ABSTRACT
There are no good markers to identify drug-induced toxicity and clinical efficacy in advanced-stage oral cancer (OC), despite the existence of well-established first-line and induction chemotherapeutics. As a result, we investigated the impact of transporter genes in (Taxane/Platinum/5-fluorouracil) TPF or (Paclitaxel/Carboplatin) PC on clinical outcomes in OC patients. One hundred and twenty OC patients who had received fixed-dose TPF or PC were included in the current study. The transporter gene’s polymorphism was evaluated using the PCR-RFLP method. The toxicity and progression-free survival of SNPs were also evaluated. There was a significant association in the gene ABCG2 (rs4693924) in both the wt/mt and mt/mt genotypes (p < .001). Meanwhile, dysphasia was significantly associated with SNPs rs943288 in ABCC4 in both wt/mt and mt/mt genotypes. A significant association was found for nausea with SNPs rs2804398 in ABCC2 (p = .008) and SNPs rs9332430 in ABCC1 (p = .001). However, there was no significant association observed between the genotypes of each SNP and progression-free survival (PFS). Clinical responses with progression-free survival and risk hazards were highly significant (p = .001). In conclusion, our data showed that the rs4693924 in ABCG2 anemia, ABCC2 (rs2804398) in diarrhea, ABCCC4 (rs943288) in dysphasia, and ABCC1 (rs9332430) in nausea were associated with individual adverse events.
Introduction
Oral cancer (OC) is an intricate and heterogeneous disease with a poor prognosis and low survival rateCitation1 Worldwide, cancer of the oral cavity is the 16th most common malignancy. (https://www.wcrf.org/cancer-trends/worldwide-cancer-data) India accounts for one-third of the world’s mouth cancer cases,Citation2 Indian oral cancer patients are 70% progressed (American Joint Committee on Cancer, Stage III-IV), and late identification reduces the prospects of cure, leaving five-year survival rates around 20%.Citation3 The major treatment options for OCC patients, even with advanced conditions, are radiation therapy and/or surgery with chemotherapy.Citation4 Chemotherapy toxicity decreases quality of life, prolongs treatment, and reinforces negative emotions. Due to fear of toxicity and side effects, many cancer patients refuse further chemotherapy and consider stopping it.Citation5 Induction chemotherapy, which is used prior to surgery or radiotherapy as the initial treatment, TPF (docetaxel/paclitaxel, cisplatin, and 5-FU) as induction, and first-line PC (paclitaxel and carboplatin) regimens are employed as adjuvant, neoadjuvant, or palliative chemotherapy.Citation6,Citation7 Chemotherapies based on platinum are effective at treating cancer, but they can cause serious side effects because of the platinum. Drugs made with platinum have side effects like nephrotoxicity, gut (GI) toxicity, and hepatotoxicity, which make the treatment less effective.Citation8 Thus, personalized therapy targets those most likely to benefit from anticancerous chemotherapy or have fewer AEs. There is growing evidence that genetic variants cause toxicity differences in chemotherapy-related drugs.Citation9 To maximize personalized therapy, association of genetic polymorphism and taxane/platinum/fluorouracil-induced drug toxicity must be assessed.
ATP-binding cassette (ABC) membrane transporters play a big role in how well chemotherapy works and how dangerous it is.Citation10 Single nucleotide polymorphisms are mutations that can also be found in genes that code for ABC transporters. These mutations can change the functions or expressions of the ABC proteins. It is possible that the abnormal expression of these transporter genes will have an effect on the interindividual variability of drug tissue concentration as well as the therapeutic effects of the drug. The impact of different transporter genes like ABCG2, ABCC2, ABCC4, and ABCC1 has been studied in some tumors.Citation11–13 These convincing data suggest that ABC gene polymorphisms play a crucial role in predicting toxicity in oral cancer patients undergoing taxane-platinum-fluorouracil based chemotherapy. Therefore, disclosing the genetic variations associated with ABC transporter genes is crucial for individualizing treatment in order to increase therapeutic response and decrease drug toxicity. Hence, the genetic variations associated with ABC transporter genes are crucial for individualizing treatment in order to increase therapeutic response and decrease drug toxicity. The goal of the current study is to determine how the ABC polymorphisms affect the AEs experienced by oral cancer patients receiving taxane-platinum-based fluorouracil treatment.
Materials and Methods
Study Design
At the Chittaranjan National Cancer Institute in Kolkata, India, 120 individuals diagnosed with oral cancer were sought out for participation in this prospective study between the months of June 2018 and December 2021. The following was a list of the inclusion criteria: the dosage of TPF or PC taken during the treatment period. Having a clinical or histological diagnosis of oral cancer, not being under the age of 18 and more than 70 years, living in the eastern part of the Indian population, having a WHO/ECOG performance status of less than 2, and not having any other concomitant malignant diseases A non-resolving active bacterial or viral infection, medication hypersensitivity, diabetes, a history of heart disease in the recent past, and other conditions were among the criteria for exclusion. Ethical approval was obtained from the Institutional Ethics Council of the Chittaranjan National Cancer Institute (A-4.311/1/2018–1) and all patients submitted their written informed consent for the study.
Treatment Regimes
Patients were received paclitaxel 175 mg/m2 by 3-h continuous infusion in 500 ml normal saline on day 1 and cisplatin 25 mg/m2/day from day 1 to 3 (total dose: 75 mg/m2) was given after a 45’ pre-hydration with 500 ml of saline containing NaCl, 10 mEq, and 90’ post-hydration with 300 mannitol was used as a renoprotective measure followed by rehydration with 500 ml of saline, and 5-fluorouracil 500 mg/m2/day from day 1 to 3 was given as an i.v. bolus at the end of the post-hydration period in 500 ml normal saline in 3-h infusion, courses were repeated every 3 weeks for TPF chemotherapy, whereas for PC chemotherapy regimes paclitaxel were given 175 mg/m2 by 3-h continuous infusion in 500 ml normal saline on day 1 and carboplatin 375 mg/m2 (5AUC) at day 1 for 1-h infusion in 5% Dextrose solution. Young patients aged less than 45 years without any co-morbidity were given triplet chemotherapy with TPF and doublet chemotherapy with PC. Though there are no documented benefits to induction chemotherapy, it is frequently used in our institute. The clinician uses inoperable oral cancer to downstage it for ease of surgery. This resulted in less mutilating surgery and less physical and psychological trauma for patients. The number of cycles is normally 2 to 3, and patients who got clinical benefit (clinical or radiological) but were still considered inoperable continue to get more cycles as a palliative approach. All Patients Received Pegfilgrastim 6 mg Subcutaneous Injection on the Day After the Completion of Chemotherapy as per Standard Care of the Institute Protocol as a Prophylaxis.
Data Collection
Patients’ identifying information, such as height, weight, sex, performance status, illness state (according to TNM classification), progression-free survival (PFS), and adverse events (AEs), was meticulously evaluated in the presence of a clinician and recorded in their medical records. Prior to each treatment therapy, a computed tomography (CT) scan of the head and neck was carried out individually. The therapeutic response was assessed using Criteria in Solid Tumours version 1.1,Citation14 and it was determined that when the patient had a complete response (CR) the target lesion disappears in each and every one of them. Any lymph nodes that are abnormal must have their short axis cut down to 10 mm. A partial response (PR) is defined as the following: a reduction of at least 30% in the sum of the diameters of target lesions as compared to the sum of the diameters at baseline; Stable Disease (SD): a condition in which there is neither expansion nor contraction, as determined by the least sum diameters while the examination is ongoing; Under TPF/PC, the length of therapy was understood to include the follow-up period. The term “progression-free survival” (PFS) refers to the amount of time that has passed since the beginning of TPF or PC treatment until the first documented instance of disease progression or death. According to the Common Toxicity Criteria version 4.0 established by the National Cancer Institute, adverse events (AEs) were documented (http://ctep.cancer.gov/protocolDevelopment/) from patients’ medical records.
Selection of Genes and SNPs
PharmGKB is an NIH-funded resource. Using the research tool at https://www.pharmgkb.org/, it provides information about how human genetic variation affects response to medications. PharGKB collects, curates, and disseminates knowledge about clinically actionable gene-drug associations and genotype–phenotype relationships. All clinically relevant SNPs (flagged SNPs) such as rs4693924 in ABCG2, rs2804398 in ABCC2, rs943288 in ABCC4, and rs9332430 in ABCC1 were Selected. SNPs database—dbSNP—from the National Center for Biotechnology Information (NCBI).
Genetic Polymorphism Analysis
The phenol-chloroform procedureCitation15 was used to extract DNA from blood. Using the polymerase chain reaction-restriction fragmentation length polymorphism (PCR-RFLP) approach, suitable enzymes, and band patterns were used to genotype ABCG2, ABCC2, ABCC4, and ABCC1 . The PCR (25 μl) was prepared using 50–100 ng of DNA. Overall, 2.5 μl of PCR reaction buffer (500 mM KCl, 100 mM Tris-HCl, pH 8.3, 10 Mm MgCl2; as supplied by the manufacturer), 1 μl of 2.5 mM dNTPs, each of the respective primers at 10 pmol, and 0.3 U/μl of Taq polymerase (APS Lab, Pvt. Limited) were used to make the PCR mixture (25 μl). All reactions were carried out in a thermal cycler (BIO-RAD Laboratories, USA, Bio-RAD C1000 Touch Thermal Cycler). Amplified PCR products were digested with different restriction enzymes and electrophoresed on a 12% native gel using the PCR-RFLP method visualized by Chemi DocTM, BIO-RAD, USA. The ABCB1, ABCC2, ABCG2, and ABCC4 alleles were identified based on their respective DNA fragments and classified accordingly.
Table 1. Primer sequences metabolizing gene and their respective restriction enzymes.
Statistical Analysis
Descriptive statistics examined patient characteristics (clinical and pathological, transporters, and adverse effects). A multinomial logistic regression model calculated the odds ratio and 95% confidence interval for genes versus toxicity. After 3 years of survival monitoring, the patient’s characteristic factors were investigated using either Kaplan–Meier analysis (univariate) or Cox regression (multivariate). The risk (hazard) was calculated using the ratio between genes and clinical characteristics. p values under 0.05 were considered statistically significant. SPSS 16.0 analyzed all data (SPSS Inc., Chicago, IL, USA).
Results
Patient Demographics
Our study included 120 patients with oral cancer, 109 of whom were men and 11 of whom were women. The median age were 47 years (18–80 years) and weight of the patients were 48kg (32–80.5 kg), respectively. Overall, 83% of the patients were diagnosed at stage IV. A total of 38 individuals received TPF, whereas 83 patients received PC. Only 12.4% of the patients responded completely to chemotherapy, 15.7% responded partially, and 71.9% showed disease stabilization (). The most frequently observed adverse events (AEs) that developed and were recorded in our study were anemia, diarrhea, fever, dyspepsia, nausea, vomiting, mucositis, neutropenia, febrile neutropenia etc.
Table 2. Clinical and pathological characteristics.
Distribution of Genotype Frequencies
Different transporter genes such as ABCG2 (rs4693924), ABCC2 (rs2804398), ABCC4 (rs943288), and ABCC1 (rs9332430) genotypes and allele frequencies in the study population are presented in . The results showed a frequency of mutated genotypes of 38% in the ABCG2 gene, with ABCC4 and ABCC1 heterozygous wt/mt genotypes present at 81.8% and 72.7%, respectively, for ABCC2 at 54.54%, and for ABCC2 homozygous wild-type genotypes at 37.2%, the highest number. The frequency of homozygous mutated genotypes was high in the ABCG2 gene compared to the ABCC4, ABCC2, and ABCC1 genes. The frequency of the dominant mutated genotypes in the population is 0.56 in ABCG2 and wild type 0.55 in ABCC4 ( (a) ABCG2, (b) ABCC2, (c) ABCC4, and (d) ABCC1).
Figure 1. (a) 12% Native gel RFLP of ABCG2(rs 4693924) M-50bp Marker, 1, 4,5-mt/mt genotypes type;2,3-wt/mt genotypes; (b) ABCC2(rs2804398) M-50bp Marker 2-wt/wt genotype, 1, 3-8-mt/mt genotypes; (c) ABCC4 (rs 943288) M-50bp Marker, 1,2,3,5,6-mt/mt genotypes; 4-wt/mt; genotype;7-wt/wt genotype (d) ABCC1 (rs 9332430) M-50bp Marker, 1,2,4,5,8-mt/mt genotypes; 6-wt/mt genotype, 7-wt/wt genotype
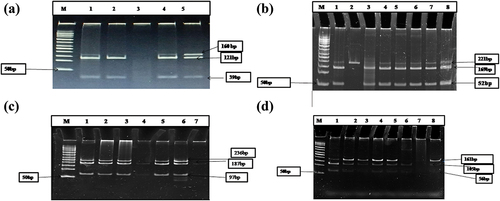
Table 3. Transporter genes and allele frequency of SNPs.
Effect of Genetic Polymorphisms on Adverse Reactions
The most frequent AEs were anemia (11.6%), diarrhea (39.16%), fever (31.6%), dyspepsia (30%) nausea (36.6%), vomiting (34.16%), mucositis (6.66%), neutropenia (4.16%), and febrile neutropenia (4.16%). Our findings show that ABCG2 and ABCC4 polymormphisms associated with increased increased the likelihood of developing anemia, but there was no significant association with ABCC2 or ABCC1. Nausea was linked to ABCC2 and ABCC1 polymorphisms, and dysphasia was associated with ABCC4. The severity of adverse events was only associated with polymorphisms in the ABCG2 gene with anemia (p = .001) in both heterozygous mutant allele (wt/mt) and homozygous mutant allele (mt/mt) genotypes and dysphasia in ABCC4 (p = .004, p = .011) in wt/mt and mt/mt genotypes, while rs2804398 in ABCC2 was associated with nausea (p = .008) and nausea (p = .01) with rs 9,332,430 in the ABCC1 gene with mt/mt genotypes, but no other significant was found in different adverse events. For wt/mt genotypes, the odds ratio (OR) of ABCG2 for anemia was 1.237 (95% CI: 1.134–1.349), and for mt/mt genotypes, it was 1.270 (95% CI: 1.164–1.385). However, with SNP rs943288 in ABCC4 in both wt/mt and mt/mt genotypes, the odds ratios were as follows: OR: 2.72 (95% CI: 1.306–3.954) and OR: 9.11 (95% CI: 1.675–49.57). But there was minimal risk of nausea in the ABCC2 and ABCC1 gene polymorphisms. No significant correlation was observed between other adverse events, such as mucositis, neutropenia, and febrile neutropenia. The heterozygous mutant genotypes (wt/mt) of SNPs (rs943288) in ABCC4 had a greater risk of developing mucositis (OR: 5.00; 95%CI 0.584–42.797; p = .142) and SNPs (rs4693924) in ABCG2 for homozygous mutant (mt/mt) genotypes increased the probability of neutropenia development (OR: 3.00; 95%CI 0.312–28.841; p = .341) ().
Table 4. Association between genetic polymorphisms and toxicity.
Effect of Genetic Polymorphisms on Progression-Free Survival (PFS)
In our study, the relationship between characteristics and PFS was analyzed. In the Kaplan-Meier Survival (univariate) analysis, there was no significant association between the SNPs in ABCG2, ABCC2, ABCC4, or ABCC1 and progression-free survival. But a strong association was seen between patients with an ECOG performance status (p = .009) and clinical outcome (p = .000) with PFS . (). In the multivariate analysis, patient age and ECOG performance status were associated with higher risk (HRs of 1.104 and 1.246, respectively) and shorter survival, whereas SNPs of ABCG2, ABCC2, ABCC4, and ABCC1 had no significant correlation (p > .05).
Figure 2. (a) The Kaplan—Meier curve showing the association between the ABCG2 rs4693924genotypes and progression -free survival of patients treated with induction chemotherapy. (b) The Kaplan—Meier curve showing the association between the ABCC2 rs2804398 genotypes and progression -free survival of patients treated with induction chemotherapy. (c) The Kaplan—Meier curve showing the association between the ABCC4 rs943288 genotypes and progression -free survival of patients treated with induction chemotherapy. (d) The Kaplan—Meier curve showing the association between the ABCC1 rs9332430 genotypes and progression -free survival of patients treated with induction chemotherapy
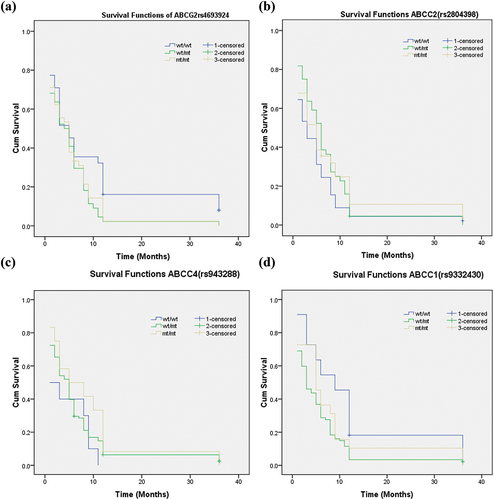
Table 5. Univariate and multivariate analysis of progression-free survival of chemotherapy treated oral cancer patients.
Discussion
In this study, the genetic and clinical characteristics of toxicities caused by TPF/PC chemotherapy in oral cancer patients were studied. Furthermore, the relationship between ABC polymorphisms and toxicity in patients with oral cancer receiving platinum based chemotherapy were also been studied. Variations in ABC transporter genes have an impact on chemotherapy treatment outcomes as well.Citation16–18 Individual variations in the pharmacotherapy and polymorphism of efflux of efflux transporters could be caused by genetic mutations, resulting in population-specific drug transport disparities. Due to toxicity, few studies have examined how mutations in the ABC gene influence cancer patients. Therefore, it is crucial and clinically useful to understand and reduce interindividual drug receptivity, sensitivity, and toxicity.
According to our findings, the homozygous mutated allele of rs4693924 in ABCG2 had an increased risk of developing nausea. Sharma et al.Citation19 showed polymorphism of ABCG2 C421A had the protective effect of GI toxicity in people harboring heterozygous genotypes in lung cancer patients undergoing platinum-based chemotherapy. This is contradictory to our findings. Cisplatin, as an emetic agent, produces nausea and vomiting in approximately 90% of the patients who are not given antiemetics (50 mg/m2 or above). According to our observations, there was a significant correlation with anemia in mt/mt genotypes. A study by Wang et al.Citation20 reported that SNPs in the ABCG2 gene had significant relations with mild or severe neutropenia or anemia, which was found to be similar to our results. Both heterozygous and homozygous mutant alleles had significant correlations with hematological toxicities such as anemia in a recessive model with higher risk. This finding is similar to our results. Several studies showed that polymorphism of ABCC2 variation has a significant association with diarrhea in different drugs and types of cancer, which was similar to our findings.Citation21–23 Our observations showed that rs2804398 SNPs in ABCC2 had a significant correlation with diarrhea. It is interesting to note that individuals who are predicted to have a high capacity to glucuronidate and a low capacity to transport via ABCC2 are relatively protected from severe diarrhea. On the other hand, individuals who have a low capacity to glucuronidate and a low capacity to transport are relatively more likely to experience severe diarrhea. It has been assumed that the SNPs in rs2804398 reduced the function of ABCC2, but our findings suggest that the presence of variation in this gene results in significantly increased systemic exposure to docetaxel. This finding suggests that other transporters did not completely compensate for the loss of apical transport capacity and that overall hepatobiliary secretion is impaired.
ABCC4 has a significant effect on the urine excretion and release of 5-fluorouracil and its metabolites into the bloodstream from the liver.Citation24 Any genetic mutation in ABCC4 disrupts drug elimination through urine and hepatic efflux, raising blood levels of its metabolites, which ultimately causes TPF-induced toxicity in individuals.Citation25,Citation26 In our study, we found that heterozygous mutant alleles rs943288 in ABCC4 were strongly associated with TPF-induced hematological toxicity like anemia and gastrointestinal toxicity like dysphagia which is similar to the study by Islam et al.Citation10 that showed that GT and TT genotypes increased the risk of EFC (epirubicin, fluorouracil, and cyclophosphamide)-induced anemia, neutropenia, leukopenia, and gastrointestinal toxicity in Bangladeshi breast cancer patients. Another study conducted by Low et al.Citation27 in the Japanese population showed a statistically significant result, ABCC4 in rs9561778 with hematological toxicities. The impairment of ABCC4 may be the reason for an insufficient clearance of fluorouracil, which may then lead to an increase in the concentration of 5-FU in the body; however, this hypothesis has to be confirmed by additional research. It is possible that the influence of SNPs on tissue-specific expression of ABCC4 and the tissue-specific clearance of the medication are responsible for the stronger correlation with one phenotype. However, this hypothesis should be validated by association analysis using larger samples as well as by a functional analysis of these SNPs.
ABCC1 is responsible for modifying the action of 5-fluorouracil.Citation28,Citation29 Cechin et al.Citation30 showed in their study that there was a significant association between rs35587 in ABCC1 and neurological toxicity and neutropenia. Another study by Vulsteke et al.Citation31 reported that the association of SNPs rs246221, rs4148350, and rs45511201 of ABCC1 were strongly associated with hematological toxicity during FEC treatment, which was contradictory to our results? Our study results showed that there was a significant association of homozygous mutant alleles of rs9332430 with nausea. This may be due to the fact that the overexpression of the ABCC1 protein was associated with in vitro resistance to 5-fluorouracil. This may be due to the ability of ABCC1 to expel folates, depleting their intracellular availability for 5-fluorouracil activity.Citation32 This may help explain the effect of ABCC1–rs9332430 in part. Additional studies at the clinical and molecular levels should be carried out to confirm the clinical associations and the mechanisms underlying them.
Apart from polymorphism with toxicity, we assessed the SNPs of transporter gene’s relationship to survival, however we found no evidence of a significant link with PFS. Kaplan-Meier analysis and log-rank test revealed that each gene in charge of drug transport had a p -value greater than 0.05. In research by Wang et al.,Citation20 lung cancer patients receiving platinum-based therapy exhibited similar results for the transporter gene, whereas Sharma et al.Citation19 showed homozygous mutant genotypes showed higher survival when compared with wild-type genotypes. That was contradictory to our result. Thus, larger cohorts are needed to validate these results.
Conclusion
According to the findings of our research, a single SNP does not have any predictive influence on efficacy or survival for patients who are treated with TPF or PC. However, the variants rs4693924 in the ABCG2 gene, rs2804398 in ABCC2, rs943288 in ABCC4, and rs9332430 in ABCC1 were linked with individual toxicity to TPF/PC-based treatment. Thus, genotype-based stratification of OCC patients may reduce side effects and improve tumor responses. There has to be a large-scale investigation with more patients and more genes to ensure optimal treatment and prevent drug-induced adverse reactions in oral cancer patients in this country.
Author’s Contributions
Study concepts by VDN, PKS; Study Design by VDN, PKS, SM, SD; Data acquisition by PKS RP, TM, SG, TC, AKB, PN, KKM Quality control of data and algorithms by PKS, AKB, PN, KKM, VDN; Data analysis and interception by AKB, PKS, SS, SD Statistical analysis PKS, RSK, VDN, Manuscript preparation PKS, VDN; Manuscript editing VDN, RSK, TM, PN SD. Manuscript review PKS, VDN.
Ethics Approval Statement
The study was approved by the Institutional Ethics Committee of the Chittaranjan National Cancer Institute, ethics approval no. (A-4.311/1/2018–1).
Acknowledgments
We thank all patients and their families, who participated in this study, and all the physicians, nurses, pharmacists, and study coordinators who enabled the conduct of this research. The authors acknowledge the Indian Council of Medical Research for awarding the Senior Research Fellowship to the first author.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Additional information
Funding
References
- Wong TSC, Wiesenfeld D. Oral cancer. Aust Dent J. 2018;63(S1):S91–11. doi:10.1111/adj.12594.
- Gupta B, Bray F, Kumar N, Johnson NW. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: a case–control study from India. Cancer Epidemiol. 2017;51:7–14. doi:10.1016/J.CANEP.2017.09.003.
- Veluthattil A, Sudha S, Kandasamy S, Chakkalakkoombil S. Effect of hypofractionated, palliative radiotherapy on quality of life in late-stage oral cavity cancer: a prospective clinical trial. Indian J Palliat Care. 2019;25(3):383–90. doi:10.4103/IJPC.IJPC_115_18.
- Li CC, Shen Z, Bavarian R, Yang F, Bhattacharya A. Oral cancer: genetics and the role of precision medicine. Dent Clin North Am. 2018;62(1):29–46. doi:10.1016/J.CDEN.2017.08.002.
- Mustian KM, Devine K, Ryan JLJanelsins M.C, Sprod L.K, Peppone L.J, Candelario G.D, Mohile S.G, Morrow G.R. Treatment of nausea and vomiting during chemotherapy. US Oncol Hematol. 2011;7(2):91. doi:10.17925/OHR.2011.07.2.91.
- Zhong LP, Zhang CP, Ren GX, Guo W, William WN, Sun J, Zhu H-G, Tu W-Y, Li J, Cai Y-L, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31(6):744–51. doi:10.1200/JCO.2012.43.8820.
- Pêtre A, Dalban C, Karabajakian A, Neidhardt EM, Roux PE, Poupart M, Deneuve S, Zrounba P, Fayette J. Carboplatin in combination with weekly paclitaxel as first-line therapy in patients with recurrent/metastatic head and neck squamous cell carcinoma unfit to EXTREME schedule. Oncotarget. 2018;9(31):22038. doi:10.18632/ONCOTARGET.25157.
- Cao S, Wang C, Ma H, Yin R, Zhu M, Shen W, Dai J, Shu Y, Xu L, Hu Z, et al. Genome-wide association study on platinum-induced hepatotoxicity in non-small cell lung cancer patients. Sci Rep. 2015;5(1):5. doi:10.1038/SREP11556.
- Xiong Y, Huang BY, Yin JY. Pharmacogenomics of platinum-based chemotherapy in non-small cell lung cancer: focusing on DNA repair systems. Med Oncol. 2017;34(4). doi:10.1007/S12032-017-0905-6.
- Islam MS, Islam MS, Parvin S, Ahmed MU, Sayeed MSB, Uddin MMN, Hussain SMA, Hasnat A. Effect of GSTP1 and ABCC4 gene polymorphisms on response and toxicity of cyclophosphamide-epirubicin-5-fluorouracil-based chemotherapy in Bangladeshi breast cancer patients. Tumor Biol. 2015;36(7):5451–57. doi:10.1007/S13277-015-3211-Y.
- Hampras SS, Sucheston L, Weiss J, Baer MR, Zirpoli G, Singh PK, Wetzler M, Chennamaneni R, Blanco JG, Ford L, et al. Genetic polymorphisms of ATP-binding cassette (ABC) proteins, overall survival and drug toxicity in patients with acute myeloid leukemi. Int J Mol Epidemiol Genet. 2010;1(3):201–07. [accessed 2023 May 28]. https://pubmed.ncbi.nlm.nih.gov/21311724/.
- Kato H, Sassa N, Miyazaki M, Takeuchi M, Asai M, Iwai A, Noda Y, Gotoh M, Yamada K. Association of axitinib plasma exposure and genetic polymorphisms of ABC transporters with axitinib-induced toxicities in patients with renal cell carcinoma. Cancer Chemother Pharmacol. 2016;78(4):855–62. doi:10.1007/S00280-016-3145-0.
- De Mattia E, Toffoli G, Polesel J, D’Andrea M, Corona G, Zagonel V, Buonadonna A, Dreussi E, Cecchin E. Pharmacogenetics of ABC and SLC transporters in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment. Pharmacogenet Genomics. 2013;23(10):549–57. doi:10.1097/FPC.0B013E328364B6CF.
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. doi:10.1016/J.EJCA.2008.10.026.
- Molecular cloning: a laboratory manual. In: Sambrook JF, and Russell DW, editors. Includes bibliographical references and index. 3rd ed., Vol. 1–3. Cold Spring Harbor Laboratory Press; 2001. p. 2100. Soft cover | sigma-aldrich. [accessed 2023 Feb 7].https://www.sigmaaldrich.com/IN/en/product/sigma/m8265?gclid=Cj0KCQiA54KfBhCKARIsAJzSrdp8ZOYtqvt85MyqjIXVvHzdhprJfxsG_fYaly5DRls4vsEyxMOZi9AaAqjXEALw_wcB&gclsrc=aw.ds.
- Kiyotani K, Mushiroda T, Kubo M, Zembutsu H, Sugiyama Y, Nakamura Y. Association of genetic polymorphisms in SLCO1B3 and ABCC2 with docetaxel-induced leukopenia. Cancer Sci. 2008;99(5):967–72. doi:10.1111/J.1349-7006.2008.00765.X.
- Choi JR, Kim JO, Kang DR, Shin J-Y, Zhang XH, Oh JE, Park J-Y, Kim K-A, Kang J-H. Genetic variations of drug transporters can influence on drug response in patients treated with docetaxel chemotherapy. Cancer Res Treat. 2015;47(3):509–17. doi:10.4143/CRT.2014.012.
- Chew SC, Singh O, Chen X, Ramasamy RD, Kulkarni T, Lee EJD, Tan E-H, Lim W-T, Chowbay B. The effects of CYP3A4, CYP3A5, ABCB1, ABCC2, ABCG2 and SLCO1B3 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of docetaxel in nasopharyngeal carcinoma patients. Cancer Chemother Pharmacol. 2011;67(6):1471–78. doi:10.1007/S00280-011-1625-9.
- Sharma P, Singh N, Sharma S. Genetic variations in ABC transporter genes as a predictive biomarker for toxicity in North Indian lung cancer patients undergoing platinum-based doublet chemotherapy. J Biochem Mol Toxicol. 2023;37(3). doi:10.1002/JBT.23269.
- Wang L, Sun C, Li X, Mao C, Qian J, Wang J, Wu J, Li Q, Bai C, Han B, et al. A pharmacogenetics study of platinum-based chemotherapy in lung cancer: ABCG2 polymorphism and its genetic interaction with SLC31A1 are associated with response and survival. J Cancer. 2021;12(5):1270–83. doi:10.7150/JCA.51621.
- Han JY, Lim HS, Yoo YK, Shin ES, Park YH, Lee SY, Lee J-E, Lee DH, Kim HT, Lee JS, et al. Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer. 2007;110(1):138–47. doi:10.1002/CNCR.22760.
- De Jong FA, Scott-Horton TJ, Kroetz DL, McLeod HL, Friberg LE, Mathijssen RH, Verweij J, Marsh S, Sparreboom A. Irinotecan-induced diarrhea: functional significance of the polymorphic ABCC2 transporter protein. Clin Pharmacol Ther. 2007;81(1):42–49. doi:10.1038/SJ.CLPT.6100019.
- Han JY, Lim HS, Park YH, Lee SY, Lee JS. Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung cancer. Lung Cancer. 2009;63(1):115–20. doi:10.1016/J.LUNGCAN.2007.12.003.
- Borst P, De Wolf C, Van De Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2007;453(5):661–73. doi:10.1007/S00424-006-0054-9.
- Zhao X, Guo Y, Yue W, Zhang L, Gu M, Wang Y. ABCC4 is required for cell proliferation and tumorigenesis in non-small cell lung cancer. Oncotargets Ther. 2014;7:343–51. doi:10.2147/OTT.S56029.
- Van Aubel RAMH, Smeets PHE, Van Den Heuvel JJMW, Russel FGM. Human organic anion transporter MRP4 (ABCC4) is an efflux pump for the purine end metabolite urate with multiple allosteric substrate binding sites. Am J Physiol Renal. 2005;288(2):F327–33. doi:10.1152/AJPRENAL.00133.2004.
- Low SK, Kiyotani K, Mushiroda T, Daigo Y, Nakamura Y, Zembutsu H. Association study of genetic polymorphism in ABCC4 with cyclophosphamide-induced adverse drug reactions in breast cancer patients. J Hum Genet. 2009;54(10):564–71. doi:10.1038/JHG.2009.79.
- Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP–dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi:10.1038/NRC706.
- Theile D, Grebhardt S, Haefeli WE, Weiss J. Involvement of drug transporters in the synergistic action of FOLFOX combination chemotherapy. Biochem Pharmacol. 2009;78(11):1366–73. doi:10.1016/J.BCP.2009.07.006.
- Cecchin E, D’Andrea M, Lonardi S, Zanusso C, Pella N, Errante D, De Mattia E, Polesel J, Innocenti F, Toffoli G, et al. A prospective validation pharmacogenomic study in the adjuvant setting of colorectal cancer patients treated with the 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) regimen. Pharmacogenomics J. 2013;13(5):403–09. doi:10.1038/TPJ.2012.31.
- Vulsteke C, Lambrechts D, Dieudonné A, Hatse S, Brouwers B, van Brussel T, Neven P, Belmans A, Schöffski P, Paridaens R, et al. Genetic variability in the multidrug resistance associated protein-1 (ABCC1/MRP1) predicts hematological toxicity in breast cancer patients receiving (neo-)adjuvant chemotherapy with 5-fluorouracil, epirubicin and cyclophosphamide (FEC). Ann Oncol. 2013;24(6):1513–25. doi:10.1093/ANNONC/MDT008.
- Schreurs WHP, Odink J, Egger RJ, Wedel M, Bruning PF. The influence of radiotherapy and chemotherapy on the vitamin status of cancer patients. Int J Vitamin Nutr Res. 1985; 55(4):425–32. [accessed 2023 May 28]. https://pubmed.ncbi.nlm.nih.gov/4086213/.

