Abstract
Objective
Many previously reported publications mentioned that oral lesion in COVID-19 patients was varied. The term oral manifestations refer to pathognomonic features that are found consistently with a specific cause and effect. In this context, the oral manifestation of COVID-19 was inconclusive. This systematic review aimed to analyse previously reported publications related to oral lesions in COVID-19 patients to define as oral manifestations or not. The PRISMA guidelines were implemented in this review.
Methods
All umbrella reviews, systematic reviews, systematic reviews and meta-analyses, comprehensive reviews, and original and non-original studies were included. Twenty-one of systematic review, 32 original studies and 68 non-original studies reported the oral lesion in COVID-19 patients.
Results
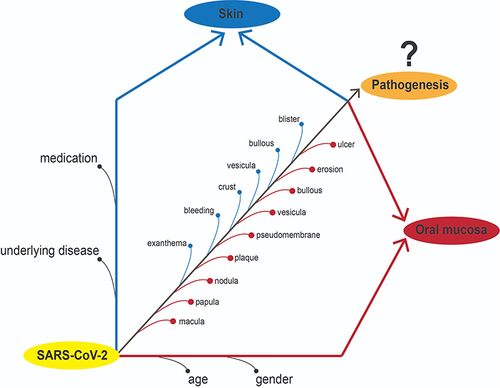
Most of the publications mentioned that ulcers, macular, pseudomembranes and crusts were frequent oral lesions. The reported oral lesions in COVID-19 patients did not show any pathognomonic features and might be unrelated directly to COVID-19 infections, however, more likely due to gender, age, underlying diseases, and medication.
Conclusion
The oral lesions found in previous studies do not have pathognomonic features and are inconsistent. Therefore, the reported oral lesion, in present time, cannot be defined as an oral manifestation.
Graphical Abstract
Introduction
Some viruses have a specific manifestation in the oral mucosa or pathognomonic features that can lead a dentist, oral pathologist, or oral medicine specialist to lead to clinical diagnosis. The herpes simplex virus is the cause of primary herpes infection in children. The pathognomonic feature is oral ulceration in the entire mucosa and gingiva,Citation1 and it is called primary gingiva stomatitis.Citation2 The secondary infection presents a specific ulceration in the vermilion of the lips called herpes labialis.Citation3 In other virus infections, like a varicella-zoster infection, the pathognomonic feature was segmental oral ulceration in oral mucosaCitation4 and facial area.Citation5 The measles infection also has pathognomonic features in the oral mucosa called Koplik’s spot and cannot be found in other virus infections.Citation6
Coronavirus infectious disease (COVID-19) is a disease that has been haunting the world for nearly three years. The disease is caused by a viral named SARS-CoV-2.Citation7 The main symptoms are fever, cough, dyspnea, malaise and fatigue, while more serious conditions like respiratory failure and pneumonia could lead to mortality.Citation8,Citation9 SARS-CoV-2 infection, like other viral infections described in a recent report, considered has pathognomonic features in the oral mucosa called COVID tongue.Citation10–13 Further, this condition is known as benign migratory glossitisCitation11–13 and is unable to be considered an oral manifestation. Many kinds of literature have described the oral manifestation or pathognomonic features of COVID-19. But until today, none have concluded the pathognomonic features of COVID-19 because various oral lesion was found in the patient, both hospitalizedCitation14 and non-hospitalized, like a casualty.Citation15 The most common oral symptom was dysgeusiaCitation16 and xerostomia,Citation17 while the oral lesion was an oral ulcer.Citation16 Further, other oral lesions, vesiculobullous, blisters, and pseudomembranes (Candida albicans infections)Citation14 were reported and more frequent in hospitalized patients.Citation15 The oral lesion looks not specific; in the pediatric patient, the maculopapular (erythematous lesions), ulcers, desquamations (dry and cracked lips), and depapilation lesion (strawberry tongue) were found.Citation18 The doubt of oral manifestation of pathognomonic features arises when accompanied by skin lesions. Most patients have skin lesions similar to herpes simplex virus infection or autoimmune diseases.Citation19 This finding also created doubtfully regarding oral lesions whether is a causality of SARS-CoV-2 (pathognomonic features) or just the casualty.
The oral ulcers, as the common oral lesion found in COVID-19 patients, are mentioned as causality (oral manifestations or pathognomonic features) because of the presence of angiotensin-converting enzyme 2 (ACE-2) in the oral epithelial tissue. It is suspected to be the first receptor for developing oral lesions in SARS-CoV-2-infected patients.Citation20 However, until today, the pathogenesis and interaction between the ACE-2 and SARS-CoV-2 in the oral mucosa has not been able to explain.Citation21,Citation22 The development of various oral lesions in COVID-19 patients looks like a casualty, because it is influenced by various factors such as underlying disease,Citation23,Citation24 immunological and psycho-social factors,Citation25 medication,Citation26–28 and age and gender.Citation29 Nevertheless, various literature has referred to the lesions found as causality, oral manifestation, or pathognomonic features of COVID-19.Citation15–17,Citation19,Citation22,Citation24,Citation26–28 For this reason, this systematic review was composed of various reports regarding oral lesions in COVID-19 and whether the reported lesions can be referred to as oral manifestations or pathognomonic features or not.
Materials and Methods
Search Strategy
In this report, PRISMA guidelines for systematic reviews were implemented. The PubMed (https://pubmed.ncbi.nlm.nih.gov), Science Direct (https://www.sciencedirect.com), and Scopus documents (https://www.scopus.com/search/form.uri?display=basic#basic) were searched up to December 22, 2022. All databases were searched using the following terms:(“COVID-19” [All Fields] OR “Sars-Cov-2” [All Fields]) AND “oral manifestation” [All Fields] OR “oral lesion” [All Fields]).
The researchers implemented language restrictions when assessing the records, and only the full-text articles in English were finally qualified for further evaluation. Additionally, a manual search of the bibliographies and the publications identified from a database search for potentially eligible references was performed. In order to identify missing information or data, we attempted to contact the authors of the relevant studies.
Study Assessment and Analysis
All types of articles, including umbrella reviews, systematic reviews, systematic review and meta-analysis and comprehensive review, were included to collect all the evidence. Initially, the records were assessed by two independent authors according to the relevance of the title and/or abstract (A.S and M.D.C.S). At this stage, the full reports were validated independently by another author (D.A), especially in doubtful cases. Studies considered potentially eligible by at least one of the authors in the initial search were then verified in their entirety by all authors.
The umbrella, systematic, systematic, meta-analysis, and comprehensive reviews have analyzed the description and collected the conclusion. The comprehensive review must follow the PRISMA guideline while collecting the data. While the original (pilot, cohort, observational, prospective, retrospective and cross-sectional) and non-original (case reports, case series, letters to the editor, correspondences and clinical images) studies analysed the patient demographic and related like gender, age, underlying disease, history of medication, oral lesion and skin lesion were listed as a primary outcome. Any disagreements between authors (A.S and M.D.C.S) were resolved after consultation with the third author (D.A).
Results
The 21 reviews (umbrella review, systematic review, meta-analysis and comprehensive review) discussed the oral lesion of COVID-19 patients. While the 32 original studies (pilot, cohort, observational, prospective, retrospective and cross-sectional) and 68 non-original studies (case reports, case series, letters to the editor, correspondences and clinical images) reported the oral lesion found in COVID-19 patients with the demographic data like ages, gender, underlying disease, medication and skin lesions ().
The Systematic Review and Systematic Review and Meta-Analysis Report on Oral Lesions in COVID-19 Patients
Systematic reviews and meta-analysis literature that analysed COVID-19 and its oral manifestation and their conclusions are summarized in . Among 21 studies published until 2022, three were systematic reviews and meta-analyses, 16 were systematic reviews, one was a systematic review of systematic reviews (umbrella review), and one was a form of a comprehensive review.
Table 1 A Systematic and Meta-Analysis Result of Correlation Between COVID-19 and Oral Manifestation
Around two reviews concluded on clinical findings in the oral cavity that the most prevalent symptom was dry mouth,Citation21 with oral lesions occurring in various sites of the oral mucosa.Citation26 Four studies concluded on data irregularityCitation33 and also unspecificCitation34 and unclearCitation24 lesions with no clear association with COVID-19.Citation22 Around five studies analysed the potential direct causality of COVID-19 infection to oral lesions and concluded that the lesions are related to the disease,Citation14,Citation19 despite not being scientifically proven.Citation27,Citation36 Five studies concluded that oral lesions in COVID-19 likely resulted from various external factors (casualty), such as co-infection,Citation35 medical devices and treatments,Citation15 comorbidities,Citation32 immunosuppression and medicationsCitation28,Citation32 that could mimic other inflammatory diseases.Citation18 Around three studies concluded in the urge of further research, including clinical evidence-based researchCitation30,Citation31 and observational studiesCitation17 to confirm the association between COVID-19 and oral lesions.
The Original Studies Report on Oral Lesions in COVID-19 Patients
Thirty-two original studies include one pilot study, one cohort study, five observational studies, four retrospective studies and fourteen cross-sectional studies (). The pilot study and cohort study reported all patients with all oral lesions.Citation37,Citation38 The observational study reported that the prevalence of oral lesions was 70.34–100% among COVID-19 patients.Citation39–44 The prospective study reported oral lesions from all patients.Citation45 The retrospective reported 1.70–100% among COVID-19 patients,Citation46–50 and the cross-sectional reported 0.67–100% ().Citation51–68
Table 2 The Original Study of a Reported Oral Lesion in a COVID-19 Patient
The Oral Lesions that Were Found in the Original Studies
Most oral lesions reported in the original study were ulcers.Citation37,Citation39,Citation40,Citation42,Citation43,Citation45,Citation48,Citation52,Citation53,Citation60,Citation63,Citation66–68 Some of the cases were found as atrophy,Citation42,Citation60 erosion,Citation42,Citation48,Citation60 pseudomembrane,Citation39,Citation46,Citation59,Citation60,Citation67 vesico-bullous,Citation39,Citation43 blister,Citation39,Citation67 nodule,Citation60 plaque,Citation59,Citation60 depapilation,Citation39,Citation67,Citation68 macula,Citation40,Citation42,Citation45,Citation46,Citation60,Citation67 petechiae,Citation43 ecchymosis,Citation43 fissure,Citation39 hematoma,Citation48 swelling and bleeding.Citation50 The patient was distributed equally between men and women aged 1–88 ().
Table 3 The Original Study of an Oral Lesion in a COVID-19 Patient without Underlying Disease and Mediation Related
Underlying Disease and No Medication-Related
Underlying disease, such as diabetes mellitus and hypertension, is reported as a common condition found,Citation42,Citation44,Citation45,Citation56,Citation57 followed by hyperthyroidism,Citation42 coronary artery disease,Citation44,Citation56 bronchial asthmaCitation56 and myocardial infarct.Citation57 While the oral lesions commonly found were ulcers, macula, atrophy,Citation42,Citation44,Citation45,Citation49,Citation56,Citation57 erosion and vesicle,Citation42,Citation56 crust,Citation42 ecchymosis,Citation56 noduleCitation56 and pseudomembrane.Citation42 Three studies did not mentioned details about the underlying disease.Citation54,Citation59,Citation64 This condition is found in 18–70 years old patients ().
Table 4 The Original Study of an Oral Lesion in a COVID-19 Patient with an Underlying Disease
The cohort study showed that the oral lesion found in underlying diseases like hypertension, diabetes mellitus, obesity, pulmonary diseases, hypothyroidism, AIDS, and dyslipidemia was ulcers, pseudomembranous, depapilation, erosions and crusts.Citation38
The Medication-Related
The medication-related to oral lesions reported was anti-viral, anti-malaria (hydroxychloroquine), antibiotic and corticosteroid. The skin lesion was found as exanthema, and the oral lesion was anathema (macula and petechiae)Citation51 ().
Table 5 The Original Study of an Oral and Skin Lesion in a COVID-19 Patient with Medication-Related
One report mentioned that a skin dan oral lesion was found in the patient without any underlying disease or medication-related. The oral lesions were ulcers, depapilation, crust, pseudomembrane and macula, while the skin lesion was exanthemaCitation65 ().
Table 6 The Original Study of an Oral and Skin Lesion in a COVID-19 Patient without Underlying Disease and Medication-Related
Underlying Disease and Medication-Related
Related to underlying diseases, diabetes mellitus is reported as a common condition found,Citation41,Citation58,Citation61,Citation62 and hypertension,Citation41,Citation55,Citation58,Citation61,Citation62 cardiovascular disease,Citation41,Citation58,Citation62 asthma,Citation55,Citation58,Citation61 obesityCitation41,Citation58 and renal disease.Citation41,Citation62 The medication-related to the oral lesion was antibiotic,Citation41,Citation62 anticoagulant,Citation41,Citation62 antimalarial,Citation58,Citation62 antiviral,Citation55,Citation58,Citation62 and corticosteroids.Citation41,Citation55,Citation62 Other underlying diseases and medications are listed in .
Table 7 The Original Study of an Oral Lesion in a COVID-19 Patient with Underlying Disease and Medication-Related
The oral lesion was found as an ulcer,Citation41,Citation55,Citation58,Citation61,Citation62 atrophy, pseudomembrane,Citation55,Citation61,Citation62 erosion,Citation41,Citation55,Citation61 macula,Citation55,Citation62 petechiaeCitation61,Citation62 vesico-bullous,Citation61,Citation62 crust, depapilation, ecchymosis, andCitation41 papule.Citation55
The Non-Original Studies Report on Oral Lesions in COVID-19 Patients
The non-original research was 39 case reports (70 cases),Citation10,Citation69–106 6 case series (64 cases),Citation107–112 18 letters to the editor (23 cases),Citation113–130 2 correspondences (2 cases)Citation131,Citation132 and 3 clinical images (3 cases)Citation133–135 ().
Table 8 The Individual Case of Oral Lesion in COVID-19 Patient
No Underlying Disease and Medication
The oral lesion in COVID-19 patients without any underlying disease and medication was reported as not different based on age and gender. Demographic analysis shows that the studies involved 30 females and 29 males ranging from 16 to 78 years old. For the female, the youngest patient was reported as 16 years old,Citation109 and the oldest was 78.Citation127 Generally, ulcerations occur most frequently in the oral cavity of COVID-19 patients, whether in single or multiple ulcers,Citation93,Citation107,Citation109,Citation112,Citation124,Citation127,Citation135 vesiculobullous,Citation131 edematous,Citation114 necrosis,Citation114 bleeding,Citation94,Citation114 depapilation,Citation127 macula,Citation127 erosion,Citation127 pseudomembraneCitation127 and non-white specific lesion.Citation124 The most common site was the tongue, lips or labial, gingiva, palatal, buccal and commissure of the lips ().
Table 9 The Oral Lesion in COVID-19 Patients without Systematic Condition and Medication
For the male, the youngest patient was reported as 19 years old,Citation71 and the oldest was 69 years old.Citation109 One study did not mention the details of patients’ ages.Citation106 The oral lesion was reported as an oral ulcer as the typical lesion,Citation71,Citation89,Citation93,Citation95,Citation101,Citation104,Citation107,Citation109,Citation130 swelling,Citation120 petechia,Citation94,Citation134 macula,Citation69 desquamation, crust and papulaCitation104 and also pustula.Citation102 The most common site was the tongue, labial mucosa, gingiva, palatal, oropharynx, and floor of the mouth ().
Oral lesions and skin lesions were also observed in the patient with COVID-19. The oral lesion was ulcer,Citation76,Citation98,Citation117,Citation118,Citation122,Citation127 erosion and hemorrhagic crust,Citation98 blister,Citation98 depapilation,Citation72 desquamationCitation117 and erythemaCitation76,Citation117—the skin lesion including the erythematous macula, urticaria, exanthema and perioral ulcer ().
Table 10 The Oral Lesion with Skin Lesion in COVID-19 Patients without Underlying Disease and Medication
The Medication-Related
The oral lesion of COVID-19 was also related to medication. Antibiotics and analgesics were reported to be the most used drugs for COVID-19 patients. Antibiotics include amoxicillin-clavulanic,Citation101 azithromycin,Citation73,Citation77,Citation81,Citation83,Citation101,Citation116,Citation126 moxifloxacin,Citation91 levofloxacin,Citation77 penicillin,Citation96 ceftriaxone,Citation95 cefixime,Citation110 piperacillin-tazobactam,Citation100 doxycyclineCitation100 and cefadroxilCitation105 ().
Table 11 The Oral Lesion in COVID-19 Patients Related to Medication
The anti-inflammatory and antipyretic drugs include dipyrone,Citation83,Citation113 acetaminophen,Citation90,Citation96,Citation101,Citation119,Citation132 ibuprofen,Citation81 acetylsalicylic acid,Citation77 paracetamolCitation73,Citation95,Citation101 Steroids also prescribe dexamethasone,Citation77,Citation111,Citation113 prednisoneCitation77 and methylprednisolone.Citation100 Other types of drugs include proton pump inhibitors,Citation91 anti-malaria,Citation73,Citation95,Citation116 anti-virus,Citation73,Citation100,Citation116 antihistamine,Citation90 mucolytic,Citation77,Citation101,Citation111 anticholinergic,Citation77 anti-coagulant,Citation95,Citation96,Citation100,Citation111 antiparasitic,Citation126 anti-gout,Citation111 anti-gerd,Citation111 anti-depressantCitation110,Citation111 and multivitaminCitation73,Citation81,Citation91,Citation95,Citation101,Citation105,Citation111 ().
The use of medication during COVID-19 treatment also has a side effect on the oral mucosa as oral lesions, observed in male and female patients in diverse age groups. In the female, the oral manifestation was crust,Citation83,Citation101 macula,Citation113 depapilation,Citation100,Citation101,Citation113 bullous,Citation90 ulcerCitation77,Citation101 and pseudomembrane.Citation77 In the male, there was depapilation,Citation100 bleeding,Citation95 ulcers,Citation94,Citation100,Citation111,Citation126 maculaCitation90 and pseudomembraneCitation111 ().
Oral lesion-related medication is sometimes also found with a skin lesion. The most common lesion was ulcer,Citation73,Citation81,Citation96,Citation105,Citation110,Citation116,Citation119 crust,Citation96,Citation105,Citation116,Citation132 macula,Citation136 vesicle,Citation132 and vesiculobullous,Citation96 with skin lesions in the form of petechiae,Citation81 macula,Citation96,Citation110,Citation119 papula,Citation96,Citation119,Citation132 exanthemCitation73,Citation110 and targetoid lesions.Citation116 The oral lesion mostly affected on lip,Citation96,Citation105,Citation116,Citation119,Citation132 while others were on other mucosaeCitation73 ().
Table 12 The Oral and Skin Lesion in COVID-19 Patients Related Medication
Underlying Disease
Underlying Disease and No Medication-Related
Oral lesions found in the COVID-19 patient with the underlying disease were also reported. Most of the lesion was ulcer,Citation42,Citation74,Citation85,Citation89,Citation99,Citation110,Citation123 vesicle,Citation42,Citation80 erythema,Citation42,Citation80 pseudomembrane,Citation42 erosion,Citation42,Citation80 crustCitation42,Citation111 and atrophy.Citation42,Citation94 Other lesions were depapilation,Citation74 oedema,Citation42 macule,Citation42 petechiaeCitation94 and plaqueCitation103 ().
Table 13 The Oral Lesion Found in COVID-19 Patient with Underlying Disease
The underlying disease found in men and women was different. Most hypertension,Citation42,Citation80,Citation89 diabetes mellitus,Citation42,Citation85,Citation89,Citation111 hyperthyroidism,Citation42,Citation74 osteoarthritis,Citation80 hypothyroidism,Citation111 rheumatoid arthritis,Citation94 severe dystonia,Citation123 epilepsy,Citation123 arterial hypertension,Citation110 chronic hepatopathy,Citation110 hypercholesterolemia,Citation110 gastroesophageal reflux disease,Citation110 HIVCitation103 and asthmaCitation99 ().
One study only reported oral and skin lesions found in COVID-19 patients with underlying disease. The males of 60 and 63 years old with chronic cholecystitis, renal cyst, and inguinal hernia found an erosive and radiating stria in the buccal and tongue. In contrast, the skin lesion was found as a pruritic macule in the arm’s skin, arm and flexure surfaceCitation79 ().
Table 14 The Oral Lesion Accompanies Skin Lesions Found in COVID-19 Patient with Underlying Disease
Underlying Disease and Medication-Related
The case presented 13 females with a range of 42–84 years old and 14 males with a range of 46–86 years old. The underlying disease frequently reported was diabetes mellitus,Citation84,Citation87,Citation91,Citation92,Citation94,Citation95,Citation107,Citation115,Citation129 hypertension,Citation70,Citation82,Citation84,Citation87,Citation90,Citation92,Citation94,Citation95,Citation100,Citation107,Citation108,Citation115 stroke,Citation100 obesity,Citation47,Citation84,Citation92,Citation107 CVD,Citation82,Citation121,Citation129 hypothyroidism,Citation92,Citation108,Citation129 COPD,Citation92,Citation100,Citation107 carcinoma,Citation92,Citation107 renal diseaseCitation92,Citation107 and cardiac disease.Citation10 Other conditions such as rheumatoid arthritis,Citation91 allergies,Citation94 chronic sinusitis,Citation70 coronary and peripheral artery disease,Citation92 vascular disease,Citation91 hypercholesterinemia,Citation121 hyperlipidemia,Citation92 pancreatitis,Citation107 Parkinson's disease,Citation107 peripheral neuropathy,Citation91 rectal tumour,Citation108 HIV,Citation78 depression,Citation91 follicular lymphoma,Citation125 kidney transplantCitation82 and autosomal dominant polycystic kidney diseaseCitation82 were also reported ().
Table 15 The Oral Lesion is Found in COVID-19 Patient with Underlying Disease and Medication Related
Most of the prescribed drug was antibiotics,Citation47,Citation70,Citation82,Citation87,Citation90,Citation91,Citation107,Citation108,Citation115,Citation121,Citation125,Citation129 anti-coagulant,Citation47,Citation82,Citation91,Citation107 anti-viral,Citation78,Citation92,Citation100,Citation115 NSAIDs,Citation70,Citation90 steroid,Citation47,Citation90,Citation92,Citation100,Citation107,Citation125 anti-diabetic,Citation94,Citation95 anti-hypertension,Citation94,Citation95 or cardiac drug,Citation10 anti-malaria,Citation82,Citation125 anti-allergic,Citation94 and bronchodilator.Citation70 Another study only mentions intensive care medicineCitation84 and covalent plasma administration.Citation92 Azithromycin, Ceftriaxone, dexamethasone and remdesivir are antibiotics, steroids and anti-virals that are frequently prescribed ().
The oral lesion was ulcer,Citation47,Citation78,Citation82,Citation87,Citation92,Citation100,Citation107,Citation108,Citation115,Citation121,Citation125 papula-plaque,Citation82,Citation90,Citation125,Citation129 pseudomembrane,Citation92,Citation94,Citation129 crust,Citation84,Citation100,Citation107,Citation108 erosion,Citation94,Citation125 hemorrhagic,Citation95,Citation107 depapilation,Citation10 ecchymosis,Citation94 macula,Citation90 vesicleCitation70 and white patches.Citation91 The location of the lesion dominated in the tongue,Citation10,Citation82,Citation87,Citation91,Citation92,Citation94,Citation107,Citation115,Citation129 lip,Citation47,Citation70,Citation84,Citation94,Citation100,Citation107,Citation108 palateCitation90,Citation91,Citation94,Citation95,Citation129 and labialCitation91,Citation92,Citation100,Citation125 ().
The oral lesion in COVID-19 patients with underlying disease and medication are also found with skin lesions. Six studies reported that the condition consists of three females and two males, ages 41 to 82. The oral lesion found was ulcer,Citation86,Citation88,Citation108,Citation110,Citation128 blister and bullae,Citation89 crusted,Citation108 maculaCitation128 and white patches.Citation110 The skin lesion found as bullae,Citation86 rashCitation89 and exanthema,Citation110 petechia-like and vesiculobullous.Citation128 Other cases reported perioral ulcersCitation88 and fungal infectionCitation108 ().
Table 16 The Oral and Skin Lesion Found in COVID-19 Patient with Underlying Disease and Medication Related
The underlying disease found was hypertension,Citation88,Citation89,Citation108,Citation128 obesity,Citation89,Citation110 Hodgkin’s lymphoma stage II,Citation86 hyperlipidemia,Citation88 dyslipidaemia,Citation108 hypothyroidism,Citation108 diabetes mellitus,Citation128 arterial hypertension,Citation110 myocardial infarctionCitation110 and septic shock.Citation110 The medication prescribes like chemotherapy medication,Citation86,Citation88 antibiotic,Citation108 antivirus,Citation86,Citation89 antimalarial,Citation89 corticosteroid,Citation128 anti-vomitingCitation128 and anti-hypertensionCitation110 ().
Discussion
COVID-19 patients are reported to suffer from various oral lesions throughout or preceding the disease onset.Citation28 Various questions and hypotheses emerged along with the increasing report of the incidence, especially regarding whether it is a manifestation of the viral infection (causality) or a result of large numbers of unidentified risk factors (casualty). Attempts to analyze the lesions and their correlation to COVID-19 are resulting to thereabouts inconclusive results. Various systematic reviews termed that the lesions are COVID-19 oral manifestations,Citation15–17,Citation19,Citation22,Citation24,Citation25,Citation27,Citation28,Citation123 that is also determined by individual and environmental factors,Citation16 secondary infection and psychosocial factors,Citation25,Citation27 immunosuppressive conditionsCitation15 caused by medicationsCitation15,Citation28 and diseases,Citation23,Citation24,Citation26,Citation29 or might be as primary direct causality since ACE2 is expressed in the oral cavity.Citation15,Citation17,Citation19,Citation22,Citation25–27 We report contradictory findings based on analyses of the patterns of COVID-19 patients with clinical oral lesions and by further breaking down the reported various factors that might be involved. The lesions reported by 70 works of literature were analyzed to see whether they are symptoms or conditions resulting from COVID-19 infection as an oral manifestation or pathognomonic features.
Preceding systematic reviews were summarized to understand the current understanding of oral lesions in COVID-19 patients (). All systematic reviews give varying conclusion but generally show uncertainty and skepticism towards the concept of oral lesions as COVID-19 manifestation. A meta-analysis also discovered that data on oral lesion prevalence was highly heterogenous, while data on xerostomia show lower heterogeneity,Citation17 indicating that the most common symptom found on COVID-19 was dry mouth, as also concluded by another review.Citation21 A systematic review concludes that oral lesions suffered by COVID-19 patients were very diverse and indistinctive,Citation24,Citation34 and mostly unrelated to the SARS-CoV-2 virus.Citation17,Citation22,Citation30 The indistinctive lesions would explain the heterogeneity of oral lesion prevalence in COVID-19 patients. Various concepts regarding the correlation between COVID-19 and oral lesions were also stated, such that the virus infected ACE2 receptors in the oral mucosal tissue, thus leading to lesion onset through inflammatory mechanism,Citation19,Citation30 and that the lesions might progress along with the disease progression.Citation29 However, despite the statement of direct causality, all these studies mostly concluded the doubt that COVID-19 infection might result in oral manifestations, as no substantial evidence could be found regarding it.Citation17 These studies suggest that other factors such as comorbidities,Citation32 medication and immunosuppressionCitation28,Citation32 are potentially the leading cause of these lesions as various interrelated factors’ casualty. This notion led to inconclusive conclusions in these reviews that oral lesions in COVID-19 patients might not be an oral manifestation of the viral infection. Detailed investigation and analysis of these oral lesions and their clinical signs along with COVID-19 patterns is needed to be done on each case published from various case reports, case series, cross-sectional, letters to editors and observational studies to uncover more of the relation between clinical oral lesion with COVID-19 conditions.
A plethora of oral diseases had been found to occur in the oral cavity of patients infected with SARS-CoV-2. We found that oral lesions in COVID-19 are very diverse but mostly in the form of ulcersCitation37–43,Citation47,Citation52–57,Citation59–64,Citation71,Citation73,Citation74,Citation76–78,Citation81,Citation82,Citation85–89,Citation92–94,Citation96,Citation98,Citation107–112,Citation115,Citation116,Citation118,Citation119,Citation121–128,Citation131,Citation135 and erosion.Citation38,Citation41,Citation42,Citation47,Citation55,Citation56,Citation60,Citation61,Citation80,Citation94,Citation96,Citation98,Citation125,Citation127,Citation132 Some studies also reported an occurrence of recurrent oral lesions, aphthous-like ulcers,Citation53 hemorrhagic ulcersCitation38,Citation41,Citation42,Citation83,Citation84,Citation96,Citation107,Citation108,Citation111,Citation116,Citation117,Citation132 and macula (erythema).Citation42,Citation55,Citation56,Citation76,Citation80,Citation81,Citation91 While the findings often took place as an ulcer, other oral findings are also found in the form of plaques,Citation55,Citation59,Citation60,Citation82,Citation90,Citation125,Citation129 pseudomembrane,Citation38,Citation39,Citation42,Citation46,Citation55,Citation59–62,Citation77,Citation81,Citation91,Citation92,Citation94,Citation111,Citation127,Citation129 depapilation,Citation38,Citation39,Citation41,Citation72,Citation74,Citation113,Citation127 or bleeding lesion.Citation37,Citation94,Citation95,Citation114 Most of these lesions were found in the tongue,Citation71,Citation72,Citation79,Citation81,Citation82,Citation87,Citation90–92,Citation94,Citation98,Citation107,Citation109–113,Citation115,Citation116,Citation118,Citation120,Citation122,Citation127–129,Citation132,Citation134 palate,Citation80,Citation85,Citation89–91,Citation94,Citation107,Citation112,Citation115,Citation128,Citation129 lipCitation70,Citation77,Citation81,Citation83,Citation84,Citation88,Citation89,Citation94,Citation96,Citation98,Citation107,Citation108,Citation110,Citation111,Citation117,Citation119,Citation128,Citation132 and buccal mucosa.Citation71,Citation80,Citation98,Citation112,Citation126,Citation128,Citation129,Citation132 In this context, the diversity of types of oral lesions and locations in COVID-19 patients raises a question regarding its causality and casualty. This shows that COVID-19 patients did not show particular clinical patterns and tendencies that could be assumed as pathognomonic lesions of COVID-19.
Unsolved hypotheses of the mechanism of the lesion formation could possibly be solved by analyzing underlying diseases and medications that underwent by COVID-19 patients with oral lesions. Most of these patients have various medical conditions that alter and worsen their immune status to respond to viral infection. We found that most of the patients included in the study suffered from cardiovascular diseases,Citation38,Citation41,Citation42,Citation55–58,Citation61,Citation62,Citation70,Citation80,Citation82,Citation84,Citation87–92,Citation94,Citation107,Citation108,Citation110,Citation115,Citation121,Citation128 pulmonary diseasesCitation38,Citation92 and diabetes,Citation38,Citation41,Citation42,Citation55–58,Citation61,Citation62,Citation84,Citation85,Citation87,Citation89,Citation91,Citation92,Citation94,Citation107,Citation111,Citation115,Citation128,Citation129 that could worsen their immune status whether independent or dependent to COVID-19 infection and resulted in the lesion due to wane host defense.Citation137 Observations on these patients also show the diversity of the reported underlying diseases that seem to likely be inconsistent and unrelated to the oral lesions. Patients with underlying diseases were separately analysed () to see whether it could be a key player in the lesion progression, but it was later found that no evident differences in clinical patterns were found in the oral cavity in patients without underlying diseases. This leads to the notion that underlying diseases might not aid the lesion manifesting in COVID-19 patients.
Most patients with underlying diseases received high doses of various medications, like antibiotics,Citation41,Citation62 immunosuppressants,Citation41,Citation55,Citation62 NSAIDs,Citation58 and anti-virals.Citation55,Citation58,Citation62 Meanwhile, in patients without underlying diseases who received medications, we found that most of them used antibiotics,Citation77,Citation83,Citation91,Citation95,Citation126 NSAIDs,Citation83,Citation113 vitamins,Citation91,Citation95,Citation111 corticosteroids,Citation77,Citation94,Citation111,Citation113 analgesics,Citation85,Citation95 and antivirals.Citation95 We found that these drugs resulted in mostly ulcers in patients without underlying diseases ( and ) or with underlying diseases (), despite the large diversity of the following lesion forms. We also found the same inconclusive lesions in patients without underlying diseases and without medications ( and ). These drugs may not directly cause the specific manifestation of lesions in COVID-19 patients. Previous systematic reviews stated that steroids could cause immunosuppression that could lead to oral lesion formation.Citation15,Citation24,Citation26 Lesions occur in those patients could also occur in non-COVID-19 patients or in patients without steroid prescriptions. This can be inferred that medication is not a plausible factor that could aid COVID-19 manifesting in the oral cavity.
COVID-19 patients often receive multiple medications, which could promote the risk of drug reactions.Citation138 Hydroxychloroquine has been used to treat COVID-19 and was reported to be one of the most prevalent erythema multiforme-triggering drugs and various other side effects in patients.Citation138–142 The lesion was caused by promoted CD8+ lymphocyte infiltration to the epithelial tissue, thus leading to the necrosis of the cells and subepithelial cleft forming as a hypersensitivity reaction to the drugs consumed.Citation140 This led to the hypothesis that the crusts suffered by the patients potentially are actually not an oral manifestation of SARS-CoV-2, but probably erythema multiforme, which is the distinct pathognomonic features that also include hemorrhagic crusts along with targetoid lesions on the skin.Citation138,Citation142 Our findings highlighted that most of the skin lesion-related medications were exanthema and skin or genital ulceration ( and ). In contrast, some cases also reported similar skin lesions in the patient without any medication ( and ).
Despite the effort to break down possible influencing factors of oral COVID-19 lesions through underlying diseases and patients’ medications, we found no relevance to specific manifestations in the oral tissue. Oral lesions in patients with and without underlying diseases and medications vary with no particular patterns or pathognomonic pattern. The lesions might be resulted from various unidentified interrelated factors with unknown mechanisms, resulting in varying forms of lesions. These lesions are unlikely to be called oral manifestations of COVID-19 since data show clinical signs that are not in accordance with the viral infection itself.
Contrary to previous studies, we also found no correlation between COVID-19 severity and oral lesions.Citation29 Patients with severe COVID-19 symptoms were often to be hospitalized. Oral ulcers in these patients may also occur because of mechanical ventilation and intubation. However, there is no distinguishing oral lesion found in patients with severe COVID-19 symptoms compared to non-hospitalized patients, as both presented with mostly ulcers.Citation41 This corroborates our statement that oral lesions in COVID-19 patients are not dependent on the viral infection to manifest whether in a mild or advanced stage of the disease. However, despite some studies reported regarding the matter, the number of studies that include the intubation treatment in their reports is limited to make a proper analysis and conclusion to the exact cause of the lesion and how significant the mechanical trauma to the ulcers that reported.
Compared to other diseases with established and distinct oral clinical patterns, such as herpes zoster, primary gingiva stomatitis, and measles, COVID-19 did not show clear and consistent causality to the oral tissue and casualty to form distinct pathognomonic features. Based on the analyses done of the lesions and their clinical patterns, it is difficult and unlikely to conclude that these oral lesions occur as the result of COVID-19 infection, and SARS-CoV-2 does not seem to have any specific manifestations in the oral cavity. We found 121 works of literature (21 reviews, 32 original studies and 68 non-original studies) that could lead to these findings on the inconclusiveness of oral lesions in COVID-19 patients. However, we found irregularity and unclarity in these reports regarding lesion descriptions and terms, such as their type, shape, size, and location. This makes the data heterogeneous and difficult to analyse in a more accurate way. This could be due to the fact that most of the authors who reported the oral lesion in those studies probably were not dentists, let alone oral medicine specialists, thus possibly lead to inaccurate lesion descriptions. The findings of oral lesions in COVID-19 patients has prompted numerous authors to hypothesize that these lesions are attributable to COVID-19 infection itself. Through meticulous analysis on the clinical signs, we found that it is crucial to emphasize that the presence of these lesions does not necessarily establish a causal relationship with COVID-19 infection itself, especially when the clear cause and effect are not found yet. Further comprehensive investigations are imperative to discern potential confounding factors and establish a clearer understanding of the etiology behind these oral lesions in COVID-19 patients. Dentists and oral medicine specialists need an active role in uncovering more about these oral lesions in COVID-19 patients in the future research, especially in its pathogenesis.
On the other hand, it is still debatable whether all reported oral lesions are solely a result of the SARS-CoV-2 infection. One piece of evidence demonstrated that, out of 14 patients, the oral lesions in 13 expressed the spike protein of SARS-CoV-2 and exhibited higher ACE2 expression.Citation43 This indicates the presence of SARS-CoV-2 components in the oral mucosa, but the subsequent processes that occurred have not been determined. Although the study conducted by Soares et al identified the presence of SARS-CoV-2 components within oral lesions, further analyses and investigations involving a larger population of patients and other types of oral lesions are required to comprehensively determine the potential significance and impact of these viral components on oral lesion development. Hence, the direct causality of SARS-CoV-2 in the oral mucosa remains uncertain. Other studies have also corroborated that oral lesions in COVID-19 patients may be secondary lesions associated with trauma events, immune impairment, or adverse reactions to therapeutic interventions.Citation143 In the subsequent study, if it can elucidate how the interplay between ACE2, the SARS-CoV-2 spike protein, and the initiation of oral lesions occurs, then the identified lesions can be confidently classified as oral manifestations.
This review has strengths as we conducted comprehensive systematic analyses from various kinds of original and non-original literature. Various types of literature, such as pilot study, cohort, observational, prospective, retrospective, cross-sectional, case reports, case series, and even letters to editor, that report COVID-19 patients with specific oral lesion conditions were included and analyzed. Through these approaches, detailed analysis and observation on oral lesions on COVID-19 could be done, and a conclusive conclusion could be reached that the lesions that suffered by COVID-19 patients are not oral manifestations of the disease. However, we were unable to determine the bias in this study, as we adhere to descriptive approaches to explain the conformity between oral lesions and COVID-19. Therefore, extensive, and comprehensive research is needed to know the cause of these lesions in COVID-19 patients and discover their pathognomonic features.
Conclusion
Oral lesions in reported studies do not have pathognomonic features and are vary, so in present time they cannot be defined as an oral manifestation. The suspicious factors such as underlying diseases and medications might be classified as predisposing factors. This would arise several possibilities for pathogenesis stacked across one another, making it possible to indirect causality of oral lesion development.
Disclosure
The authors report no conflicts of interest in this work.
References
- Huang CW, Hsieh CH, Lin MR, Huang YC. Clinical features of gingivostomatitis due to primary infection of herpes simplex virus in children. BMC Infect Dis. 2020;20(1):782. doi:10.1186/s12879-020-05509-2
- Crimi S, Fiorillo L, Bianchi A, et al. Herpes virus, oral clinical signs and QoL: systematic review of recent data. Viruses. 2019;11(5):463. doi:10.3390/v11050463
- Leung AKC, Barankin B. Herpes labialis: an update. Recent Pat Inflamm Allergy Drug Discov. 2017;11(2). doi:10.2174/1872213X11666171003151717
- Lamichhane S, Humagain M, Subba M, Neupane M, Dawadi A. Necrotizing stomatitis in varicella zoster infection. Kathmandu Univ Med J. 2020;18(70):210–213.
- Clarkson E, Mashkoor F, Abdulateef S. Oral Viral Infections. Dent Clin North Am. 2017;61(2):351–363. doi:10.1016/j.cden.2016.12.005
- Tanaka M, Harada T. Koplik spots in measles. Postgrad Med J. 2019;95(1126):454. doi:10.1136/postgradmedj-2019-136739
- Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23(1):3–20. doi:10.1038/s41580-021-00418-x
- da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133(7–8):377–382. doi:10.1007/s00508-020-01760-4
- Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19. J Chine Med Ass. 2020;83(3):217–220. doi:10.1097/JCMA.0000000000000270
- Sharma S, Bhardwaj A. COVID tongue. J Indian Soc Periodontol. 2022;26(5):498–500. doi:10.4103/jisp.jisp_437_21
- Scotto G, Fazio V, Spirito F, Lo Muzio E, Lo Muzio L. COVID Tongue: suggestive hypothesis or clinical reality? Oral Dis. 2022;28:2618–2619. doi:10.1111/odi.14134
- Hathway RW. COVID tongue. Br Dent J. 2021;230(3):114. doi:10.1038/s41415-021-2666-z
- Surboyo MDC, Santosh ABR, Kuntardjo Y, Indriyani I, Ayna VKP, Ernawati DS. COVID tongue: reports, debate, and scope for research. Dental J Advan Stud. 2022;2022:1.
- Cuevas-Gonzalez MV, Espinosa-Cristóbal LF, Donohue-Cornejo A, et al. COVID-19 and its manifestations in the oral cavity. Medicine. 2021;100(51):e28327. doi:10.1097/MD.0000000000028327
- Orilisi G, Mascitti M, Togni L, et al. Oral manifestations of COVID-19 in hospitalized patients: a systematic review. Int J Environ Res Public Health. 2021;18(23):12511. doi:10.3390/ijerph182312511
- Nijakowski K, Wyzga S, Singh N, Podgórski F, Surdacka A. Oral manifestations in SARS-CoV-2 positive patients: a systematic review. J Clin Med. 2022;11(8):2202. doi:10.3390/jcm11082202
- Aragoneses J, Suárez A, Algar J, Rodríguez C, López-Valverde N, Aragoneses JM. Oral manifestations of COVID-19: updated systematic review with meta-analysis. Front Med. 2021;8. doi:10.3389/fmed.2021.726753
- Nascimento RB, Araujo NS, Silva JC, Xavier FCA. Oral manifestations of multisystemic inflammatory syndrome in children (MIS‐C) and Kawasaki disease associated to COVID‐19: a systematic review. Spec Care Dentist. 2022;42(3):266–280. doi:10.1111/scd.12669
- Erbaş GS, Botsali A, Erden N, et al. COVID‐19‐related oral mucosa lesions among confirmed SARS‐CoV‐2 patients: a systematic review. Int J Dermatol. 2022;61(1):20–32. doi:10.1111/ijd.15889
- Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12(1):8. doi:10.1038/s41368-020-0074-x
- Qi X, Northridge ME, Hu M, Wu B. Oral health conditions and COVID-19: a systematic review and meta-analysis of the current evidence. Aging Health Res. 2022;2(1):100064. doi:10.1016/j.ahr.2022.100064
- Di Spirito F, Iandolo A, Amato A, et al. Prevalence, features and degree of association of oral lesions in COVID-19: a systematic review of systematic reviews. Int J Environ Res Public Health. 2022;19(12):7486. doi:10.3390/ijerph19127486
- Temgoua MN, Endomba FT, Nkeck JR, Kenfack GU, Tochie JN, Essouma M. Coronavirus disease 2019 (COVID-19) as a multi-systemic disease and its impact in low- and middle-income countries (LMICs). SN Compr Clin Med. 2020;2(9):1377–1387. doi:10.1007/s42399-020-00417-7
- Doceda MV, Gavriiloglou M, Petit C, Huck O. Oral health implications of SARS-CoV-2/COVID-19: a systematic review. Oral Health Prev Dent. 2022;20(1):207–218. doi:10.3290/j.ohpd.b2960801
- Uzêda-E-Silva VD, de Sá IB, Martins J, Pedreira N, Vieira VPS, de Silva BHM. Oral lesions associated with COVID-19: a systematic review. Stomatologija. 2021;23(1):3–8.
- Bhujel N, Zaheer K, Singh RP. Oral mucosal lesions in patients with COVID-19: a systematic review. Br J Oral Maxillofac Surg. 2021;59(9):1024–1030. doi:10.1016/j.bjoms.2021.06.011
- Sharma P, Malik S, Wadhwan V, Gotur Palakshappa S, Singh R. Prevalence of oral manifestations in COVID‐19: a systematic review. Rev Med Virol. 2022;32(6). doi:10.1002/rmv.2345
- Fakhruddin KS, Samaranayake LP, Buranawat B, Ngo H. Oro-facial mucocutaneous manifestations of Coronavirus Disease-2019 (COVID-19): a systematic review. PLoS One. 2022;17(6):e0265531. doi:10.1371/journal.pone.0265531
- da Santana LAM, Vieira W, Gonçalo RIC, Lima Dos Santos MA, Takeshita WM, Miguita L. Oral mucosa lesions in confirmed and non-vaccinated cases for COVID-19: a systematic review. J Stomatol Oral Maxillofac Surg. 2022;123(5):e241–50. doi:10.1016/j.jormas.2022.05.005
- Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID-19: a 6-month update. J Dent Res. 2021;2021:002203452110296.
- Dar-Odeh N, Bobamuratova DT, Alnazzawi A, et al. Jaw-related complications in COVID-19 patients; a systematic review. CRANIO®;2022. 1–8. doi:10.1080/08869634.2022.2031438
- Samaranayake LP, Fakhruddin KS, Ngo HC, Bandara HM, Leung YY. Orofacial mycoses in coronavirus disease-2019 (COVID-19): a systematic review. Int Dent J. 2022;72(5):607–620. doi:10.1016/j.identj.2022.02.010
- Di Spirito F, Caggiano M, Di Palo MP, et al. Oral lesions in pediatric subjects: SARS-CoV-2 Infection and COVID-19 Vaccination. Appl Sci. 2022;12(18):8995. doi:10.3390/app12188995
- Surboyo MDC, Ernawati DS, Budi HS. Oral mucosal lesions and oral symptoms of the SarS-coV-2 infection. Minerva Dent Oral Sci. 2021;70(4):161–168. doi:10.23736/S2724-6329.21.04493-9
- Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. 2020;2020:002203452095728.
- Reis VP, Bezerra AR, Maia ABP, Marques LC, Conde DC. An integrative review of oral manifestations in patients with COVID‐19: signs directly related to SARS‐CoV‐2 infection or secondary findings? Int J Dermatol. 2022;61(3):278–290. doi:10.1111/ijd.15881
- Kady DM, Gomaa EA, Abdella WS, Ashraf Hussien R, ElAziz RH A, Khater AGA. Oral manifestations of COVID-19 patients: an online survey of the Egyptian population. Clin Exp Dent Res. 2021;2020:1–9.
- Schwab G, Palmieri M, Zerbinati RM, et al. Lack of direct association between oral mucosal lesions and SARS-CoV- 2 in a cohort of patients hospitalised with COVID-19. J Oral Microbiol. 2022;14(1). doi:10.1080/20002297.2022.2047491
- Favia G, Tempesta A, Barile G, et al. Covid-19 symptomatic patients with oral lesions: clinical and histopathological study on 123 cases of the university hospital policlinic of bari with a purpose of a new classification. J Clin Med. 2021;10(4):757. doi:10.3390/jcm10040757
- Fidan V, Koyuncu H, Akin O. Oral lesions in Covid 19 positive patients. Am J Otolaryngol. 2021;42(3):102905. doi:10.1016/j.amjoto.2021.102905
- Batista AAF, Ramos KPP, Do Amaral MA, et al. Oral lesions in patients with COVID-19 hospitalized in an intensive care unit: a case-series study. Braz Oral Res. 2022;36:e108. doi:10.1590/1807-3107bor-2022.vol36.0108
- Subramaniam T, Nikalje M, Jadhav S. Oral manifestations among COVID-19: an observational study of 713 patients. Dent Res J (Isfahan). 2021;18(1):67. doi:10.4103/1735-3327.324026
- Soares CD, Souza LL, de Carvalho MGF, et al. Oral manifestations of coronavirus disease 2019 (COVID-19). Am J Surg Pathol. 2022;46(4):528–536. doi:10.1097/PAS.0000000000001825
- Bianco E, Maddalone M, Ferdeghini C, Mirabelli L, Hari S. Oral Manifestations in Hospitalized COVID Patients. World J Dent. 2022;13(5):434–440. doi:10.5005/jp-journals-10015-2082
- Naser AI, Al-Sarraj MN, Deleme ZH. Oral and maxillofacial lesions in COVID 19 infection from Mosul Hospital in Iraq: epidemiological study and approach to classification and treatment. J Oral Res. 2021;10(6):1–14. doi:10.17126/joralres.2021.069
- Bardellini E, Bondioni MP, Amadori F, et al. Non-specific oral and cutaneous manifestations of Coronavirus Disease 2019 in children. Med Oral Patol Oral Cir Bucal. 2020;2020:1.
- de Paula Eduardo F, Gobbi MF, Bergamin LG, Migliorati CA, Bezinelli LM. Oral care and photobiomodulation protocol for the prevention of traumatic injuries and lip necrosis in critically ill patients with COVID-19: an observational study. Lasers Dent Sci. 2021;5(4):239–245. doi:10.1007/s41547-021-00144-9
- Eduardo F, Bezinelli P, Gobbi MF, Bergamin LG, de Carvalho DLC, Corrêa L. Oral lesions and saliva alterations of COVID‐19 patients in an intensive care unit: a retrospective study. Spec Care Dentist. 2022;42(5):494–502. doi:10.1111/scd.12705
- Santos NMV, Brito DH, Santos TG, et al. Oral manifestations in hospitalized children with COVID-19. Braz Oral Res. 2022;36:e139. doi:10.1590/1807-3107bor-2022.vol36.0139
- Saraf S, Nalawade T, Mallikarjuna R, Al Kashmiri A. The retrospective pilot study of the prevalence of olfactory dysfunction or loss of smell, loss of taste and oral manifestations among COVID-19 positive health workers in Muscat, Oman. Indian J Otolaryngol Head Neck Surg. 2022;2022:1.
- Jimenez-Cauhe J, Ortega-Quijano D, de Perosanz-Lobo D, et al. Enanthem in patients with COVID-19 and skin rash. JAMA Dermatol. 2020;156(10):1134. doi:10.1001/jamadermatol.2020.2550
- Abubakr N, Salem ZA, Kamel AHM. Oral manifestations in mild-to-moderate cases of covid-19 viral infection in the adult population. Dent Med Probl. 2021;58(1):7–15. doi:10.17219/dmp/130814
- Katz J, Yue S. Increased odds ratio for COVID-19 in patients with recurrent aphthous stomatitis. J Oral Pathol Med. 2021;50(1):114–117. doi:10.1111/jop.13114
- Alade O, Folayan MO, Adeniyi A, et al. Differences in oral lesions associated with tobacco smoking, E-Cigarette Use and COVID-19 infection among adolescents and young people in Nigeria. Int J Environ Res Public Health. 2022;19(17):10509. doi:10.3390/ijerph191710509
- Villarroel-Dorrego M, Chacón L, Rosas R, Barrios V, Pernía Y, Vélez H. Hallazgos bucales en pacientes COVID-19. Actas Dermosifiliogr. 2022;113(2):183–186. doi:10.1016/j.ad.2021.08.007
- Chawla J, N Y, Bakshi SS, et al. Oral manifestations associated with COVID-19 disease: an observational cross sectional study. J Oral Biol Craniofac Res. 2022;12(2):279–283. doi:10.1016/j.jobcr.2022.03.008
- Muthyam A, Reddy M, Kulkarni S, Srilatha A, Sahithi K, Satyanarayana D. Oral manifestations in COVID-19 patients: an observational study. J Family Med Prim Care. 2022;11(3):1000. doi:10.4103/jfmpc.jfmpc_1264_21
- Tuter G, Yerebakan M, Celik B, Kara G. Oral manifestations in SARS-CoV-2 infection. Med Oral Patol Oral Cir Bucal. 2022;27(4):e330–9. doi:10.4317/medoral.25259
- El Tantawi M, Sabbagh HJ, Alkhateeb NA, et al. Oral manifestations in young adults infected with COVID-19 and impact of smoking: a multi-country cross-sectional study. PeerJ. 2022;10:e13555. doi:10.7717/peerj.13555
- Ganesan A, Kumar S, Kaur A, et al. Oral Manifestations of COVID-19 infection: an analytical cross-sectional study. J Maxillofac Oral Surg. 2022;21:1326–1335. doi:10.1007/s12663-021-01679-x
- Binmadi NO, Aljohani S, Alsharif MT, Almazrooa SA, Sindi AM. Oral Manifestations of COVID-19: a cross-sectional study of their prevalence and association with disease severity. J Clin Med. 2022;11(15):4461. doi:10.3390/jcm11154461
- Elamrousy W, Nassar M, Issa D. Prevalence of oral lesions in COVID-19 Egyptian patients. J Int Soc Prev Community Dent. 2021;11:6.
- Folayan MO, Zuniga RAA, Ezechi OC, et al. Associations between emotional distress, sleep changes, decreased tooth brushing frequency, self-reported oral Ulcers and SARS-Cov-2 infection during the first wave of the COVID-19 pandemic: a global survey. Int J Environ Res Public Health. 2022;19(18):11550. doi:10.3390/ijerph191811550
- Hans M, Hans VM, Kahlon N, Sagar M, Pandey AK, Das A. Gustatory dysfunction and oral ulceration in COVID-19 patients: a cross sectional study. Dent Res J. 2022;19:43. doi:10.4103/1735-3327.346401
- Özen T, Kahraman FC, Öcal S, Ovalı HF. Skin, mucosa and nail findings in hospitalized pediatric patients with Coronavirus disease-2019 (COVID-19). An Bras Dermatol. 2022;98:208–215. doi:10.1016/j.abd.2022.03.006
- Bhuyan R, Bhuyan SK, Mohanty JN, et al. A preliminary survey on the oral manifestation of COVID-19 in the First and Second Waves in Bhubaneswar, City of Odisha, India. Nat J Commun Med. 2022;13(5):294–297. doi:10.55489/njcm.130520221617
- Natto ZS, Afeef M, Khalil D, et al. Characteristics of oral manifestations in symptomatic non-hospitalized COVID-19 Patients: a cross-sectional study on a sample of the Saudi Population. Int J Gen Med. 2021;14:9547–9553. doi:10.2147/IJGM.S331611
- Nuno‐Gonzalez A, Martin‐Carrillo P, Magaletsky K, et al. Prevalence of mucocutaneous manifestations in 666 patients with COVID‐19 in a field hospital in Spain: oral and palmoplantar findings. Br J Dermatol. 2021;184(1):184–185. doi:10.1111/bjd.19564
- Kahraman FC, Çaşkurlu H. Mucosal involvement in a COVID ‐19‐positive patient: a case report. Dermatol Ther. 2020;33:4.
- Eghbali Zarch R, Hosseinzadeh P. COVID ‐19 from the perspective of dentists: a case report and brief review of more than 170 cases. Dermatol Ther. 2021;34(1):1–6. doi:10.1111/dth.14717
- Dominguez‐Santas M, Diaz‐Guimaraens B, Fernandez‐Nieto D, Jimenez‐Cauhe J, Ortega‐Quijano D, Suarez‐Valle A. Minor aphthae associated with SARS‐CoV‐2 infection. Int J Dermatol. 2020;59(8):1022–1023. doi:10.1111/ijd.15004
- Chaughtai S, Chaughtai Z, Asif A. Conservative treatment with mouthwashes followed by tongue photo biomodulation therapy in Covid-19: a case report. J Med Case Rep. 2022;16(1):367. doi:10.1186/s13256-022-03519-z
- Putra BE, Adiarto S, Dewayanti SR, Juzar DA. Viral exanthem with “Spins and needles sensation” on extremities of a COVID-19 patient: a self-reported case from an Indonesian medical frontliner. Int J Infect Dis. 2020;96:355–358. doi:10.1016/j.ijid.2020.05.020
- Al-Khanati NM, Riad A, Sahloul ME, Klugar M. Aphthous-like stomatitis of COVID-19 patients. Braz J Oral Sci. 2020;19:1–4. doi:10.20396/bjos.v19i0.8661354
- Chérif MY, de Filette JMK, André S, Kamgang P, Richert B, Clevenbergh P. Coronavirus disease 2019–related Kawasaki-like disease in an adult: a case report. JAAD Case Rep. 2020;6(8):780–782. doi:10.1016/j.jdcr.2020.06.023
- Malih N, Hajinasrollah G, Zare M, Taheri M. Unexpected Presentation of COVID-19 in a 38-year-old male patient: a case report. Case Rep Dermatol. 2020;12(2):124–131. doi:10.1159/000509994
- Berlingieri G, Alvares CMA, Serrano RV, Palma LF, Campos L. Phototherapies for COVID-19-associated opportunistic oral infections. Photodiagnosis Photodyn Ther. 2022;37:102678. doi:10.1016/j.pdpdt.2021.102678
- Campeanu AT, Dumea E, Rus M, et al. A rare case of plasmablastic lymphoma in a patient with HIV and SARS-CoV-2 infections. Curr Oncol. 2022;29(3):1537–1543. doi:10.3390/curroncol29030129
- Saleh W, SHawky E, Halim GA, Ata F. Oral lichen planus after COVID-19, a case report. Ann Med Surg. 2021;72:103051. doi:10.1016/j.amsu.2021.103051
- Saleh W, Ata F, Elashry MM. Is COVID-19 infection triggering oral herpes zoster? A case report. SAGE Open Med Case Rep. 2021;9:2050313X2110657. doi:10.1177/2050313X211065793
- Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID-19: case report. Int J Infect Dis. 2020;100:154–157. doi:10.1016/j.ijid.2020.08.071
- Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, et al. Oral mucosal lesions in a COVID-19 patient: new signs or secondary manifestations? Int J Infect Dis. 2020;97:326–328. doi:10.1016/j.ijid.2020.06.012
- Kitakawa D, Oliveira FE, Neves de Castro P, Carvalho LF. Short report - Herpes simplex lesion in the lip semimucosa in a COVID-19 patient. Eur Rev Med Pharmacol Sci. 2020;24(17):9151–9153. doi:10.26355/eurrev_202009_22863
- Ramires MC, Mattia MB, Tateno RY, Palma LF, Campos L. A combination of phototherapy modalities for extensive lip lesions in a patient with SARS-CoV-2 infection. Photodiagnosis Photodyn Ther. 2021;33(January):102196. doi:10.1016/j.pdpdt.2021.102196
- Pauli MA, Pereira LM, Monteiro ML, de Camargo AR, Rabelo GD. Painful palatal lesion in a patient with COVID-19. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(6):620–625. doi:10.1016/j.oooo.2021.03.010
- De Medeiros VLS, Monteiro-Neto AU, França DDT, Castelo Branco R, de Miranda Coelho ÉO, Takano DM. Pemphigus Vulgaris After COVID-19: a case of induced autoimmunity. SN Compr Clin Med. 2021;3(8):1768–1772. doi:10.1007/s42399-021-00971-8
- Nejabi MB, Noor NAS, Raufi N, et al. Tongue ulcer in a patient with COVID-19: a case presentation. BMC Oral Health. 2021;21(1):1–5. doi:10.1186/s12903-021-01635-8
- Siotos C, Bonett AM, Hansdorfer MA, Siotou K, Kambeyanda RH, Dorafshar AH. Medical device related pressure ulcer of the lip in a patient with COVID-19: case report and review of the literature. J Stomatol Oral Maxillofac Surg. 2020;14(4):337–339.
- Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2021;27(S3):710–712. doi:10.1111/odi.13382
- Tapia ROC, Labrador AJP, Guimaraes DM, Valdez LHM. Oral mucosal lesions in patients with SARS‐CoV‐2 infection. Report of four cases. Are they a true sign of COVID‐19 disease? Spec Care Dentist. 2020;3(July):12520.
- Riad A, Gomaa E, Hockova B, Klugar M. Oral candidiasis of COVID-19 patients: case report and review of evidence. J Cosmet Dermatol. 2021;20(6):1580–1584. doi:10.1111/jocd.14066
- Yeom J, Wolk R, Griffin L, Freedman PD, Reich RF. Atypical herpetic ulcerations in COVID-19 positive patients: a report of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2022;135:268–271. doi:10.1016/j.oooo.2022.07.015
- Khodavirdipour A, Asadimanesh M, Masoumi SA. Impact of SARS-CoV-2 genetic blueprints on the oral manifestation of COVID-19: a case report. Glob Med Genet. 2021;08(4):183–185. doi:10.1055/s-0041-1735538
- Rafałowicz B, Wagner L, Rafałowicz J. Long COVID oral cavity symptoms based on selected clinical cases. Eur J Dent. 2022;16(02):458–463. doi:10.1055/s-0041-1739445
- Manzalawi R, Alhmamey K, Abdelrasoul M. Gingival bleeding associated with COVID‐19 infection. Clin Case Rep. 2021;9(1):294–297. doi:10.1002/ccr3.3519
- Dalipi ZS, Dragidella F, Dragidella DK. Oral manifestations of exudative erythema multiforme in a patient with COVID-19. Case Rep Dent. 2021;2021:1–8.
- Garcez AS, Delgado MGT, Sperandio M, Dantas Silva FT, de Assis JSR, Suzuki SS. Photodynamic therapy and photobiomodulation on oral lesion in patient with coronavirus disease 2019: a case report. Photobiomodul Photomed Laser Surg. 2021;39(6):386–389. doi:10.1089/photob.2020.4977
- Palaia G, Pernice E, Pergolini D, et al. Erythema multiforme as early manifestation of COVID-19: a case report. Pathogens. 2022;11(6):654. doi:10.3390/pathogens11060654
- Al-Akhali MS, Halboub E, Ibraheem W, Khan HK, Hummadi AM. Recurring oral erythema multiforme-like lesions elicited by COVID-19 infection: a case report. Braz Dent Sci. 2022;25(1):e2960. doi:10.4322/bds.2022.e2960
- Sircar K, Popli DB, Jha OK, Sircar M, Hasan S. Oral mucosal lesions in moderate-to-severe COVID-19 Disease - An Indian critical care unit experience. J Datta Meghe Inst Med Sci Univ. 2022;17(5):S63–6. doi:10.4103/jdmimsu.jdmimsu_137_22
- Mahmoud MS, Taha MS, Mansour OI, et al. Oral mucosal lesions during SARS-CoV-2 infection: a case series and literature review. Egypt J Otolaryngol. 2022;38(1):18. doi:10.1186/s43163-022-00203-3
- Ingale Y, Bommanavar S, Ingale M. Mucormycotic osteomyelitis involving maxilla in SARS-CoV-2 prediabetic patient: a case report 1 2* 3 4. J Krishna Inst Medical Sci Univ. 2022;11(1):105–110.
- Ding X, Ma X, Xu Y, Xu L. HIV-associated mycobacterium Avium Complex, Oral Candida, and SARS-CoV-2 Co-Infection: a rare case report. Infect Drug Resist. 2022;15:7037–7042. doi:10.2147/IDR.S390333
- Emelyanova N, Isayeva G, Komir I, Shalimova A, Buriakovska O, Vovchenko M. Changes in the oral cavity of a patient after suffering from coronavirus infection COVID-19: a clinical case. Acta Med Mediterr. 2021;37:827–831.
- Talahatu LB, Kaban BE, Ayuningtyas NF, et al. Management of patients with aphthous-like ulcers related to aplastic anaemia in the COVID-19 pandemic era through teledentistry: a case report. Dental J. 2022;55(1):49–55. doi:10.20473/j.djmkg.v55.i1.p49-55
- Indu S. Multiple oral ulcerations – an initial manifestation of COVID 19 infection: a personal experience!! J.Oral Maxillofac Pathol. 2020;24(2):227. doi:10.4103/jomfp.JOMFP_324_20
- Brandão TB, Gueiros LA, Melo TS, et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;00(00):1–7.
- Teixeira IS, Leal FS, Tateno RY, Palma LF, Campos L. Photobiomodulation therapy and antimicrobial photodynamic therapy for orofacial lesions in patients with COVID-19: a case series. Photodiagnosis Photodyn Ther. 2021;34:102281. doi:10.1016/j.pdpdt.2021.102281
- Riad A, Kassem I, Hockova B, Badrah M, Klugar M. Tongue ulcers associated with SARS‐CoV‐2 infection: a case series. Oral Dis. 2020;2020:13635.
- Hocková B, Riad A, Valky J, et al. Oral Complications of ICU Patients with COVID-19: case-series and review of two hundred ten cases. J Clin Med. 2021;10(4):581. doi:10.3390/jcm10040581
- Sachet P, Rocha BA, Lima FS, et al. Management of orofacial lesions with antimicrobial photodynamic therapy and photobiomodulation protocols in patients with COVID-19: a multicenter case series. Photodiagnosis Photodyn Ther. 2022;38:102743. doi:10.1016/j.pdpdt.2022.102743
- Riad A, Kassem I, Stanek J, Badrah M, Klugarova J, Klugar M. Aphthous stomatitis in COVID‐19 patients: case‐series and literature review. Dermatol Ther. 2021;34(1):1–4.
- Tomo S, Miyahara GI, Simonato LE. Oral mucositis in a SARS‐CoV‐2‐infected patient: secondary or truly associated condition? Oral Dis. 2020;2020:13570.
- Patel J, Woolley J. Necrotizing periodontal disease: oral manifestation of COVID‐19. Oral Dis. 2020;2020:13462.
- Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID‐19). Oral Dis. 2020;27:13465.
- Demirbaş A, Elmas ÖF, Atasoy M, Türsen Ü, Lotti T. A case of erythema multiforme major in a patient with COVID 19: the role of corticosteroid treatment. Dermatol Ther. 2020;13:21–23.
- Labé P, Ly A, Sin C, et al. Erythema multiforme and Kawasaki disease associated with COVID‐19 infection in children. J Eur Acad Dermatol Venereol. 2020;34. doi:10.1111/jdv.16666
- Gueiros LA, Neves MCS, Marques CDL. Chikungunya fever and COVID‐19: oral ulcers are a common feature. Oral Dis. 2022;28(S1):1008–1009. doi:10.1111/odi.13717
- Hockova B, Riad A, Klugar M, Azar B. Self-case report of oral and skin lesions associated with positivity of COVID-19. J Cosmet Dermatol. 2021;20:1–2.
- McGoldrick DM, Sarai R, Green J. Tongue and floor of mouth swelling: a potential rare manifestation of COVID-19. Br J Oral Maxillofac Surg. 2021;59(4):500–501. doi:10.1016/j.bjoms.2021.03.001
- Kämmerer T, Walch J, Flaig M, French LE. COVID‐19‐associated herpetic gingivostomatitis. Clin Exp Dermatol. 2021;46(1):174–176. doi:10.1111/ced.14402
- Chaux-Bodard AG, Deneuve S, Desoutter A. Oral manifestation of Covid-19 as an inaugural symptom? Oral Surg Oral Med Oral Radiol. 2020;26(2):18. doi:10.1051/mbcb/2020011
- Cant A, Bhujel N, Harrison M. Oral ulceration as presenting feature of paediatric inflammatory multisystem syndrome associated with COVID-19. Br J Oral Maxillofac Surg. 2020;58(8):1058–1059. doi:10.1016/j.bjoms.2020.06.037
- Glavina A, Biočina‐Lukenda D, Mravak‐Stipetić M, Markeljević J. Oral symptoms and lesions in SARS‐CoV‐2‐positive patient. Oral Dis. 2020;15:odi.13596.
- Gabusi A, Gissi DB, Rossi R, Foschini MP, Montebugnoli L. Persistent lesions in oral cavity after SARS-CoV-2 infection. Oral Dis. 2021;2020:1–2.
- Bezerra TM, Feitosa SG, Carneiro DTO, Costa FWG, Pires FR, Pereira KMA. Oral lesions in COVID‐19 infection: is long‐term follow‐up important in the affected patients? Oral Dis. 2020;28:2570–2571
- Rodríguez MD, Romera AJ, Villarroel M. Oral manifestations associated with COVID‐19. Oral Dis. 2020;28:960.
- Soares C, Carvalho RA, Carvalho KA, Carvalho MG, Almeida O. Letter to Editor: oral lesions in a patient with Covid-19. Med Oral Patol Oral Cir Bucal. 2020;25(4):e563–4. doi:10.4317/medoral.24044
- Riad A, Gad A, Hockova B, Klugar M. Oral candidiasis in non‐severe COVID‐19 patients: call for antibiotic stewardship. Oral Surg. 2020;15:465.
- Aranda Romo S, Rizo VHT, Noyola Cherpitel DE, et al. Oral lesions as the only signs of recurrent SARS‐CoV‐2 infection. Oral Dis. 2022;28(S2):2614–2615. doi:10.1111/odi.14098
- Soares CD, Mosqueda-Taylor A, de Carvalho MGF, de Almeida OP. Oral vesiculobullous lesions as an early sign of COVID-19: immunohistochemical detection of SARS-CoV-2 spike protein. Br J Dermatol. 2021;184(1):e6. doi:10.1111/bjd.19569
- Aghazadeh N, Homayouni M, Sartori‐Valinotti JC. Oral vesicles and acral erythema: report of a cutaneous manifestation of COVID‐19. Int J Dermatol. 2020;2020:15047.
- Dilsiz A, Parlak E, Gül SS. Oral and ocular manifestations in a patient with coronavirus disease-2019: clinical presentation and management. Rev Soc Bras Med Trop. 2022;55:e06992021. doi:10.1590/0037-8682-0699-2021
- Casu C, Orrù G. Tongue papillitis and volatile sulfur compounds (VSC) values in a COVID-19 patient. Pan Afrn Medl J. 2022;41. doi:10.11604/pamj.2022.41.5.28915
- Sweet S, Moayedi S, Torres M. Oral aphthous ulcers associated with COVID-19. Vis J Emerg Med. 2022;29:101423. doi:10.1016/j.visj.2022.101423
- Muñoz-Corcuera M, Esparza-Gómez G, González-Moles MA, Bascones-Martínez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part I. Acute ulcers. Clin Exp Dermatol. 2009;34(3):289–294. doi:10.1111/j.1365-2230.2009.03220.x
- Liu D, Yuan X, Gao F, et al. High number and specific comorbidities could impact the immune response in COVID-19 Patients. Front Immunol. 2022;13:899930.
- Ergun T, Ergenç İ, Seven S, et al. Drug eruption: a mimicker of Coronavirus disease-2019 rash. Turkderm Turk Arch Dermatol Venereol. 2022;56(1):34–38.
- Mohammad Zadeh N, Mashinchi Asl NS, Forouharnejad K, et al. Mechanism and adverse effects of COVID-19 drugs: a basic review. Int J Physiol Pathophysiol Pharmacol. 2021;13(4):102–109.
- Bennardo L, Nisticò SP, Dastoli S, et al. Erythema multiforme and covid‐19: what do we know? Medicina. 2021;57(8):2–7.
- Bouabdella S, Benkaraache M, Almheirat Y, Zizi N, Dikhaye S. Erythema multiforme eruption due to SARS-COV 2: case report. Ann Med Surg. 2021;68(June):102591. doi:10.1016/j.amsu.2021.102591
- Etaee F, Eftekharian M, Naguib T, Daveluy S. Erythema multiforme inCOVID-19 patients and following COVID-19 vaccination: manifestations, associations and outcomes. J Eur Acad Dermatol Venereol. 2022;36(7):e522–4. doi:10.1111/jdv.18063
- Zarpellon A, Matuck BF, Dolhnikoff M, et al. Oral lesions and SARS‐CoV‐2: a postmortem study. Oral Dis. 2022;28(S2):2551–2555. doi:10.1111/odi.14047


