ABSTRACT
Purpose:
The aim of this scoping review was to identify the physical function assessment scales for the rehabilitation treatment of upper limb lymphedema. This review was conducted to establish a consensus on assessment scales for use in clinical practice worldwide.
Methods:
Four electronic databases (PubMed, Cochrane Library, PEDro, and OTseeker) were searched. Search strategy included identified search terms. Articles were selected based on specific inclusion and exclusion criteria.
Results:
A total of 411 results were retrieved, and 16 articles were analysed after screening. We identified 13 types of physical function assessment scales and six functional categories. Disabilities of arm, shoulder, and hand (n = 9), range of motion (n = 6), and grip strength (n = 4) were the most commonly reported assessment scales.
Conclusions:
Current assessment scales in upper limb lymphedema are dominated by patient-reported outcome measures, motion, and strength. However, the gold standard for physical function assessment scales in upper limb lymphedema remains uncertain. In the future, there is a need to standardize or develop physical function assessment scales that reflect the effectiveness of rehabilitation treatment in patients with upper limb lymphedema.
Introduction
Lymphedema is a chronic localized form of tissue swelling that results from excessive retention of lymphatic fluid in the interstitial compartment and is caused by impaired lymphatic drainage [Citation1]. Lymphedema can occur anywhere in the body and is generally classified as either primary (genetic) or secondary (acquired) lymphedema, with many patients suffering negative effects on their physical activities of daily living and quality of life. In particular, secondary lymphedema of the upper limbs following breast cancer treatment that often causes functional disability and psychological and social problems [Citation2].
Complex decongestive physiotherapy is commonly used in lymphedema rehabilitation. This technique includes skin care, manual lymph drainage, compression therapy, and exercise therapy under compression. Furthermore, patient education is also important to enable patients to manage edema adequately their own. Previous reports have shown that rehabilitation for upper or lower limb lymphedema reduces the circumference or volume of the lymphedema [Citation3,Citation4].
However, little is known regarding the effects of rehabilitation on physical function. No consensus has been reached on the assessment scales for measuring physical function in patients with lymphedema. Importantly, to establish evidence or rehabilitation of lymphedema, the assessment scales of physical function must be determined.
This article presents a scoping review aimed to identify the physical function assessment scales used in published research articles on upper limb lymphedema rehabilitation.
Materials and methods
Study design
This scoping review was conducted in accordance with the five-stage framework initially presented by Arksey and O’Malley [Citation5] further developed by Levac [Citation6]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping review (PRISMA-ScR) guidelines were also followed [Citation7,Citation8].
Framework stage 1: identifying the research question
We defined the research question in this study as follows: “which physical function assessment scales are used when rehabilitation of upper limb lymphedema?”.
Framework stage 2: identifying relevant studies
The article search was conducted on June 1, 2023. All articles were written in English and published by the time of the search date. Four electronic databases (PubMed, Cochrane Library, PEDro, and OTseeker) were searched. In the database search, the key concepts were divided into five items, namely, pathological condition, treatment, site, physical function, and outcome measurement, and the search was conducted. In the pathological condition section, one term was used, namely, “lymphedema”. In the treatment section, one term was used, namely, “rehabilitation”. In the site section, two terms were used, namely, “upper limb” and “upper extremity”. In the physical function section, three terms were used, namely, “physical”, “function*”, and “performance”. In the outcome measure section, five terms were used, namely, “outcome”, “parameter”, “measurement”, “task”, and “assessment”. The Boolean operators “OR” and “AND” were used to link the search terms from each concept. A list of key concepts and search terms is presented in . In addition, for the database search, we obtained references through manual searching.
Table 1. List of the key concepts and search terms.
The following criteria were used for inclusion in the study: (1) inclusion of physical function assessment scales, (2) use of non-invasive and commonly used assessments in rehabilitation practice, (3) original articles with a study design demonstrating a level of evidence higher than that typically found in observational studies, and (4) the author or evaluators were not limited to rehabilitation-related professions.
The exclusion criteria were as follows: (1) use of subjective measurements alone, such as Visual Analogue Scale and Numerical Rating Scale, (2) exclusively measuring the circumference or volume of the affected limb for physical function assessment, (3) intervention or treatment utilizing invasive approaches, (4) study designs, including case reports, review articles, systematic reviews, and meta-analyses, and (5) conference abstracts.
Framework stage 3: study selection
Independent reviewers examined the inclusion and exclusion criteria for all relevant articles. Title and abstract screening were conducted independently by two authors (Y.F. and H.T.), followed by full-text screening, with discrepancies resolved by a third arbiter (Y.Y.).
Framework stage 4: charting the data
The reviewers collaboratively developed a data-extraction method. Base on the research question and review purpose, the extraction categories were as follows: first author, year of publication, article title, study location, study design, number of participants, intervention, lymphedema measurement (circumference and/or volume), outcome measure of physical function, and other measurement tools.
Framework stage 5: collating, summarizing, and reporting the results
Data were extracted from all eligible articles by an independent author and the verified by another author, and the results were analysed numerically and thematically. For the thematic analysis, we identified how the articles were related to the research question and created codes (labels) that best reflected the assessment of the physical function assessment scales. After repeating the code creation process and successfully identifying patterns among the codes, categories were created. The results of the numerical and thematic analyses are presented in .
Table 2. Synthesis of eligibility article characteristics.
Results
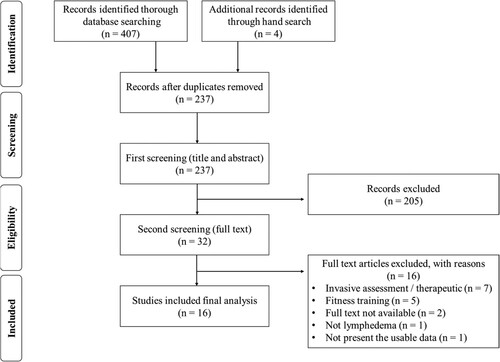
A total of 411 results were retrieved from all the sources. Duplicate articles were removed (n = 174), yielding 237 articles for eligibility screening. The titles and abstracts were screened, and 205 articles were excluded. Thirty-two articles were examined in full text and assessed for eligibility, and an additional 16 articles were excluded. Finally, 16 eligible articles were analysed in the present study. The number and strategy of articles reviewed, selected, and checked at each stage are shown in the PRISMA flow diagram [Citation7,Citation8] ().
Synthesis of eligibility article characteristics
summarizes the characteristics of the eligible articles. Sixteen articles published between 2005 and 2023 were included in this review. The studies were conducted in Turkey (n = 6), USA (n = 2), Spain (n = 2), South Korea (n = 2), Germany (n = 1), Canada (n = 1), Bahrain (n = 1), and Saudi Arabia (n = 1). The study designs were randomized controlled trial (n = 11), pretest-posttest design (n = 2), cross-sectional study (n = 2), and case–control study (n = 1). A total of 900 patients with upper limb lymphedema were included in this review. All the studies included Breast Cancer-Related Lymphedema (BCRL) as a factor in the pathogenesis of upper limb lymphedema. The interventions in the articles were complex physical therapy or complex decongestive therapy (n = 9), kinesio taping (n = 2), negative pressure massage (n = 1), myofascial release (n = 1), and none for observational studies (n = 3). The morphometric measurements of lymphedema included circumference (n = 2), volume (n = 12), circumference and volume (n = 1), and none (n = 1).
Reported physical function assessment scales
The reported physical function assessment scales are presented in . We identified 13 types of physical function assessment scales and six functional categories (motion, strength, sensory, manual dexterity, fine motor skill, and patient-reported outcome measures). The three most commonly reported assessment scales were disabilities of arm, shoulder, and hand (DASH) (n = 9), range of motion (n = 6), and grip strength (n = 4).
Table 3. Reported physical function assessment scales in upper limb lymphedema.
Discussion
We conducted a scoping review to identify physical function assessment scales in upper limb lymphedema. As a result, 16 articles met the inclusion criteria. This article provides important insight into the assessment of rehabilitation in patients with upper limbs lymphedema.
Patient-reported outcome measures (PROMs), which are subjective scales, are the physical function assessment scales used in most articles. This concept and terminology have recently emerged as a health outcome measures, particularly in the evaluation of technologies such as drugs and medical devices, as this concept is more practical and easier to share compared with quality of life [Citation25]. Among the various PROMs, the DASH, including the Quick-DASH, was the most frequently used assessment scales in this review [Citation9,Citation10,Citation12,Citation13,Citation15,Citation17,Citation19–24]. The DASH is a typical PROM designed to measure functional disability resulting from upper limb condition. It evaluates not just the specific site of disability but also subjectively measures activities of daily living involving the entire upper limb. Moreover, the DASH is frequently used not only for lymphedema but also for other diseases [Citation26]. Its versatility in addressing many upper limb dysfunctions and its ease of assessment likely account for its use in lymphedema studies. Another noteworthy PROM for measuring upper limb function is the shoulder pain and disability index [Citation16], which has demonstrated excellent reliability akin to the DASH [Citation27,Citation28]. Therapy for lymphedema improves a patient’s quality of life, as evidenced by improvements in the patient’s well-being. Consequently, PROM studies assessing patient subjectivity are poised to gain increasing importance in the future. However, this method has several problems. For example, solely relying on PROM measurements many obscure the actual physical function. Therefore, the combination of measurement of PROMs and the assessment of actual physical function is necessary to effectively demonstrate the impact of rehabilitation on lymphedema. In the present study, all selected eligible articles assessed not only PROMs but also physical functions.
The next category for measuring physical function assessment scales in upper limb lymphedema was Motion. In the Motion category, ROM was measured in all articles. All the articles included in this review focused on had patients with BCRL as the study subjects. During the perioperative period of breast cancer, patients often present with a loss of shoulder joint motion [Citation29,Citation30]. Furthermore, upper limb lymphedema is expected to result in the loss of ROM due to swelling, weight, and mechanical properties of the skin. Among the selected eligible articles, there were studies that measured ROM before and after lymphedema therapy to determine treatment efficacy [Citation9–12,Citation14]. They also compared ROM between lymphedema and non-lymphedema patients [Citation13], all of which showed loss of ROM in patients with upper extremity lymphedema.
The third most common category was Strength. (The Sensory category had the same number, but many duplicate articles were identified.) Grip strength is a widely used and accessible test that can be performed in hospital practices and specialty clinical settings [Citation31,Citation32]. It is commonly used in clinical practice as a diagnostic or screening tool to detect low skeletal muscle weakness caused by sarcopenia and/or frailty [Citation33]. In upper limb lymphedema, edema may cause difficulties with full finger grip and decreased muscle output. Among the selected eligible articles, there were reports indicating that therapy for lymphedema resulted in increased grip strength [Citation15,Citation16], suggesting that patients may have had decreased grip strength prior to treatment.
Other categories used were Sensory, Manual dexterity, and Fine motor skill. The sensory category consisted of two duplicate articles, each using five outcome measures, which represented the highest number of outcome measures among all categories. Among the five outcome measures, both articles used the Semmes-Weinstein Monofilament test (SWM) [Citation13,Citation18], a sensory test that applies tactile stimuli to the skin and to determine the threshold or static tactile sensation based on the response. The SWM, as a quantitative test, demonstrates high reliability in diagnosing and evaluating therapeutic efficacy, being the only method with established reliability and validity in assessing sensory function [Citation34,Citation35]. Given the often-unclear sensory functions of lymphedema, these two articles sought to clarify these functions using the SWM, a test recognized for its reliability and validity. The results indicated a trend toward mild sensory impairment in both studies [Citation13,Citation18]. Nonetheless, other sensory tests were explored and have not yet achieved standardization in lymphedema assessments.
Manual dexterity and fine motor skills were used in the same article [Citation13]. Although finger lymphedema is expected to reduce hand usability, few studies have assessed this aspect of the disease. Therefore, the most suitable assessment method is unclear, and the results of this review cannot be generalized. Moreover, no study has used the Box and Block Test, a highly reliable and validated tool used in measuring hand usability for various upper limb dysfunctions [Citation36–38].
This study had some limitations. First, we searched the electronic databases from their inception until June 1, 2023. Thus, documents published after these dates were excluded. Second, this scoping review excluded non-English articles, grey literature, and previous review articles. Finally, we did not assess the effectiveness of lymphedema therapy in patients with upper limb lymphedema using each physical function assessment scales, because it is optional in the PRISMA-ScR guidelines [Citation7,Citation8]. This is because scoping studies do not aim to assess the quality of evidence and, consequently, cannot determine whether particular studies provide robust or generalizable findings.
In conclusion, we demonstrated the physical function assessment scales that are used in the treatment of upper limb lymphedema. This study classified these physical function assessment scales into six categories and 13 types. Current scales in upper limb lymphedema are dominated by patient-reported outcome measures, motion, and strength. However, no consensus has been reached on the physical function assessment scale in upper limb lymphedema which remains a subject of uncertain. In the future, there is a need to standardize or develop physical function assessment scales that reflect the effectiveness of rehabilitation treatment in patients with upper limb lymphedema.
Data availability statement
Data are available upon reasonable request from corresponding author.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Grada AA, Phillips TJ. Lymphedema: pathophysiology and clinical manifestations. J Am Acad Dermatol. 2017;77(6):1009–1020. doi:10.1016/j.jaad.2017.03.022
- Eaton LH, Narkthong N, Hulett JM. Psychosocial issues associated with breast cancer-related lymphedema: a literature review. Curr Breast Cancer Rep. 2020;12(4):216–224. doi:10.1007/s12609-020-00376-x
- Rogan S, Taeymans J, Luginbuehl H, et al. Therapy modalities to reduce lymphoedema in female breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016;159(1):1–14. doi:10.1007/s10549-016-3919-4
- Lasinski BB, McKillip Thrift K, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R. 2012;4(8):580–601. doi:10.1016/j.pmrj.2012.05.003
- Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract. 2005;8(1):19–32. doi:10.1080/1364557032000119616
- Levac D, Colquhoun H, O'Brien K. Scoping studies: advancing the methodology. Implement Sci. 2010;5(1):69. doi:10.1186/1748-5908-5-69
- Moher D, Liberati A, Tetzlaff J, et al. PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi:10.1371/journal.pmed.1000097
- Tricco AC, Lillie E, Zarin W, et al. Prisma extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/M18-0850
- Kim Y, Park EY, Lee H. The effect of myofascial release in patients with breast cancer-related lymphedema: a cross-over randomized controlled trial. Eur J Phys Rehabil Med. 2023;59(1):85–93. doi:10.23736/S1973-9087.22.07698-5
- Pajero Otero V, García Delgado E, Martín Cortijo C, et al. Intensive complex physical therapy combined with intermittent pneumatic compression versus kinesio taping for treating breast cancer-related lymphedema of the upper limb: a randomised cross-over clinical trial. Eur J Cancer Care (Engl). 2022;31(5):e13625. doi:10.1111/ecc.13625
- Pajero Otero V, García Delgado E, Martín Cortijo C, et al. Kinesio taping versus compression garments for treating breast cancer-related lymphedema: a randomized, cross-over, controlled trial. Clin Rehabil. 2019;33(12):1887–1897. doi:10.1177/0269215519874107
- Sezgin Ozcan D, Dalyan M, Unsal Delialioglu S, et al. Complex decongestive therapy enhances upper limb functions in patients with breast cancer-related lymphedema. Lymphat Res Biol. 2018;16(5):446–452. doi:10.1089/lrb.2017.0061
- Smoot B, Wong J, Cooper B, et al. Upper extremity impairments in women with or without lymphedema following breast cancer treatment. J Cancer Surviv. 2010;4(2):167–178. doi:10.1007/s11764-010-0118-x
- Didem K, Ufuk YS, Serdar S, et al. The comparison of two different physiotherapy methods in treatment of lymphedema after breast surgery. Breast Cancer Res Treat. 2005;93(1):49–54. doi:10.1007/s10549-005-3781-2
- Tastaban E, Soyder A, Aydin E, et al. Role of intermittent pneumatic compression in the treatment of breast cancer-related lymphoedema: a randomized controlled trial. Clin Rehabil. 2020;34(2):220–228. doi:10.1177/0269215519888792
- Tantawy SA, Abdelbasset WK, Nambi G, et al. Comparative study between the effects of kinesio taping and pressure garment on secondary upper extremity lymphedema and quality of life following mastectomy: a randomized controlled trial. Integr Cancer Ther. 2019;18:1534735419847276. doi:10.1177/1534735419847276
- Lee D, Hwang JH, Chu I, et al. Analysis of factors related to arm weakness in patients with breast cancer-related lymphedema. Support Care Cancer. 2015;23(8):2297–2304. doi:10.1007/s00520-014-2584-6
- Baran E, Özçakar L, Özgül S, et al. Upper limb sensory evaluations and ultrasonographic skin measurements in breast cancer-related lymphedema receiving complex decongestive physiotherapy. Support Care Cancer. 2021;29(11):6545–6553. doi:10.1007/s00520-021-06235-4
- Korucu TS, Ucurum SG, Tastaban E, et al. Comparison of shoulder-arm complex pain, function, and scapular dyskinesia in women with and without unilateral lymphedema after breast cancer surgery. Clin Breast Cancer. 2021;21(3):e285–e293. doi:10.1016/j.clbc.2020.10.008
- Lampinen R, Lee JQ, Leano J, et al. Treatment of breast cancer-related lymphedema using negative pressure massage: a pilot randomized controlled trial. Arch Phys Med Rehabil. 2021;102(8):1465–1472.e2. doi:10.1016/j.apmr.2021.03.022
- Buragadda S, Alhusaini AA, Melam GR, et al. Effect of complete decongestive therapy and a home program for patients with post mastectomy lymphedema. J Phys Ther Sci. 2015;27(9):2743–2748. doi:10.1589/jpts.27.2743
- Dayes IS, Whelan TJ, Julian JA, et al. Randomized trial of decongestive lymphatic therapy for the treatment of lymphedema in women with breast cancer. J Clin Oncol. 2013;31(30):3758–3763. doi:10.1200/JCO.2012.45.7192
- King M, Deveaux A, White H, et al. Compression garments versus compression bandaging in decongestive lymphatic therapy for breast cancer-related lymphedema: a randomized controlled trial. Support Care Cancer. 2012;20(5):1031–1036. doi:10.1007/s00520-011-1178-9
- Sen EI, Arman S, Zure M, et al. Manual lymphatic drainage may not have an additional effect on the intensive phase of breast cancer-related lymphedema: a randomized controlled trial. Lymphat Res Biol. 2021;19(2):141–150. doi:10.1089/lrb.2020.0049
- U.S. Food and Drug Administration. Patient-reported outcome measures: use in medical product development to support labeling claims. 2009; [cited 1 Nov 2023].
- Valdes K, MacDermid J, Algar L, et al. Hand therapist use of patient report outcome (PRO) in practice: a survey study. J Hand Ther. 2014;27(4):299–308. doi:10.1016/j.jht.2014.07.001
- Roy JS, MacDermid JC, Woodhouse LJ. Measuring shoulder function: a systematic review of four questionnaires. Arthritis Rheum. 2009;61(5):623–632. doi:10.1002/art.24396
- Franchignoni F, Vercelli S, Giordano A, et al. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (QuickDASH). J Orthop Sports Phys Ther. 2014;44(1):30–39. doi:10.2519/jospt.2014.4893
- Olsson Möller U, Beck I, Rydén L, et al. A comprehensive approach to rehabilitation interventions following breast cancer treatment - a systematic review of systematic reviews. BMC Cancer. 2019;19(1):472. doi:10.1186/s12885-019-5648-7
- De Groef A, Van Kampen M, Dieltjens E, et al. Effectiveness of postoperative physical therapy for upper-limb impairments after breast cancer treatment: a systematic review. Arch Phys Med Rehabil. 2015;96(6):1140–1153. doi:10.1016/j.apmr.2015.01.006
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi:10.1093/ageing/afq034
- Takamoto K, Morizaki Y, Fukuda A, et al. Hand grip strength differences in geriatric subjects with and without hand diseases. Prog Rehabil Med. 2023;8:20230030. Published 2023 Sep 21. doi:10.2490/prm.20230030
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis [published correction appears in Age Ageing. 2019 Jul 1;48(4):601]. Age Ageing. 2019;48(1):16–31. doi:10.1093/ageing/afy169
- Bell-Krotoski J, Tomancik E. The repeatability of testing with Semmes-Weinstein monofilaments. J Hand Surg Am. 1987;12(1):155–161. doi:10.1016/S0363-5023(87)80189-2
- Jerosch-Herold C. Assessment of sensibility after nerve injury and repair: a systematic review of evidence for validity, reliability and responsiveness of tests. J Hand Surg Br. 2005;30(3):252–264. doi:10.1016/J.JHSB.2004.12.006
- Huang J, Ji JR, Liang C, et al. Effects of physical therapy-based rehabilitation on recovery of upper limb motor function after stroke in adults: a systematic review and meta-analysis of randomized controlled trials. Ann Palliat Med. 2022;11(2):521–531. doi:10.21037/apm-21-3710
- Connell LA, Tyson SF. Clinical reality of measuring upper-limb ability in neurologic conditions: a systematic review. Arch Phys Med Rehabil. 2012;93(2):221–228. doi:10.1016/j.apmr.2011.09.015
- Platz T, Pinkowski C, van Wijck F, et al. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer test, action research Arm test and box and block test: a multicentre study. Clin Rehabil. 2005;19(4):404–411. doi:10.1191/0269215505cr832oa