ABSTRACT
Background
Earlier timing, and possibly faster tempo, of puberty is associated with overweight and obesity (Ow/Ob). However, most studies consider these concepts separately when investigating the implications to adolescent weight gain.
Aim
To assess pubertal timing and tempo associations with weight gain over early-mid adolescence.
Subjects and methods
This study analyzed data from 263 healthy adolescents (10–13y at recruitment) who were followed annually for three years. Growth models were employed to estimate timing and tempo of peak height growth and self-reported Tanner stage (TS) change. Timing and tempo variables were assessed against anthropometry and body composition change via mixed models (data: estimate [95% confidence interval]).
Results
In girls, earlier height and TS change were associated with higher BMI z-score (height: −0.51 [−0.85, −0.17], p = 0.004; TS: −0.43 [−0.67, −0.20], p < 0.001) and waist-to-height ratio (height: −0.02 [−0.04, −0.00]) 0.025; TS: −0.01 [−0.03, −0.00]; p = 0.028). There were no consistent findings for pubertal tempo among girls. In boys, earlier timing and slower tempo of height growth consistently related to higher adiposity across all anthropometric and body fat variables (all p < 0.01). Timing and tempo of TS change showed no consistent findings among boys.
Conclusion
Relative to pubertal tempo, girls with earlier height and TS change exhibited significantly higher BMI z-score and waist-to-height ratio. This finding corroborates strong evidence linking earlier female puberty with elevated adiposity. In boys, timing and tempo of height growth showed independent but compensatory relationships with anthropometry and body composition. This suggests the risk of excess weight gain in boys may be less attributable to puberty compared to other risk factors.
Introduction
Puberty is a time of rapid growth and body composition change. Appetites increase to meet the energy demands of growing bodies (Cheng et al. Citation2016). Simultaneously, lifestyle behaviours change with increasing adolescent autonomy – evolving under peer and societal influences (Neufeld et al. Citation2022). Such changes may predispose to excess weight gain, and for many adolescents, this is an important risk period for the development and progression of overweight and obesity (Ow/Ob).
Earlier timing of pubertal events like the growth spurt, breast budding, testicular enlargement and menarche have well-documented relationships with Ow/Ob, cardiometabolic disease and other morbidity and mortality outcomes (Prentice and Viner Citation2013; Day et al. Citation2015; Li et al. Citation2017; Reinehr and Roth Citation2019), especially in females. Recent advances in mathematical modelling of puberty suggest rapid pubertal progression (or tempo) may also be linked to increased adiposity (Cheng et al. Citation2020) although evidence is less strong than for timing. Few studies have considered both timing and tempo when investigating the associations of puberty with adiposity. Even fewer studies have examined whether timing and tempo obtained from different descriptors, e.g. height versus breast/genital change (which fall under slightly different hormone regulatory systems), (Cheng et al. Citation2020) may be variably associated with adolescent weight and adiposity trajectories.
This study aimed to examine whether timing and tempo of puberty, determined by the descriptors of height growth and breast/genital development, are associated with anthropometry and body composition in a cohort of pubertal adolescents. We hypothesized that earlier timing and faster tempo of puberty, as determined by both descriptors, would be independently associated with measures indicative of higher adiposity.
Materials and methods
Study design and participants
This study analysed data from the prospective Adolescent Rural Cohort study of Hormones, Health, Education, Environments and Relationships (ARCHER). ARCHER recruited a community sample of 342 healthy adolescents aged 10–13 years from the central and surrounding areas of two regional townships in the state of New South Wales, Australia. Adolescents were followed up for three years with the aim of studying puberty hormone change and its impact on health, wellbeing and behaviour. Adolescents attended one baseline and three annual follow-ups where they completed a physical assessment, a battery of health and behaviour questionnaires and provided a blood sample. The study protocol and cohort characteristics (including their sociodemographic profile) are published (Steinbeck et al. Citation2012; Luscombe et al. Citation2017)
Anthropometry, body composition and pubertal assessment
Height, weight, waist circumference, body composition (percent body fat, absolute fat mass and absolute fat-free mass via bioelectrical impedance), Tanner breast/genital stages (self-reported) and serum gonadal steroids (mass spectrometry) were assessed annually (see Appendix). BMI z-score (zBMI) and waist-to-height ratio (WtHR) were calculated as measures of whole-body and central adiposity, respectively.
Ethics
ARCHER was approved by The University of Sydney Human Research Ethics Committee (HREC 2010/13,094 and 2015/199). All research was undertaken according to the Principles of the Declaration of Helsinki. A parent/guardian provided initial written informed consent. Adolescents provided signed initial assent and verbal assent at each visit.
Statistical analysis
Modelling height growth
Annual height data were modelled using the SuperImposition by Translation And Rotation (SITAR) growth curves via the “sitar” package of the R language (R Core Team, 2021, v. 4.0.5) (Cole et al. Citation2010; Cole Citation2018). This non-linear modelling methodology describes an individual’s pubertal height growth trajectory using three geometric parameters; two of which are relevant to the current work. The first parameter concerns genetic height variation and is unrelated to the aims of this study. The second parameter represents timing of the growth spurt, from which the age at peak height velocity (APHV) can be derived. The third parameter relates to the tempo of the growth spurt, from which the magnitude of the peak height velocity (PHV; expressed in cm/y) can be derived. APHV and PHV were thus used as proxies for the timing and tempo of the growth spurt, respectively.
Modelling sexual maturation
Annual Tanner stage data were modelled using a published logistic growth curve methodology via PROC NLMIXED on SAS Version 9.4 (SAS Institute Inc., Cary, NC) (Marceau et al. Citation2011). This methodology was deemed the most appropriate for obtaining sexual maturation trajectories in our cohort, given that our study follow-ups were not timed to capture the first appearance of each Tanner stage. Logistic curves are also reported to be more realistic than linear models of sexual maturation, yielding better model-fit (Beltz et al. Citation2014)
Logistic Tanner stage modelling uses two mathematical parameters to describe how an individual’s sexual maturation trajectory deviates from the mean curve; these lend themselves naturally as estimates of timing and tempo. The first parameter represents the age at Tanner breast/genital stage 3 (B3/G3), or the point at which an adolescent is halfway between the lower (B1/G1) and upper (B5/G5) asymptotes of their sexual maturation curve. The second parameter represents the slope of the logistic curve at the same halfway point, interpreted as the theoretical rate of maturation at B3/G3 expressed in Tanner stages per year. For our current research, age and slope at B3/G3 were used as the proxies for timing and tempo of sexual maturation, respectively. Due to the self-reported nature of our Tanner stage data, manual data cleaning was undertaken prior to model fitting to remove participants with implausible or uninformative TS trajectories. These included those with (i) less than three TS data points needed for non-linear analysis (n = 40); (ii) TS that stagnated (n = 22) or decreased (n = 54) over the course of the study.
Pubertal timing and tempo associations with anthropometry and body composition
Mixed-effects models assessed the relationship of pubertal timing and tempo to longitudinal measures of anthropometry and body composition via IBM SPSS Statistics Version 24 (IBM Corporation, Armonk, NY). The models were separated by sex and pubertal descriptor.
The outcome variables (weight, zBMI, waist circumference, WtHR, percent body fat, fat mass and fat-free mass) were each regressed against a set of fixed effects including (i) age; (ii) age2 ; (iii) timing of height or breast/genital change; and (iv) tempo of height or breast/genital change. All anthropometric and body composition data points were used in the analytic models, regardless of whether they were measured before or after age at PHV/B3/G3. Age variables were centred on individual age at PHV/B3/G3 and treated as the time exposure variables. All models included participant ID and intercept as random effects. Descriptive data are presented as mean ± SD, median (interquartile range [IQR]) or percentages. Mixed model results are presented as estimates (95% confidence interval), with significance set at p < 0.05.
Results
Participant characteristics
Of the 342 participants in the full ARCHER cohort, growth curves were successfully fitted for 263 adolescents (height only: n = 37; Tanner stage only: n = 95; both: n = 131; see Appendix). Mean age at baseline was 11.7 ± 1.0 years. Baseline Ow/Ob prevalence was 26%, consistent with state-wide figures at the time (Hardy et al. Citation2011). Compared to girls who were excluded from analyses, included girls had lower baseline oestradiol and lower baseline measures of all anthropometric, body fat and fat-free mass variables except WtHR (). Included boys exhibited lower Tanner stage (p < 0.001) but higher serum testosterone (p = 0.033) at baseline compared to excluded boys.
Table 1. Baseline characteristics of ARCHER participants who were included versus excluded from the pubertal timing and tempo analysis.
Longitudinal analyses of the included participants showed an increase in all anthropometric and body composition measures over time. Trajectories of height, fat mass and fat-free mass diverged significantly between the sexes, as did changes in serum testosterone and oestradiol (see Appendix).
Estimates of pubertal timing and tempo
Height growth modelling yielded a median APHV of 12.2 y (IQR 11.7–12.7) for girls and 13.3 y (IQR 12.7–13.8) for boys. The PHV was estimated at a median of 8.4 cm/y (IQR 7.6–9.2) for girls and 10.7 cm/y (IQR 10.0–11.4) for boys.
Tanner stage modelling yielded a median age at B3 of 12.7 y (IQR 12.1–13.5) in girls and an age at G3 of 12.6 y (IQR 12.1–13.1) in boys. Slope at B3/G3 was estimated at a median of 0.98 stages/y (IQR 0.8–1.1) for girls and 1.17 stages/y (IQR 1.0–1.3) for boys.
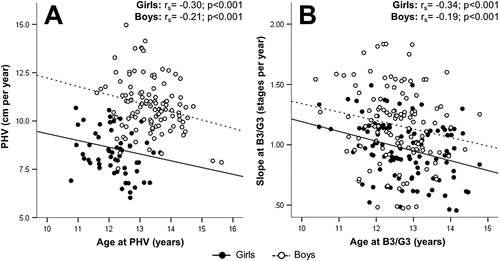
In both sexes, a significant negative correlation was observed between APHV and PHV, and also between age and slope at B3/G3 ().
Figure 1. Bivariate correlations between pubertal timing and pubertal tempo parameters derived from non-linear modelling of height and Tanner stage change. (a) Timing and tempo of height change as represented by the age at peak height velocity and absolute peak height velocity, respectively. (b) Timing and tempo of Tanner stage change as represented by age and slope at breast/genital stage 3, respectively. APHV, age at peak height velocity; B3, Tanner breast stage 3; G3, Tanner genital stage 3; PHV, peak height velocity; rs, Spearman’s rho.
Timing and tempo associations with anthropometry and body composition
The APHV was significantly and inversely associated to BMI z-score (−0.51 [−0.85, −0.17]; p = 0.004) and WtHR (−0.02 [−0.04, −0.00]; p = 0.025) in girls, and all anthropometric and body composition variables in boys except for fat-free mass. The PHV was inversely associated with BMI z-score among girls (−0.11 [−0.21, −0.00]; p = 0.045) and was significantly and inversely associated with all anthropometric and body composition variables in boys ().
Table 2. Mixed-effects models assessing anthropometric and body composition change in relation to the timing and tempo of the height growth spurt.
Age at B3 in girls was inversely associated with BMI z-score (−0.43 [−0.67, −0.20]; p < 0.001) and WtHR (−0.01 [−0.03, −0.00]; p = 0.028). Age at G3 in boys was positively associated with weight (3.54 [0.36, 6.72]; p = 0.029) and fat-free mass (2.61 [1.06, 4.17]; p = 0.001). Slope at B3/G3 did not show any significant association with anthropometry or body composition in either sex ().
Table 3. Mixed-effects models assessing anthropometric and body composition change in relation to the timing and tempo of Tanner stage change.
Discussion
By assessing timing and tempo within the same predictive model, this study was able to demonstrate the relative significance of pubertal timing, compared to tempo, on whole-body (BMI z-score) and central (WtHR) adiposity in girls. Findings were largely concordant across both descriptors of female puberty, suggesting that elevated adiposity in girls may coincide with alterations in both the growth hormone/insulin-like growth factor axis and the hypothalamic-pituitary-gonadal axis. Our data, however, did not allow us to further define the direction of the significant relationships identified, nor the specific roles these hormones play in fat and fat-free mass change.
We found no evidence to support a relationship between rapid pubertal tempo and elevated adiposity in either sex, regardless of the descriptor. Conversely, boys with greater PHV (indicating more rapid height growth) showed significantly lower adiposity and fat-free mass throughout the study. This finding contradicts our recent systematic review that found a propensity for rapid tempo (regardless of descriptor) to be linked with higher BMI or BMI z-scores measured at various time points across the lifespan (Cheng et al. Citation2020). In this review, there were only two studies using similar pubertal modelling to our current work, in which, conflicting findings were reported for BMI measured in adolescence (Marceau et al. Citation2011; Mathias et al. Citation2016)
This study found independent but opposing associations between earlier timing and faster tempo of height growth on measures of anthropometry and body composition in boys. This result, taken together with the significant negative correlation between APHV and PHV, suggests that some degree of timing-tempo compensation may exist among males. That is, heavier boys who experience an earlier growth spurt also tend to lose more excess weight through a rapid growth spurt. Compared to the clear Ow/Ob risks associated with earlier puberty in girls, the pattern with which a boy travels through puberty may be less concerning in terms of excess weight gain.
Strengths of this study include the longitudinal research design, recruitment of a community sample and assessment of two separate descriptors of puberty. We observed typical age- and sex-related changes in anthropometry, body composition and the hormone variables which provide assurance of a normally developing cohort. Our APHV and PHV estimates were also similar to other healthy adolescent cohorts that underwent SITAR modelling (Cole et al. Citation2014; Frysz et al. Citation2018). A key study limitation was the assessment of Tanner stage via self-report (physical examination in non-clinical cohorts was not acceptable to our Ethics Review Committee). In this cohort, we previously found Tanner stage self-reporting to be poor amongst boys, especially early in puberty (Balzer et al. Citation2019). This likely explains the higher Tanner stage but paradoxically lower testosterone levels observed at baseline in the excluded boys. Also, while our Tanner stage data has been shown to track reasonably well with serum gonadal hormone change (Balzer et al. Citation2019), self-reporting error may account for reduced accuracy in our age at B3/G3 estimates. Compared to the 0.81 y difference in age at B3 (12.1 y) and age at G3 (12.9 y) reported in a study using the same logistic modelling strategy and clinician-assessed Tanner stage (Marceau et al. Citation2011), we did not observe a similar time gap between these variables in our cohort (age at B3: 12.7 y; age at G3: 12.6 y). Our baseline comparisons also indicate that the earliest maturing girls from our cohort may have been excluded, as these participants would have exhibited inadequate height or TS progression to allow successful fitting of the growth curves.
In conclusion, this study found significantly higher BMI z-score and WtHR in girls who exhibited earlier height growth and breast development. The absence of consistent pubertal tempo associations emphasises the relative importance of pubertal timing with respect to Ow/Ob risk in girls. Among boys, significant but compensatory associations were identified for the timing and tempo of height growth with respect to anthropometry and body composition. Clinically, this is reflected in the observation that puberty may reduce adiposity in some males. The challenge remains to identify those who are more at risk of retaining higher levels of adiposity throughout and beyond puberty.
Acknowledgments
The authors thank the ARCHER study participants and their families and research assistants Lisa Riley and Janet Symons. Thank you to the schools and communities of Central Western NSW that were key to the recruitment of this cohort.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Cheng HL, Amatoury M, Steinbeck K. 2016. Energy expenditure and intake during puberty in healthy nonobese adolescents: a systematic review. Am J Clin Nutr. 104(4):1061–1074. doi:10.3945/ajcn.115.129205.
- Neufeld LM, Andrade EB, Ballonoff Suleiman A, Barker M, Beal T, Blum LS, et al. 2022. Food choice in transition: adolescent autonomy, agency, and the food environment. Lancet. 399(10320):185–197. doi:10.1016/S0140-6736(21)01687-1
- Prentice P, Viner RM. 2013. Pubertal timing and adult obesity and cardiometabolic risk in women and men: a systematic review and meta-analysis. Int J Obes (Lond). 37(8):1036–1043. doi:10.1038/ijo.2012.177.
- Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. 2017. Association between obesity and puberty timing: a systematic review and meta-analysis. Int J Environ Res Public Health. 14(10). doi:10.3390/ijerph14101266.
- Day FR, Elks CE, Murray A, Ong KK, Perry JR. 2015. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 5(1):11208. doi:10.1038/srep11208.
- Reinehr T, Roth CL. 2019. Is there a causal relationship between obesity and puberty? Lancet Child Adolesc Health. 3(1):44–54. doi:10.1016/S2352-4642(18)30306-7.
- Cheng HL, Harris SR, Sritharan M, Behan MJ, Medlow SD, Steinbeck KS. 2020. The tempo of puberty and its relationship to adolescent health and well-being: a systematic review. Acta Paediatr. 109(5):900–913. doi:10.1111/apa.15092.
- Steinbeck K, Hazell P, Cumming RG, Skinner SR, Ivers R, Booy R, Fulcher G, Handelsman DJ, Martin AJ, Morgan G, et al. 2012. The study design and methodology for the ARCHER study – adolescent rural cohort study of hormones, health, education, environments and relationships. BMC Pediatr. 12(1):143. doi:10.1186/1471-2431-12-143
- Luscombe GM, Cheng HL, Balzer BWR, Chow CM, Paxton K, Steinbeck KS, et al. The ARCHER study of health and wellbeing in young rural Australians. 14th National Rural Health Conference; Cairns, Australia 2017.
- Cole TJ, Donaldson MD, Ben-Shlomo Y. 2010. SITAR – a useful instrument for growth curve analysis. Int J Epidemiol. 39(6):1558–1566. doi:10.1093/ije/dyq115.
- Cole TJ. 2018. Optimal design for longitudinal studies to estimate pubertal height growth in individuals. Ann Hum Biol. 45(4):314–320. doi:10.1080/03014460.2018.1453948.
- Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. 2011. Individual differences in boys’ and girls’ timing and tempo of puberty: modeling development with nonlinear growth models. Dev Psychol. 47(5):1389–1409. doi:10.1037/a0023838.
- Beltz AM, Corley RP, Bricker JB, Wadsworth SJ, Berenbaum SA. 2014. Modeling pubertal timing and tempo and examining links to behavior problems. Dev Psychol. 50(12):2715–2726. doi:10.1037/a0038096.
- Hardy L, Diaz PE, King L, Cosgrove C, Bauman A NSW Schools Physical Activity and Nutrition Survey (SPANS) 2010: full report. Sydney: NSW Ministry of Health, 2011.
- Mathias CW, Charles NE, Liang Y, Acheson A, Lake SL, Ryan SR, et al. 2016. Pubertal maturation compression and behavioral impulsivity among boys at increased risk for substance use. Addict Disord Their Treat. 15(2):61–73. doi:10.1097/ADT.0000000000000077
- Cole TJ, Pan H, Butler GE. 2014. A mixed effects model to estimate timing and intensity of pubertal growth from height and secondary sexual characteristics. Ann Hum Biol. 41(1):76–83. doi:10.3109/03014460.2013.856472.
- Frysz M, Howe LD, Tobias JH, Paternoster L. 2018. Using SITAR (SuperImposition by Translation and Rotation) to estimate age at peak height velocity in Avon Longitudinal Study of Parents and Children. Wellcome Open Res. 3:90. doi:10.12688/wellcomeopenres.14708.2.
- Balzer BWR, Garden FL, Amatoury M, Luscombe GM, Paxton K, Hawke CI, et al. 2019. Self-rated Tanner stage and subjective measures of puberty are associated with longitudinal gonadal hormone changes. J Pediatr Endocrinol Metab. 32(6):569–576. doi:10.1515/jpem-2019-0017

