Abstract
Purpose
In mixed aortic valve disease (MAVD), the results of transcatheter aortic valve replacement (TAVR) are conflicting. There is limited data on the outcomes of TAVR in patients with bicuspid aortic valve (BAV) and MAVD. The objective of this study is to compare outcomes after TAVR in BAV patients with MAVD and predominant aortic stenosis (PAS).
Patients and Methods
Patients with BAV who underwent TAVR between January 2016 and April 2023 were included. The primary outcome was device success. The secondary endpoints were periprocedural mortality and other complications as defined by the Valve Academic Research Consortium-3 (VARC-3). Propensity score matching was used to minimize potential confounding.
Results
A total of 262 patients were included in this study, 83 of whom had MAVD. The median age was 72 years, and 55.7% were male. The baseline comorbidity risk files were comparable between the two groups. Patients with MAVD had more mitral regurgitation, tricuspid regurgitation and pulmonary hypertension, larger annular and left ventricular outflow tract dimensions, and more severe calcification than PAS. In the unmatched population, MAVD patients had similar device success rate (69.9% vs 79.9%, P=0.075) and 30-day mortality (3.6% vs 3.4%, P=1) compared to PAS. Propensity score matching resulted in 66 patient pairs. Device success rate were still comparable in the matched population. Other clinical outcomes, including stroke, bleeding (type 2–4), major vascular complications, acute kidney injury (stage 2–4) and permanent pacemaker implantation, were comparable between the two groups. Multivariable logistic regression analysis did not show MAVD to be an independent negative predictor of device success. At one year, survival was similar between patients with MAVD and those with PAS.
Conclusion
For the bicuspid valve, patients with MAVD had a more challenging anatomy. MAVD patients associated with comparable 30-day clinical outcomes after TAVR compared to PAS patients in patients with BAV.
Introduction
The indications for transcatheter aortic valve replacement (TAVR) have been expanded from a high surgical risk to a low surgical risk.Citation1–3 The trend towards TAVR in younger and lower-risk patients has led to an increase in the number of BAV patients encountered by cardiac teams.Citation4 In previous studies, the outcome of TAVR in patients with BAV was comparable to that in patients with tricuspid valve.Citation5,Citation6
Mixed aortic valve disease (MAVD), a combination of AS and moderate or severe aortic regurgitation (AR), was not uncommon in practice.Citation7 Moderate to severe AR was observed in 3% to 15% of patients with BAV treated with TAVR.Citation5,Citation8 Due to pressure and volume overload, MAVD is more aggressive than isolated aortic valve lesions and is associated with worse clinical outcomes.Citation9,Citation10 MAVD is currently managed according to the predominant lesion and the underlying surgical risk.Citation11,Citation12 However, in patients with MAVD, the results of TAVR were mixed.Citation13–16 In a retrospective study analysing 1133 patients (MAVD, n=688), patients with MAVD who underwent TAVR had a lower 3-year mortality rate compared with patients with AS alone (15.3% vs 20.4%), but similar early safety.Citation15 A meta-analysis of six studies involving 58,879 patients showed that MAVD patients had lower odds of device success, while 30-day and 1-year mortality and other complications were similar between MAVD and severe AS.Citation17 Demirel et al showed in their study that MAVD patients had worse long-term survival compared to isolated AS, with a HR of 1.412.Citation18
There is a lack of data on the outcome of TAVR in patients with severe AS accompanied by moderate or severe AR in the BAV. The aim of this study was to evaluate the outcomes of TAVR for BAV with MAVD compared to PAS.
Materials and Methods
This is a retrospective study that was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2019384H). Consecutive patients undergoing TAVR at Guangdong provincial people’s hospital were enrolled into a prospective institutional registry database. Written informed consent was obtained from all participants for this registry. Our study complied the Declaration of Helsinki. All patients with BAV underwent TAVR between January 2016 and April 2023 were included. Patients with a history of aortic valve surgery, pure aortic regurgitation and missing baseline data were excluded. The database was retrospectively reviewed to obtain patient’s age, sex, New York Heart Association (NYHA) heart failure, hypertension, diabetes mellitus, prior myocardial infarction, prior percutaneous coronary intervention, prior coronary artery bypass grafting, prior stroke, and peripheral arterial disease, echocardiographic variables. MAVD was defined as severe AS with associated moderate or severe AR. Patients were divided into two groups according to the presence of pre-operative AR: Predominant AS (PAS) and MAVD.
All patients underwent transthoracic echocardiography (TTE) prior to TAVR and repeat echocardiography at discharge. Mean aortic pressure gradients were calculated using the simplified Bernoulli equation. Left ventricular ejection fraction (LVEF) was calculated using Simpson’s biplane method. According to guidelines,Citation19,Citation20 we defined moderate AS (pVel: 3.0–3.9 m/s and AVA 1.0–1.5 cm2); severe AS (pVel: ≥4.0 m/s and AVA ≤1.0 cm2), AR was graded as mild, moderate or severe. To avoid bias, two cardiologists assessed the valve disease together. Multidetector computed tomography (MDCT) was performed on all study patients prior to the procedure to determine the type of native aortic valve, annulus, left ventricular outflow tract (LVOT) variables, and aortic root angles. MDCT was also used to determine the size of the prosthetic valve and the vascular access. Severe calcification was defined as the sum of calcification volumes of each native aortic valve greater than 500 mm3. The details of multidetector computed tomography (MDCT) analysis were described in our previous study.Citation21
According to the center expertise and device availability, self-expanding valve such as VenusA, VenusA-Pro, VenusA-Plus valve (Venus Medtech), Taurusone valve (Peijia Medical), ScienCrown valve (Lepu medical), Vitaflow(Microport), ballon-expanding valve such as Edwards Sapien (Edwards Lifesciences) and MuguetA™ (Xinchang medical) were implanted. Decisions about TAVI, device type and size were made by consensus by a dedicated heart team consisting of cardiac surgeons, interventional cardiologists and cardiac imaging specialists.
All outcomes were defined according to VARC-3 definitions. Technical success was defined as 1) freedom from mortality; 2) successful access, delivery of the device, and retrieval of the delivery system; 3) correct positioning of a single prosthetic heart valve into the proper anatomical location; and 4) freedom from surgery or intervention related to the device or to a major vascular or access-related, or cardiac structural complication at exit from the procedure room. The primary endpoint was device success, defined as 1) technical success; 2) 30-day freedom from mortality; 3) 30-day freedom from surgery or intervention related to the device or to a major vascular, or access related, or cardiac structural complication; and 4) less than moderate aortic regurgitation. Secondary endpoints included periprocedural mortality, stroke, major vascular complications, type 2–4 bleeding, permanent pacemaker implantation and acute kidney injury (AKI, stage 2-4) (within 30 days of TAVR).
Continuous variables were expressed as mean ± standard deviation for normal distribution and median and interquartile range for skewed variables. Categorical variables were presented as number and percent. To compare baseline variables and outcomes between PAS and MAVD groups, we used Student’s t-test for normally distributed continuous variables and Mann–Whitney U-test for non-normally distributed continuous variables. Categorical variables were compared using χ2 or Fisher’s exact test as appropriate. Logistic regression analysis was performed to determine the association between MAVD and rate of device success. Unadjusted Kaplan-Meier analysis was used to evaluate the incidence of clinical outcomes at maximal follow-up and the log rank test was used for group comparisons.
A propensity score was estimated using a logistic regression model with MAVD as the dependent variable and baseline characteristics with statistically significant difference or clinically relevant as independent variables. The variables included in the propensity analysis were age, sex, coronary artery disease, diabetes mellitus, peripheral artery disease, mean gradient, LVEF, mitral regurgitation, tricuspid regurgitation, pulmonary hypertension, severe calcification, annulus areas, LVOT areas. Patients with MAVD were matched to a PAS patient based on the nearest propensity score using the one-to-one nearest neighbor method. Calliper was 0.2 of the logit of the propensity score and there was no replacement.
We evaluated association between patient’s characteristic and device success and mortality in our study population using multivariable logistic analysis. Univariable logistic regression was performed to identify predictors for device success. Variables with P-values<0.05 in the univariable analysis were included in the multivariable analysis.
Result
A total of 274 patients with BAV underwent TAVR. 4 pure AR, 3 missing baseline data and 5 missing post-TAVR echocardiographic variables were excluded. Finally, 262 patients were included in the analysis. Of these, 83 (31.6%) were in the MAVD group and 179 (68.4%) in the PAS group. Baseline characteristics of the study population were presented in the . The median age was 72 years, and 44.3% of the total study population was female. Hypertension was the most common comorbidity in the cohort and did not differ between the study groups. Other comorbidities were comparable between MAVD and PAS.
Table 1 Demographic and Computed Tomography Characteristics of the Study Populations
The MDCT characteristics were shown in . Patients with MAVD generally had larger annulus and LVOT dimensions and more severe calcification. The echocardiographic characteristics were shown in . In the study, pre-procedure and discharge echocardiography was performed by the same cardiac ultrasound team, but not by the same doctor. Prior to TAVR, concomitant mitral regurgitation, tricuspid regurgitation and pulmonary hypertension greater than mild were more common in patients with MVAD but lower LVEF (P<0.001). After the procedure, the rate of mitral regurgitation, tricuspid regurgitation and pulmonary hypertension greater than mild was not significantly different between the two groups. However, patients with MAVD still had a lower LVEF (P<0.001). The incidence of paravalvular leak (PVL) was similar between the two groups (P=0.286).
Table 2 Echocardiographic Characteristics of the Populations
The details of procedure were summarized in . Nearly all patients (95%) in the study underwent TAVR via a transfemoral approach. Patents with MAVD were more likely to implant a larger size prosthesis (P<0.001) and a second prostheses, although there was no statistical difference (P=0.054). Clinical outcomes compared between the two groups are shown in . Technical success rates were similar between MAVD and PAS (83.1% vs 86.0%, P=0.539). Although patients with MAVD had a slightly lower device success rate than those with PAS, there was no significant statistical difference (69.9% vs 79.9%, P=0.075). There was no statistically significant difference in secondary outcomes between the two groups. 9 patients died within 30 days of TAVR, but there was no significant difference between the groups (3.6% in the MAVD group vs 3.4% in the PAS group, P=1.00). AKI was slightly higher in the MAVD group but did not reach significance (7.2% vs 2.2%, P=0.078). On multivariable analysis, only annular area, but not MAVD, was associated with lower device success, as shown in Supplementary Table 1.
Table 3 Procedure Details of Transcatheter Aortic Valve Replacement
Table 4 Clinical Outcomes According to MAVD and PAS in Unmatched Population
A total of 66 patient pairs were obtained using propensity score matching. Clinical outcomes after matching are shown in . Clinical outcomes did not differ between MAVD and PAS patients: mortality (0% vs 4.5%, P=0.244), stroke (4.5% vs 1.5%, P=0.619), major vascular complication (1.5% vs 7.6%, P=0.208), acute kidney injury (9.1% vs 3.0%, P=0.274), pacemaker implantation (7.6% vs 3.0%, P=0.44), device success (78.8% vs 72.7%, P=0.417). MAVD patients had a slightly lower rate of bleeding (3.0% vs 12.1%, P=0.048).
Table 5 Clinical Outcomes According to MAVD and PAS in Matched Population
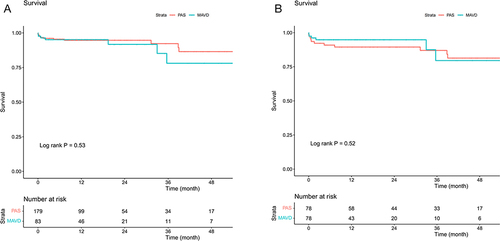
During a median follow-up of 14 months, 19 patients (7.2%) died in the entire cohort. Kaplan-Meier analysis showed that there was no significant difference in survival between MAVD and PAS in unmatched patients (Log rank P= 0.53). In matched patients, the cumulative survival rate was comparable between the two groups (Log rank P=0.52) ().
Discussion
This is the first study to compare anatomical characteristics and clinical outcomes after TAVR for mixed aortic valve disease with predominant AS in patients with BAV. Our main findings include the following: 1) Patients in the MAVD group had more calcification, larger dimensions of the ascending aorta, annulus and LVOT compared with those in the PAS group. 2) Annular area but not MAVD was associated with lower device success rate. 3) MAVD did not affect 30-day clinical outcomes or 1-year survival after TAVR.
Patients with MAVD are thought to have a worse natural history and prognosis than those with PAS.Citation7,Citation10 Clinical outcomes after TAVR in patients with MAVD are inconsistent in previous studies.Citation17,Citation22 Current guidelines recommend treatment for MAVD according to the predominant lesion. When the severity of both lesions is balanced, the indication for intervention should be based on symptoms and objective consequences. However, the timing and modalities of treatment are unclear.Citation11,Citation12 Patients with mixed aortic valve disease have been excluded from large randomized trials focusing on transcatheter aortic valve replacement.Citation23,Citation24 In a meta-analysis of six studies involving 58,879 patients, Guddeti et al found that the 30-day mortality rate after TAVR in patients with MAVD was 5.1%, with no significant difference between the MAVD and PAS groups.Citation17 A study of 1133 patients found that the incidence of 30-day mortality in patients with MAVD undergoing TAVR was 1.6% and was not significant compared with patients with PAS.Citation15 However, the above studies included both tricuspid and bicuspid aortic valves, and the majority of patients had a tricuspid valve. In the present study, which included 262 BAV (MAVD, n=83; PAS, n=179), 9(3.4%) patients died within 30 days after TAVR. 30-day mortality was similar between MAVD and PAS (3.6% vs 3.4%, P=1.00). Even in a propensity-matched analysis, the mortality rate in MAVD and PAS patients remains similar. The 30-day mortality rate in our study was consistent with previous studies.Citation17,Citation25 The present study was the first study to compare clinical outcomes after TAVR between MAVD and PAS in patients with BAV. The present results may provide clinical data to guide intervention for patients with BAV and MAVD.
Chieffo et al showed in their retrospective study that patients with MAVD had a lower device success rate compared to PAS, but this did not affect prognosis.Citation13 However, the definition of MAVD in their study is severe aortic stenosis with ≥ mild aortic regurgitation, which differs from the definition in our study. It is therefore difficult to fully compare the results of our analysis with their study. In several other analyses, MAVD was defined as severe aortic stenosis plus ≥ moderate aortic regurgitation.Citation14,Citation16,Citation26 Contrary to our study, previous studies have found that patients with MAVD have a significantly lower device success rate than those with PAS. In addition, the device success rate in our study was lower than in previous studies. Although there is a slightly lower device success rate in patients with MAVD, there is no significant difference. There is also no significant difference in peri-operative mortality, need for a second valve, moderate or severe post-procedural aortic regurgitation or major vascular complications between MAVD and PAS. Several potential factors may contribute to this. In our study, device success was defined according to the VARC-3 definition, which is stricter than the VARC-2 definition used in previous studies. BAV is a significant challenge for TAVR, with previous studies showing low rates of BAV. Therefore, the device success rate was lower than in previous studies. Guddeti et al pointed out that annulus dilation and increased valve stress caused by aortic regurgitation may affect the success of the procedure.Citation17 Vianello et al demonstrated that patients with MAVD had a predominant fibrotic pattern in the valve leaflets which may play a role in implant success.Citation27 In our study, patients with MAVD had a larger dimension of LOVT and annular and more severe calcification, which may have an impact on device success. Multivariable regression analysis indicated that annulus size was associated with lower rate of device success. After propensity score matching, there was no difference in device success between the two groups. Larroche et al showed that aortic valve calcification was significantly associated with less device success, but not with major adverse cardiac events.Citation28
PVL is common after TAVR and has been reported to be associated with worse short- and long-term clinical outcomes.Citation29,Citation30 However, there are analyses suggesting that post-TAVR aortic regurgitation is more common in the MAVD group than in the PAS group and is positive for survival in patients with MAVD.Citation15,Citation17 Pre-existing AR causes the left ventricle to adapt to volume overload and myocardial remodeling. As a result, patients with PAS who experienced PVL had a worse prognosis due to inadaptation. In the present study, the prevalence of aortic regurgitation after TAVR was higher than that reported in previous studies.Citation13,Citation15 In the present study, no significant difference in the incidence of post-TAVR aortic regurgitation ≥ moderate between patients with MAVD and PAS. Data from several observational studies and registries have shown that patients with BAV are more likely to develop PVL after TAVR compared to TAV due to the more complicated anatomy of the aortic root: greater aortic root calcification, larger annulus and ascending aortic dimensions.Citation31 A higher prevalence of post-procedural aortic regurgitation is associated with inter-operator echocardiographic variability and patient selection. Extensive calcification played a role in the under-expansion of the transcatheter valve and consequently in PVL.
In the present study, the anatomy of patients with MAVD was more challenging than that of patients with PAS. The geometry of the annular, LVOT and ascending aorta was larger in MAVD compared to PAS. In Chahine’s study of 1133 patients (MAVD, n=668), the size of the LVOT was similar between MAVD and pure aortic stenosis. However, the tricuspid aortic valve was also included and the proportion of BAV in each group was unclear in their study.Citation15 There was a lack of data to compare the anatomy of the aortic valve complex. Further cohort studies are needed to confirm our findings.
Our study had several limitations. It was a single-centre, retrospective, observational study with several known limitations. The number of advertising events was relatively low. Large prospective multicenter trials are needed to validate our findings. Data on cardiac remodeling after TAVR were not available in our study. The majority of patients in our study underwent TAVR with older-generation devices, which may overestimate the device failure rate. In addition, there may be measurement errors for the same ultrasound parameter when different examiners are involved. Although echocardiography is not performed by a single doctor, and there can be measurement errors between different operators, it is performed by the same ultrasound team and all team members have extensive experience in echocardiography.
Conclusion
For the bicuspid valve, patients with MAVD had a more challenging anatomy. MAVD patients associated with comparable 30-day clinical outcomes after TAVR compared to PAS patients in patients with BAV.
Abbreviations
AKI, acute kidney injury; AR, aortic regurgitation; AS, aortic stenosis; BAV, bicuspid aortic valve; LVOT, left ventricular outflow tract; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; MAVD, mixed aortic valve disease; MDCT, Multidetector computed tomography; NYHA, New York Heart Association; PAS, predominant aortic stenosis; PVL, paravalvular leak; TAVR, transcatheter aortic valve replacement; TTE, transthoracic echocardiography; VARC3, Valve Academic Research Consortium-3.
Ethics Approval and Informed Consent
This is a retrospective study that was approved by the Research Ethics Committee of Guangdong Provincial People’s Hospital (No. GDREC2019384H). Written informed consent was obtained from all participants for the study.
Disclosure
The authors declare that there is no conflict of interest in this work.
Acknowledgments
This study was funded by 1. Clinical Major Technology Project of Guangzhou (No.2023FTJCZ0017); 2. National Nature Science Foundation of China (No.82070478); 3. Guangdong Provincial Clinical Research Center for Cardiovascular disease (No.2020B1111170011); 4. Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention (No.Y0120220151).
References
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med. 2016;374(17):1609–1620. doi:10.1056/NEJMoa1514616
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477–2484. doi:10.1016/S0140-6736(15)60308-7
- Leon MB, Mack MJ, Hahn RT, et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J Am Coll Cardiol. 2021;77(9):1149–1161. doi:10.1016/j.jacc.2020.12.052
- Vincent F, Ternacle J, Denimal T, et al. Transcatheter Aortic Valve Replacement in Bicuspid Aortic Valve Stenosis. Circulation. 2021;143(10):1043–1061. doi:10.1161/CIRCULATIONAHA.120.048048
- Forrest JK, Kaple RK, Ramlawi B, et al. Transcatheter Aortic Valve Replacement in Bicuspid Versus Tricuspid Aortic Valves From the STS/ACC TVT Registry. JACC: Cardiovasc Interv. 2020;13(15):1749–1759. doi:10.1016/j.jcin.2020.03.022
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in Transcatheter Aortic Valve Replacement for Bicuspid Versus Tricuspid Aortic Valve Stenosis. J Am Coll Cardiol. 2017;69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Egbe AC, Luis SA, Padang R, et al. Outcomes in Moderate Mixed Aortic Valve Disease: is it Time for a Paradigm Shift? J Am Coll Cardiol. 2016;67(20):2321–2329. doi:10.1016/j.jacc.2016.03.509
- Halim SA, Edwards FH, Dai D, et al. Outcomes of Transcatheter Aortic Valve Replacement in Patients With Bicuspid Aortic Valve Disease: a Report From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2020;141(13):1071–1079. doi:10.1161/CIRCULATIONAHA.119.040333
- Honda S, Kitai T, Okada Y, et al. Impact of aortic regurgitation on the prognosis of severe aortic stenosis. Heart. 2012;98(21):1591–1594. doi:10.1136/heartjnl-2012-302089
- Egbe AC, Poterucha JT, Warnes CA. Mixed aortic valve disease: midterm outcome and predictors of adverse events. Eur Heart J. 2016;37(34):2671–2678. doi:10.1093/eurheartj/ehw079
- Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2022;43(7):561–632. doi:10.1093/eurheartj/ehab395
- Writing Committee M, Otto CM, Nishimura RA, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: executive Summary: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77(4):450–500. doi:10.1016/j.jacc.2020.11.035
- Chieffo A, Van Mieghem NM, Tchetche D, et al. Impact of Mixed Aortic Valve Stenosis on VARC-2 Outcomes and Postprocedural Aortic Regurgitation in Patients Undergoing Transcatheter Aortic Valve Implantation: results From the International Multicentric Study PRAGMATIC (Pooled Rotterdam-Milan-Toulouse in Collaboration). Catheter Cardiovasc Interv. 2015;86(5):875–885. doi:10.1002/ccd.25975
- Abdelghani M, Cavalcante R, Miyazaki Y, et al. Transcatheter aortic valve implantation for mixed versus pure stenotic aortic valve disease. EuroIntervention. 2017;13(10):1157–1165. doi:10.4244/EIJ-D-17-00328
- Chahine J, Kadri AN, Gajulapalli RD, et al. Outcomes of Transcatheter Aortic Valve Replacement in Mixed Aortic Valve Disease. JACC: Cardiovasc Interv. 2019;12(22):2299–2306. doi:10.1016/j.jcin.2019.06.020
- Heidari B, Al-Hijji MA, Alkhouli MA, et al. Transcatheter aortic valve replacement outcomes in mixed aortic valve disease compared to predominant aortic stenosis. Int J Cardiol. 2020;299:209–214. doi:10.1016/j.ijcard.2019.07.099
- Guddeti RR, Gill GS, Garcia-Garcia HM, et al. Transcatheter aortic valve replacement in mixed aortic valve disease: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. 2022;8(2):169–176. doi:10.1093/ehjqcco/qcab080
- Demirel C, Winter MP, Nitsche C, et al. Mixed aortic valve disease: association with paravalvular leak and reduced survival after TAVR. Eur Heart J Cardiovasc Imaging. 2024. doi:10.1093/ehjci/jeae005
- Zoghbi WA, Adams D, Bonow RO, et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: a Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30(4):303–371. doi:10.1016/j.echo.2017.01.007
- Baumgartner HC, Hung J, Bermejo J, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2017;18(3):254–275. doi:10.1093/ehjci/jew335
- Li J, Sun Y, Zheng S, et al. Anatomical Predictors of Valve Malposition During Self-Expandable Transcatheter Aortic Valve Replacement. Front Cardiovasc Med. 2021;8:600356. doi:10.3389/fcvm.2021.600356
- Stathogiannis K, Toutouzas K, Drakopoulou M, et al. The Effect of Mixed Aortic Valve Disease in Clinical Outcomes after Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2017;69(11):1223. doi:10.1016/S0735-1097(17)34612-0
- Meredith IT, Walters DL, Dumonteil N, et al. 1-Year Outcomes With the Fully Repositionable and Retrievable Lotus Transcatheter Aortic Replacement Valve in 120 High-Risk Surgical Patients With Severe Aortic Stenosis: results of the REPRISE II Study. JACC: Cardiovasc Interv. 2016;9(4):376–384. doi:10.1016/j.jcin.2015.10.024
- Deeb GM, Reardon MJ, Chetcuti S, et al. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2016;67(22):2565–2574. doi:10.1016/j.jacc.2016.03.506
- Grant JK, Rubin P, Chambers S, et al. Comparison of In-Hospital Outcomes and Readmission Rates of Transcatheter Aortic Valve Implantation in Mixed Aortic Valve Disease Versus Pure Aortic Stenosis. Am J Cardiol. 2022;175:72–79. doi:10.1016/j.amjcard.2022.03.052
- Seeger J, Gonska B, Mörike J, et al. Outcome of Patients with Mixed Aortic Valve Disease Undergoing Transfemoral Aortic Valve Replacement. Struct Heart. 2017;1(3–4):162–167. doi:10.1080/24748706.2017.1348648
- Vianello A, Perlini S, Cappelli S, et al. A multimarker study of degenerative aortic valve disease: stenoinsufficiency shows more indices of bad prognosis. Cardiology. 2013;124(2):126–137.
- Larroche J, Panh L, Lhermusier T, et al. Impact of aortic valve calcification severity on device success after transcatheter aortic valve replacement. Int J Cardiovasc Imaging. 2020;36(4):731–740. doi:10.1007/s10554-019-01759-7
- Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol. 2013;61(15):1585–1595. doi:10.1016/j.jacc.2013.01.047
- Kodali S, Pibarot P, Douglas PS, et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J. 2015;36(7):449–456. doi:10.1093/eurheartj/ehu384
- Bhushan S, Huang X, Li Y, et al. Paravalvular Leak After Transcatheter Aortic Valve Implantation Its Incidence, Diagnosis, Clinical Implications, Prevention, Management, and Future Perspectives: a Review Article. Curr Probl Cardiol. 2022;47(10):100957. doi:10.1016/j.cpcardiol.2021.100957