in leaves of Rice (Oryza sativa L.) cultivars differing in salinity resistance. Annals of Botany, 78(3), 389–398. https://doi.org/10.1006/anbo.1996.0134
- Machado, R. M. A., & Serralheiro, R. P. (2017). Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae, 3(2), 30. https://doi.org/10.3390/horticulturae3020030
- Mahawar, L., Ramasamy, K. P., Suhel, M., Prasad, S. M., Živčák, M., Brestic, M., Rastogi, A., & Skalický, M. (2023). Silicon nanoparticles: Comprehensive review on biogenic synthesis and applications in agriculture. Environmental Research, 232, 116292. https://doi.org/10.1016/j.envres.2023.116292
- Mahawar, L., Živčák, M., Barboricova, M., Kovár, M., Filaček, A., Ferencova, J., Vysoká, D. M., & Brestič, M. (2024). Effect of copper oxide and zinc oxide nanoparticles on photosynthesis and physiology of Raphanus sativus L. under salinity stress. Plant Physiology and Biochemistry: PPB, 206, 108281. https://doi.org/10.1016/j.plaphy.2023.108281
- Mansouri, M., & Kheloufi, A. (2017). Effect of diluted seawater on seed germination and seedling growth of three leguminous crops (pea, chickpea and common bean). Agriculture & Forestry, 63(2), 131–142.
- Miranda, D., Fischer, G., Mewis, I., Rohn, S., & Ulrichs, C. (2014). Salinity effects on proline accumulation and total antioxidant activity in leaves of the cape gooseberry (Physalis peruviana L.)Journal of Applied Botany and Food Quality, 87, 67–73. https://doi.org/10.5073/JABFQ.2014.087.010
- Mirfattahi, Z., Karimi, S., & Roozban, M. R. (2017). Salinity induced changes in water relations, oxidative damage and morpho-physiological adaptations of pistachio genotypes in soilless culture. Acta Agriculturae Slovenica, 109(2), 291–302. https://doi.org/10.14720/aas.2017.109.2.12
- Mittal, N., Thakur, S., Verma, H., & Kaur, A. (2018). Interactive effect of salinity and ascorbic acid on Brassica rapa L. plants. Global Journal of Bio-Science and Biotechnology, 7(1), 27–29.
- Mohamed, A. A., Sameeh, M. Y., & El-Beltagi, H. S. (2023). Preparation of seaweed nanopowder particles using planetary ball milling and their effects on some secondary metabolites in Date Palm (Phoenix dactylifera L.) Seedlings. Life (Basel, Switzerland), 13(1), 39. https://doi.org/10.3390/life13010039
- Molinari, H. B. C., Marur, C. J., Daros, E., de Campos, M. K. F., de Carvalho, J., Bespalhok, J. C., Pereira, L. F. P., & Vieira, L. G. E. (2007). Evaluation of the stress-inducible production of proline in transgenic sugarcane (Saccharum spp.): Osmotic adjustment, chlorophyll fluorescence and oxidative stress. Physiologia Plantarum, 130(2), 218–229. https://doi.org/10.1111/j.1399-3054.2007.00909.x
- Moran, R. (1982). Formulae for determination of chlorophllous pigments extracted with N, N-Dimetheylformamide. Plant Physiology, 69(6), 1376–1381. https://doi.org/10.1104/pp.69.6.1376
- Naliwajski, M., & Skłodowska, M. (2021). The relationship between the antioxidant system and proline metabolism in the leaves of cucumber plants acclimated to salt stress. Cells, 10(3), 609. https://doi.org/10.3390/cells10030609
- Naseer, I., Javad, S., Iqbal, S., Shah, A. A., Alwutayd, K., & AbdElgawad, H. (2023). Deciphering the role of zinc oxide nanoparticles on physiochemical attributes of Zea mays exposed to saline conditions through modulation in antioxidant enzyme defensive system. South African Journal of Botany, 160, 469–482. https://doi.org/10.1016/j.sajb.2023.07.035
- Nasrudin, N., Isnaeni, S., & Fahmi, P. (2022). The effect of high salt stress on the agronomic, chlorophyll content, and yield characteristics of several rice varieties. IOP Conference Series: Earth and Environmental Science, 995(1), 012028. https://doi.org/10.1088/1755-1315/995/1/012028
- Oktay, M., Küfreviolu, I., Kocaçalişkan, I., & ŞAKlROLU, H. (1995). Polyphenol oxidase from Amasya Apple. Journal of Food Science, 60(3), 494–496. https://doi.org/10.1111/j.1365-2621.1995.tb09810.x
- Okuma, E., Murakami, Y., Shimoishi, Y., Tada, M., & Murata, Y. (2008). Effects of exogenous application of proline and Betaine on the growth of tobacco cultured cells under saline conditions. Soil Science and Plant Nutrition, 50(8), 1301–1305. https://doi.org/10.1080/00380768.2004.10408608
- Polle, A., Otter, T., & Seifert, F. (1994). Apoplastic peroxidases and lignification in needles of Norway Spruce Picea abies L. Plant Physiology, 106(1), 53–60. https://www.jstor.org/stable/4276023 https://doi.org/10.1104/pp.106.1.53
- Priyanka, N., Geetha, N., Ghorbanpour, M., & Venkatachalam, P. (2019). Role of engineered zinc and copper oxide nanoparticles in promoting plant growth and yield: Present status and future prospects. Advances in Phytonanotechnology, 183–201. https://doi.org/10.1016/B978-0-12-815322-2.00007-9
- Rai, S., Singh, P. K., Mankotia, S., Swain, J., & Satbhai, S. B. (2021). Iron homeostasis in plants and its crosstalk with copper, zinc, and manganese. Plant Stress, 1, 100008. https://doi.org/10.1016/j.stress.2021.100008
- Rossi, L., Fedenia, L. N., Sharifan, H., Ma, X., & Lombardini, L. (2019). Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiology and Biochemistry: PPB, 135, 160–166. https://doi.org/10.1016/j.plaphy.2018.12.005
- Salama, Z., A. E. R., El-Beltagi, H. S., & El-Hariri, D.-M. (2009). Effect of Fe deficiency on antioxidant system in leaves of three flax cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 37(1), 122–128. https://doi.org/10.15835/nbha3713107
- Sarker, U., & Oba, S. (2020). The response of salinity stress-induced A. tricolor to growth, anatomy, physiology, non-enzymatic and enzymatic antioxidants. Frontiers in Plant Science, 11, 559876. https://doi.org/10.3389/fpls.2020.559876
- Shao, J., Tang, W., Huang, K., Ding, C., Wang, H., Zhang, W., Li, R., Aamer, M., Muhammad Umair Hassan, M. U., Elnour, R. O., Hashem, M., Huang, G., & Qari, S. H. (2023). How does zinc improve salinity tolerance? Mechanisms and future prospects. Plants (Basel, Switzerland), 12(18), 3207. https://doi.org/10.3390/plants12183207
- Shumilina, J., Kusnetsova, A., Tsarev, A., Van Rensburg, H. C. J., Medvedev, S., Demidchik, V., Ende, W. V. D., & Frolov, A. (2019). Glycation of plant proteins: Regulatory roles and interplay with sugar signalling? International Journal of Molecular Sciences, 20(9), 2366. https://doi.org/10.3390/ijms20092366
- Sicher, R. C., & Barnaby, J. Y. (2012). Impact of carbon dioxide enrichment on the responses of maize leaf transcripts and metabolites to water stress. Physiologia Plantarum, 144(3), 238–253. https://doi.org/10.1111/j.1399-3054.2011.01555.x
- Signorelli, S., Coitino, E. L., Borsani, O., and Monza, J. (2014). Molecular mechanisms for the reaction between (OH)-O-center dot radicals and proline: insights on the role as reactive oxygen species scavenger in plant stress. Journal of Physical Chemistry B, 118, 37–47. https://doi.org/10.1021/jp407773u
- Sparks, D. L., Page, A. L., Helmke, P. A., & Loeppert, R. H. (Eds.). (2020). Methods of soil analysis, part 3: Chemical methods (vol. 14). John Wiley & Sons.
- Srivastav, A., Ganjewala, D., Singhal, R. K., Rajput, V. D., Minkina, T., Voloshina, M., Srivastava, S., & Shrivastava, M. (2021). Effect of ZnO nanoparticles on growth and biochemical responses of wheat and maize. Plants (Basel, Switzerland), 10(12), 2556. https://doi.org/10.3390/plants10122556
- Van Zelm, E., Zhang, Y., & Testerink, C. (2020). Salt tolerance mechanisms of plants. Annual Review of Plant Biology, 71(1), 403–433. https://doi.org/10.1146/annurev-arplant-050718-100005
- Velikova, V., Yordanov, I., & Edreva, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous Poly-amines. Plant Science, 151(1), 59–66. https://doi.org/10.1016/S0168-9452(99)00197-1
- Walinga, I., Van Der Lee, J., Houba, V. J., Vanvark, W., & Novozamsky, I. (2013). Plant Analysis Manual. Springer-Science. 1–239.
- Yasar, F., Ellialtioglu, S., & Yildiz, K. (2008). Effect of salt stress on antioxidant defense systems, lipid peroxidation, and chlorophyll content in green bean. Russian Journal of Plant Physiology, 55(6), 782–786. https://doi.org/10.1134/S1021443708060071
- Zafar, S., Ashraf, M. Y., & Saleem, M. (2017). Shift in physiological and biochemical processes in wheat supplied with zinc and potassium under saline condition. Journal of Plant Nutrition, 41(1), 19–28. https://doi.org/10.1080/01904167.2017.1380825
- Zhao, S., Zhang, Q., Liu, M., Zhou, H., Ma, C., & Wang, P. (2021). Regulation of plant responses to salt stress. International Journal of Molecular Sciences, 22(9), 4609. https://doi.org/10.3389/fpls.2022.1053699


