ABSTRACT
BECN1 (BCL2 interacting myosin like coiled protein) is a major regulator of autophagy and a haploinsufficient tumor suppressor. BECN1 binds to multiple proteins and it is part of at least two different class III phosphatidylinositol (PI) 3 Kinase (PI3KC3) complexes that regulate autophagy and endocytic trafficking through the biosynthesis of phosphatidylinositol-3-phosphate. BECN1 and the activity of the PI3KC3 are regulated by post-translational modifications and/or subcellular localization. We recently discovered that GRB2 (growth factor receptor bound protein 2), an adaptor protein of several tyrosine kinases, interacts with BECN1 and regulates autophagy.Citation1
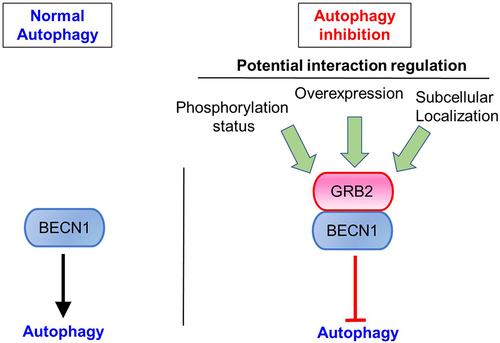
We previously found that HER2 (human epidermal growth factor receptor 2), a member of the EGFR (epidermal growth factor receptor) receptor tyrosine kinase family that is frequently amplified in breast and other cancers, interacts with BECN1 in breast cancer cell lines overexpressing HER2. We have also shown that the BECN1-HER2 interaction inhibits autophagy, affecting tumorigenesis and tumor growth. We also uncovered that the genetic induction of autophagy in mice through the knock-in of a BECN1 mutant with decreased binding to its inhibitor BCL2 prevents mammary tumorigenesis in transgenic mice overexpressing HER2 in the mammary gland. In addition, treatment of xenografts overexpressing HER2 with an autophagy-inducing peptide prevents tumor growth. In the new study,Citation1 we have investigated the molecular mechanism whereby HER2 binds to BECN1 and inhibits autophagy. A search into a comprehensive proteomic study of the autophagy network led us to hypothesize GRB2 as a potential mediator of the HER2-BECN1 interaction. Surprisingly, we found that GRB2 was not only binding to BECN1 in the context of HER2+ breast cancer cells, but also in HER2-negative breast cancer cells as well as in other tumor cells from different origins in which HER2 is not overexpressed. These data suggested that GRB2 might be a universal BECN1 binding partner (). Moreover, the catalytic unit of the PI3KC3 complexes, PIK3C3 (phosphatidylinositol 3-kinase catalytic subunit type 3/VPS34) was also co-immunoprecipitated with BECN1 and GRB2, although other BECN1 binding partners were not evaluated. Docking studies led us to propose a mechanism whereby GRB2 binds to BECN1 through the evolutionary conserved domain (ECD) of BECN1, and we validated these findings by co-immunoprecipitation experiments in cells expressing wild-type (WT) BECN1 or a variant of this protein that lacks the ECD. There are many different BECN1 binding proteins that interact through its ECD. Therefore, it is plausible that GRB2 affects the recruitment of other subunits to the complex. The regulation of this binding is most probably of an enormous complexity because of the large number of proteins that have been reported to bind and modulate both GRB2 and BECN1.
To determine whether GRB2 played a role into the function of BECN1 in autophagy, we depleted GRB2 in all the cell lines tested and found that the absence of GRB2 induced both the autophagic flux and the phosphatidylinositol 3-kinase activity of PIK3C3, suggesting that GRB2 is indeed reducing autophagy possibly by inhibiting the PIK3C3 activity. Since numerous proteins bind to BECN1, it is of great interest to determine in the future whether GRB2 depletion affects the interaction profile of BECN1 and the PI3KC3 complexes. Whether GRB2 also modulates the BECN1 function in endocytic trafficking remains to be solved.
To understand the structural determinants regulating the GRB2-BECN1 interaction, mutations in GRB2 residues potentially involved in its interaction (as determined by molecular docking studies) with BECN1 revealed that they are not individually required for such binding, even though some of them (Y52, within the N-terminal SH3 domain; R86 and S88 within the SH2 domain) are required for the autophagic modulatory function of GRB2. That is, while back-transfection of WT GRB2 in cells depleted of the endogenous GRB2 restored the autophagic flux, expression of the point mutants GRB2Y52A or GRB2R86A,S88A did not. Whether these residues are required for other known GRB2 functions remain to be solved and their effect when mutated all together still need to be tested. Moreover, it is unknown whether these mutations affect the subcellular localization of GRB2 and the GRB2-BECN1 interaction.
To test the potential consequences of these mutants in a more physiological context, we performed studies in a CAM (chicken chorioallantoic membrane) model. As expected, depletion of GRB2 strongly compromised both the tumor formation as well as the tumor growth in ovo, and decreased the sequestosome 1 (SQSTM1/p62) levels as assessed by immunohistochemistry (IHC). Although these observations indicate an induction of the autophagic flux, GRB2 knockdown is potentially affecting numerous different pathways, with autophagy possibly being only one of them. A better approach to determine the role of the autophagy-modulating function of GRB2 in ovo would be to test the effect of the GRB2 loss-of-function mutants in tumor formation and tumor growth. Tumors arising from cells overexpressing WT GRB2 were not significantly bigger that those generated from cells expressing a vector control, but significantly smaller tumors were generated when overexpressing GRB2Y52A or GRB2R86A,S88A. The results suggest that these mutant proteins might function as dominant-negative and that GRB2 autophagy-regulating role is important for tumor growth. Although we found significantly lower p62 levels in the tumors overexpressing GRB2 loss-of-function mutants, suggesting a potentially induced autophagic flux, it remains to be addressed whether GRB2 effect on the decrease of tumor size depends on autophagy. Only a handful of tumors have been annotated to harbor mutations in GRB2 in the catalogue of somatic mutations in cancer (COSMIC) database, and therefore GRB2 mutations are not really hotspots and cannot be considered as drivers of cancer. Multiple residues in GRB2 have been reported to be phosphorylated, including the Y52, S88 and Y209, and modified by kinases such as EGFR, janus kinase 2 (JAK2) and breakpoint cluster region-ABL proto-oncogene 1 non-receptor tyrosine kinase (BCR-ABL). As a result, some of these kinases might modulate the GRB2-BECN1 interaction. However, this aspect remains to be investigated.
The potential clinical relevance of our findings is highlighted by the analyses of both gene expression (using publicly available data) and protein levels (by reverse-phase protein array data [RPPA]) in human breast cancer samples, which showed that breast cancer tumors with higher GRB2 levels (and potentially having a strong inhibitory effect on autophagy) correlated with higher SQSTM1/p62 levels (potentially confirming a lower autophagy activity). In addition, patients with higher levels of both, GRB2 and SQSTM1/p62, have a significantly lower overall survival and progression-free survival when compared with patients with lower levels of both. How the BECN1-GRB2 interaction is regulated and whether this is direct, and if these two proteins interact in patient samples remains to be studied.
In summary, we discovered a new BECN1 binding protein that regulates autophagy and might be potentially a new player into multiple signaling pathways converging in autophagic flux modulation both in healthy and pathological conditions.
Abbreviations
BCR-ABL, breakpoint cluster region-ABL proto-oncogene 1 non-receptor tyrosine kinase; BECN1, BCL2 interacting myosin like coiled protein; CAM, chick chorioallantoic membrane; ECD, evolutionary conserved domain; EGFR, epidermal growth factor receptor; GRB2, growth factor receptor bound protein 2; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; JAK2, janus kinase 2; PI, phosphatidylinositol; PI3KC3, class III PI3K; PIK3C3, phosphatidylinositol 3-kinase catalytic subunit type 3/VPS34; RPPA, reverse-phase protein array; SQSTM1/p62: sequestosome 1; SH2, Src homology 2; SH3, Src homology 3; WT, wild-type.
Conflict of interest statement
The authors declare no conflict of interest.
Additional information
Funding
Reference
- Montero-Vergara J, Plachetta K, Kinch L, Bernhardt S, Kashyap K, Levine B, et al. GRB2 is a BECN1 interacting protein that regulates autophagy. Cell Death Dis. 2024; 15:14.