ABSTRACT
Conidiation mechanism confers filamentous fungus efficient dispersal and survival in natural habitats. However, the mechanisms that regulate conidial lifespan and persistence remain enigmatic. We recently revealed that autophagy functions as a sub-cellular mechanism essential for conidial longevity and vitality after maturation in Beauveria bassiana. Significantly, BbApe4 (B. bassiana aspartyl aminopeptidase) contributes to conidial persistence and recovery from dormancy. There are two isoforms for BbAtg8 (B. bassiana autophagy-related protein 8), BbAtg8-α and BbAtg-β. BbApe4 is translocated into vacuoles via its direct binding to BbAtg8-α (hereafter called BbAtg8), which occurs during conidial germination and hyphal growth. The specific interaction between BbAtg8 and BbApe4 is crucial for vacuolar targeting and functionality of BbApe4 and dependent on the autophagic function of BbAtg8. We named the BbApe4-BbAtg8 pathway as simplified cytoplasm-to-vacuole pathway (sCvt) due to the absence of the autophagy receptors that characterize classic yeast Cvt pathway. These findings demonstrate that autophagy acts as a physiological mechanism for fungal survival in nature and highlight the diversity in autophagy-related pathways for vacuolar targeting of hydrolase between fungi.
Abbreviations: Bb: Beauveria bassiana; ATG: autophagy-related gene; AIM: Atg8-family interacting motif; Cvt pathway: cytoplasm-to-vacuole targeting pathway; Ape4: aspartyl aminopeptidase
Beauveria bassiana is an archetypical filamentous entomopathogenic fungus and has been extensively used in the biological control of insect pests. Like other filamentous fungi, B. bassiana employs conidiation process for fungal propagation and survival in natural environment, and in practical application, its conidia are the infectious ingredients in various formulations. Therefore, conidial persistence is essential for higher biocontrol efficacy. After maturation, conidia enter a stage of oxidation-dependent aging during their dormancy, in which an antioxidant mechanism is involved in conidial capacity to survive. However, the mechanisms underlying conidial persistence in environments remains poorly understood.
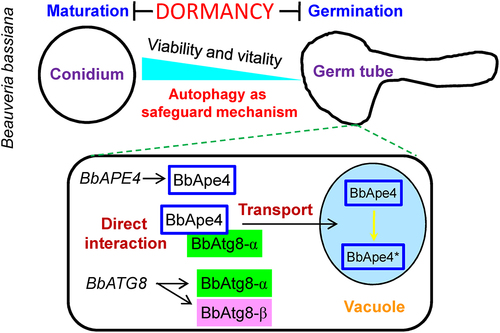
In our study [Citation1], we discovered that autophagy is essential for conidial longevity and vitality (e.g., stress responses and virulence) in B. bassiana, and the autophagy-related process mediates vacuolar targeting of proteinases during conidial exit from a long-term dormancy, based on the following main findings: 1) the fungus lacking ‘core’ autophagy-related (ATG) genes (e.g., BbATG1 and BbATG8) displays the decreased tolerance to nutrient deprivation, shortened longevity, and compromised vitality in conidia. BbATG8 generates two isoforms, i.e., BbAtg8-α and BbAtg8-β (hereafter, BbAtg8-α is referred to as BbAtg8); 2) the loss of BbApe4 (B. bassiana aspartyl aminopeptidase) results in the impaired phenotypes similar with those observed in above autophagy-null strains; 3) vacuolar targeting of BbApe4 is dependent on BbAtg1 and BbAtg8, in particular, on the physical interaction with BbAtg8; 4) the carboxyl-tripeptide (GSI) of BbAtg8 is indispensable for the BbAtg8 lipidation and vacuolar targeting of BbApe4. These observations suggest that autophagy acts as a safeguard mechanism for conidial persistence and recruits hydrolytic enzymes for conidial germination when reviving from dormancy.
Conidiation occurs under aerial condition and conidia are in a state of water restriction during dormancy. Autophagy does not take place during conidial dormancy, but is observed in germinating conidia. As for the newly-formed conidia, autophagy contributes to germination under nutrient-deprivation conditions, but not the nutrient-rich ones. During long-term dormancy, autophagy is critical for conidial viability and ability to recover, in which the Atg11-mediated selective autophagy plays a less important role in comparison to the bulk autophagic process. In addition, inhibition or enhancement of autophagy impairs conidial ability to germinate, which suggests that autophagy is finely tuned by fungal cells.
In B. bassiana, BbApe4 is localized to vacuoles and also plays a significant role in conidial lifespan and vitality after maturation. We found that BbAtg8 transports BbApe4 into vacuoles via a direct interaction. In yeast Saccharomyces cerevisae, Ape4 binds to the selective autophagy receptor Atg19 and is transported into vacuoles from the cytoplasm via the cytoplasm-to-vacuole targeting (Cvt) pathway. There are seven Atg8-interaction motifs (AIM) in BbApe4, but site mutation analyses indicated that only two of them at the C-terminus are required for its vacuolar targeting and functionality. BbApe4 and BbAtg8 co-localize during conidial germination and hyphal growth. In vacuoles, BbApe4 undergoes processing at carboxyl terminus leading to its maturation into an active enzyme, similarly to S. cerevisae Ape4. In B. bassiana, the transport and processing of BbApe4 are dependent on BbAtg1 and BbAtg8, but not BbAtg11. Importantly, the autophagic role of BbAtg8 is also essential for vacuolar targeting of BbApe4 and conidial persistence during dormancy. Thus, these observations suggest that BbApe4 is functionally associated with autophagic process via its direct binding to BbAtg8. The identified functional AIMs are conserved in other fungal Ape4 homologs, including in S. cerevisae and other filamentous fungi. This suggests that the Ape4-Atg8 pathway might be prevalent in mutiple fungi.
Among hypotheses of conidial aging, intracellular redox homeostasis has received extensive attention. Autophagy is responsible for removing the damaged proteins and organelles under stress. In B. bassiana, pexophagy is involved in fungal resistance to oxidative stress. In addition, BbAtg8 maintains intracellular superoxide dismutase activity. As a consequence, BbAtg1 and BbAtg8 contribute to conidial resistance to oxidative stress during dormancy. In S. cerevisae, Ape4 contributes to cellular response to oxidative stress. Interestingly, BbApe4 could rescue the impaired oxidation tolerance of the S. cerevisae ape4Δ mutant, which suggests that BbApe4 is the functional ortholog of yeast Ape4. These findings reinforce the notion that autophagy is required for conidial lifespan owing to its antioxidant functions. The BbApe4-BbAtg8 interaction occurs not only under starvation but also during conidial germination and hyphal growth. Given its biochemical features, Ape4 acts as an autophagy-related proteinase involved in the substrate degradation necessary for both stress tolerance and the nutrient supply for vegetative growth. In comparison with the Cvt pathway in S. cerevisae, the direct interaction between BbApe4 and BbAtg8 deserves further investigation for the potential physiological relevance.
Taken together, autophagy safeguards conidial environmental persistence and acts as a sub-cellular mechanism for conidial recovery from dormancy (). Notably, our study supports a novel mechanistic model for vacuolar targeting of hydrolase in which BbAtg8 transports BbApe4 into vacuolar lumen via its direct association with BbAtg8. This direct interaction is essential for vacuolar transport and functionality of BbApe4. The BbApe4-BbAtg8 pathway contributes to conidial chronological life span and reactivation during dormancy, and we named it as simplified Cvt pathway (sCvt), since no selective autophagy receptor is involved. Altogether, this study further advances our understanding of the mechanisms involved in the longevity of filamentous fungi.
Figure 1. Model for the sCvt pathway function during conidial dormancy in B. bassiana. Upon maturation, conidia enter a stage of dormancy and employ autophagy to safeguard cell longevity and vitality. During germinating, autophagy participates in conidial recovery from dormancy in this fungus. BbATG8 produces two isoforms, i.e., BbAtg8-α and BbAtg8-β. BbAtg8-α meditates BbApe4 transport into the vacuole via direct physical interaction. In the vacuole, BbApe4 undergoes processing that lead to its maturation into the active form (BbApe4*).
Disclosure statement
No competing interest was reported by the authors.
Additional information
Funding
Reference
- Ding JL, Lin HY, Hou J, et al. The entomopathogenic fungus Beauveria bassiana employs autophagy as a persistence and recovery mechanism during conidial dormancy. mBio. 2023; doi: 10.1128/mbio.03049-22

