ABSTRACT
Accumulation of Lewy bodies in dopaminergic neurons is associated to Parkinson disease (PD). The main component of Lewy bodies appears to be aggregates of alpha-synuclein (α-syn). Several mutations of the gene encoding this protein promote its aggregation. Thus, clustering of α-syn is considered a central event in the onset of PD. An old theory also postulates that mitochondrial dysfunction represents another cause of PD pathogenesis. However, the impact of α-syn aggregates on mitochondria remains poorly understood considering the technical difficulties to discriminate between the different forms of α-syn. In this punctum, we describe our recent work in which we used a newly developed optogenetic tool to control the aggregation of α-syn and examine the impact on mitochondria. This work revealed that α-syn aggregates dynamically interact with mitochondria, triggering their depolarization and leading to cardiolipin translocation to the surface of mitochondria and mitophagy.
Abbreviations: α-syn: alpha-synuclein; BNIP3L: BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like; FUNDC1: FUN14 domain-containing protein 1; IMM: inner mitochondrial membrane; LIPA: light-induced protein aggregation; OMM: outer mitochondrial membrane; PD: Parkinson disease; SNc: substantia nigra par compacta;
Parkinson disease (PD) is characterized by the degeneration of dopaminergic neurons in the substantia nigra par compacta (SNc), leading to motor impairment. In the surviving neurons, Lewy bodies, which are mainly composed of alpha-synuclein (α-syn) aggregates, are observed. The genetic A30P, E46K, A53T, H50Q or G51D mutation in α-syn accelerate its aggregation and are associated with familial forms of PD. Thus, the aggregation of α-syn appears as one of the important molecular hallmarks of PD.
Considering the role of mitochondria in ATP production, metabolism of different neurotransmitters, control of Ca2+ fluxes within cells, it is not surprising that mitochondrial dysfunctions can lead to neurodegeneration and PD. Activity of complex I, a component of the electron transport chain involved in oxidative phosphorylation, is reduced in PD patients and models. Mitochondrial toxins, including the inhibitors of complex I, can lead to PD-like symptoms. Deficiencies in mitophagy is also a common hallmark of PD. For instance, mutations of PRKN and PINK1 encoding for PARKIN and PINK1, respectively, which regulate the induction of mitophagy upon specific cues, are associated to familial forms of PD. Overall, mitochondrial dysfunctions also appear to be an important hallmark of PD. The exact mechanism linking mitochondria and aggregation of α-syn, however, remains obscure. First, many models mimic PD physiopathology via overexpression of α-syn without controlling its aggregation. Also, antibodies specific for α-syn do not distinguish between monomeric, fibrillar or oligomeric α-syn species. In these contexts, the specific impact of α-syn aggregates on mitochondria is difficult to examine.
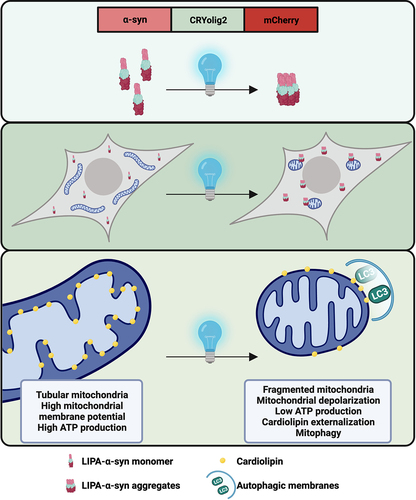
To address this gap, the group of A. Oueslati (Université Laval, Canada) developed a new light-induced protein aggregation (LIPA) system enabling to control and follow the aggregation of α-syn. This LIPA system is based on the photoreceptor cryptochrome protein 2, which rapidly clusters when exposed to blue light. Thus, fusion of the photoreceptor with α-syn and mCherry allows to induce and visualize α-syn clustering in cellular and animal models. In our latest workCitation1, this system was used to examine how α-syn aggregates impact mitochondria. Briefly, we induced the clustering of α-syn in different cell lines, human dopaminergic neurons and mouse SNc. Then, we examined how mitochondrial activity, morphology and degradation were impacted.
Live imaging showed that α-syn aggregates transiently interact with mitochondria in a kiss-and-run manner, suggesting that α-syn aggregates are not imported within mitochondria but rather contact with the cytosolic face of the mitochondrial outer membrane membrane (OMM). Contacts between the LIPA-α-syn aggregates and the OMM lead to mitochondrial depolarization, lower ATP production, mitochondrial fragmentation and mitophagy ().
Figure 1. The LIPA system is based on the fusion of the photoreceptor CRYolig2, which undergoes robust clustering upon stimulation with blue light. Fusion of CRYolig2 with α-syn and mCherry allows to control and visualize LIPA-α-syn aggregation. LIPA-α-syn aggregates contact with the cytosolic face of mitochondria in a kiss-and-run manner, leading to mitochondrial dysfunction. This causes a translocation of cardiolipin to the OMM, triggering mitophagy.
Multiple pathways can trigger mitophagy. In the PINK1-PARKIN pathway, PINK1 accumulates in depolarized mitochondria and recruits the ubiquitin ligase PARKIN. Then, PARKIN poly-ubiquitylates proteins at the surface of mitochondria allowing the recruitment of selective autophagy receptors. However, the PINK1-PARKIN pathway is likely not involved in the degradation of mitochondria induced by LIPA-α-syn aggregation. Indeed, increased ubiquitylation of mitochondrial proteins is not observed upon LIPA-α-syn aggregation. Moreover, overexpression of an ubiquitin mutant unable to form polyubiquitin chains does not prevent degradation of this organelle. These findings suggest that PARKIN and ubiquitylation are not involved in mitophagy triggered upon LIPA-α-syn aggregation.
Thus, we explored other pathways regulating mitophagy. Mitochondrial proteins that act as selective autophagy receptors such as FUNDC1 (FUN14 domain-containing protein 1) and BNIP3L (BCL2/adenovirus E1B 19 kDa protein-interacting protein 3-like) can initiate mitophagy. However, the silencing of these proteins does not block mitochondrial degradation after aggregation of LIPA-α-syn. Cardiolipin is a phospholipid specifically present within the inner mitochondrial membrane (IMM). It can be translocated to the OMM during various stresses where it can directly bind to LC3 (Microtubule-associated proteins 1A/1B light chain 3B) to trigger mitophagy. Interestingly, the abundance of cardiolipin increases at the surface of mitochondria after LIPA-α-syn aggregation. Also, downregulation of PLSCR3 (phospholipid scramblase 3), the enzyme responsible for cardiolipin transport between IMM and OMM, completely prevent mitochondrial degradation upon LIPA-α-syn aggregation. Overall, our findings suggest that the dynamic interaction between LIPA-α-syn aggregates and mitochondria induces mitochondrial alterations which favor cardiolipin externalization and mitophagy. However, α-syn has been shown to interact with endoplasmic reticulum (ER) and mitochondria-associated ER membranes, which are hardly distinguishable from mitochondria in standard fluorescent microscopy. It will be important to examine whether LIPA-α-syn aggregates interact with these structures to trigger mitochondrial dysfunctions.
Cardiolipin might also have a dual effect on how α-syn aggregates impact on mitochondria and mitophagy. For instance, α-syn can directly bind to cardiolipin to favor mitochondrial membrane disruption and, consequently, mitophagy. However, α-syn and the autophagy protein LC3 can also compete for binding to cardiolipin, resulting is diminished mitophagy when α-syn binds to mitochondrial membranes. In other words, binding of α-syn to cardiolipin may prevent mitophagy. It will be important to examine whether the α-syn aggregates interact with cardiolipin on mitochondrial surface to induce mitochondrial dysfunction and, in turn, mitophagy.
In conclusion, the LIPA-α-syn system allows to specifically examine how α-syn aggregates induce mitochondrial dysfunctions and mitophagy. Our findings suggest that cardiolipin externalization is necessary in the mitophagy triggered by α-syn aggregation
In future work, it will also be important to test whether untagged LIPA constructs have the same consequences since the protein mCherry could impact how the LIPA-α-syn aggregates induce mitochondrial dysfunctions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Reference
- Lurette O, Martín-Jiménez R, Khan M, et al. Aggregation of alpha-synuclein disrupts mitochondrial metabolism and induce mitophagy via cardiolipin externalization. Cell Death Dis. 2023; 14:729.

