Abstract
Lemierre syndrome (LS) is a rare and life-threatening condition predominantly caused by Fusobacterium necrophorum. Currently, there are no standardized clinical guidelines for LS management. Here, we describe the case of a 40-year-old male with fever, productive cough, and dyspnea but no sore throat. Diagnostic radiological examinations revealed multiple pulmonary cavitary nodules and an internal jugular vein occlusion. Metagenomic Next-Generation Sequencing (mNGS) of the alveolar lavage fluid identified Fusobacterium necrophorum, thereby confirming the diagnosis of LS. Intriguingly, the patient exhibited a delayed clinical response despite receiving the appropriate antibiotic. After integrating tigecycline into the treatment to address potential co-infecting bacteria, we observed a marked improvement in his clinical symptoms. Subsequent follow-up over 12 weeks post-discharge revealed complete alleviation of symptoms, and a chest CT scan showed marked regression of the lung lesions.
Introduction
Lemierre syndrome (LS), originally described by Prof. Lemierre in 1936, is characterized by septic thrombophlebitis, predominantly affecting the internal jugular vein, which subsequently embolizes and causes peripheral septic lesions such as metastatic lung abscesses.Citation1 The predominant causative agent of LS is Fusobacterium necrophorum, a gram-negative anaerobic bacillus frequently located in the oropharyngeal region.Citation2 Predominantly affecting young and healthy individuals, LS has an annual incidence of 14.4 cases per million among adolescents aged 15–24.Citation3,Citation4 Notably, it has been hypothesized that the incidence of LS may have risen over recent decades possibly owing to antibiotic resistance, increased syndrome awareness, and fewer tonsillectomies.Citation5
However, not all LS cases present typical clinical symptoms, which can result in misdiagnoses. Patients with LS have a higher risk of thromboembolic complications and mortality.Citation6 Timely recognition and standard antibiotic regimens are vital for improving patient prognosis. The primary treatment modalities for LS comprise antibiotic and anticoagulant therapies. We detail a case where a prompt resolution of clinical symptoms was challenging even with adherence to a standardized antibiotic regimen.
Case Presentation
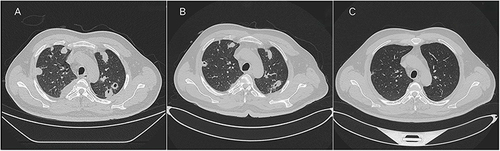
A 40-year-old male was admitted to the emergency room with an 8-day history of fever, productive cough, and dyspnea but without a sore throat. He reported no pharyngeal pain prior to admission and had not undergone any recent dental procedures. Physical examination revealed bilateral coarse breath sounds with scattered rales and edema in the lower extremities. The oropharyngeal examination was unremarkable. Laboratory analysis showed a white cell count of 20.31×10^9 cells/L with 87.7% neutrophils. Procalcitonin was 44.91 ng/mL, C-reactive protein was 54.40 mg/L, interleukin 6 was 117.20 pg/mL, thrombocytopenia (platelet count of 17×10^9/L) and D-Dimer concentration was 9.12mg/L. Immediate radiologic assessment included a chest CT revealing multiple cavitated nodules and moderate pleural effusion (). Empirical treatment with meropenem and moxifloxacin was initiated, alongside chest drainage and admission to the hospital ward for further management.
Figure 1 (A) Chest CT upon admission reveals scattered nodules and encapsulated pleural effusion in both lungs. (B) After a 10-day course of oral ceftriaxone and metronidazole, chest CT indicates a partial reduction of the pulmonary lesions. (C) Subsequent chest CT at 12 weeks post-antibiotic treatment, demonstrating significant and sustained regression of pulmonary lesions.
Three days later, the blood cultures yielded growth of both gram-negative rods and gram-positive cocci. This prompted an adjustment in the antibiotic regimen, transitioning to vancomycin and imipenem. The patient’s lower extremity edema persisted asymmetrically, despite no significant findings on lower extremity venous ultrasonography. Laboratory tests showed an increased platelet count of 452 × 10^9/L and a further elevated D-dimer concentration of 12.3 mg/L. Given these laboratory changes, prophylactic anticoagulation with enoxaparin (4000 AXaIU/day) was commenced to prevent possible thrombosis. Nonetheless, the patient continued to experience febrile episodes despite broad-spectrum antibiotic coverage.
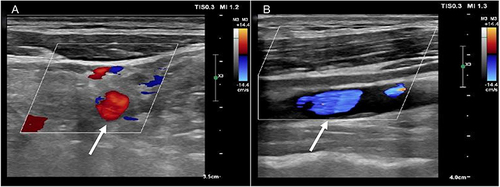
Nine days later, metagenomic sequencing of bronchoalveolar lavage fluid identified Fusobacterium necrophorum as the predominant pathogenic microorganism with a sequence count of 5097. Subsequent ultrasonographic examinations, including jugular venous and transesophageal cardiac ultrasounds, revealed patent flow in the proximal portion of the left internal jugular vein () and occlusion in the middle and distal portions (), without notable cardiac abnormalities. These findings confirmed the diagnosis of Lemierre syndrome. Given the patient’s known allergy to piperacillin/tazobactam, the antibiotic course was adjusted to ceftriaxone (2g/day) and metronidazole (1.5g/day). Additionally, the dose of enoxaparin was increased to 8000 AXaIU per day. Despite the antibiotic change, the fever persisted intermittently, and inflammatory markers remained elevated (CRP 94.10 mg/L, interleukin 6 43.60 pg/mL, procalcitonin 0.12 ng/mL). A repeat chest CT demonstrated a partial resolution of pulmonary lesions (). Adding tigecycline to the treatment protocol resulted in significant clinical improvement and the cessation of fever. Discharged on day 26 with a regimen of oral metronidazole and amoxicillin-clavulanate, the patient had a follow-up chest CT one month later that showed insufficient improvement of the lung lesions. Consequently, the antibiotic therapy was extended due to the continued symptoms and suboptimal lesion resolution. By the end of the 12-week antibiotic course, two months post-discharge, the patient was symptom-free, and imaging revealed substantial regression of the lung abscess ().
Discussion
The classic triad of Lemierre syndrome is pharyngotonsillitis, internal jugular vein thrombosis, and metastatic abscess.Citation7 It is essential to note that these symptoms are characteristic but not universal.Citation8 This variability can pose challenges to early detection and intervention. The diagnostic approach usually integrates various tools like Doppler ultrasound of the internal jugular vein, blood cultures, and chest imaging. Occasionally, there’s a need for more advanced or invasive measures.Citation9 In the case we discussed, the mNGS of the alveolar lavage fluid provided clarity regarding the LS diagnosis. Therefore, in instances where diagnosis remains elusive, techniques like sampling from embolic locations and leveraging mNGS on alveolar lavage fluid, especially in cases where cultures are negative, can prove invaluable.Citation10
There is no standardized treatment consensus for LS, but the primary approach often involves antibiotics and anticoagulation. Fusobacterium necrophorum exhibits susceptibility to penicillin, amoxicillin, imipenem, and clindamycin.Citation11 Given that some strains of Fusobacterium necrophorum and potential co-infecting pathogens might exhibit beta-lactamase production, recommendations strongly advocate incorporating beta-lactamase inhibitors into the LS antibiotic regimen.Citation12 Initial treatment strategy typically commences with broad-spectrum antibiotics, which are then switched to a pathogen-specific regimen following diagnostic results. Carbapenems or piperacillin/tazobactam in combination with metronidazole is currently recommended.Citation13 For patients with beta-lactam allergies, combining empiric quinolones with metronidazole stands as a viable alternative.Citation14 Antibiotic therapy for LS typically spans between 3–6 weeks. For those who respond well within the initial 2 weeks of IV treatment, transitioning to oral therapy is an option to consider.Citation9,Citation15 In the case we present, severe infection was evident based on clinical evaluation and diagnostic tests. Adhering to our institution’s protocol for managing severe respiratory infections, we initiated broad-spectrum antibiotics, specifically meropenem and moxifloxacin, to cover a diverse range of pathogens. This empirical treatment was later refined based on pathogen identification. Nevertheless, 10 days into the antibiotic regimen, the patient did not exhibit marked improvement, persisting with intermittent fever. Prior case reports have suggested delayed clinical responses despite adequate antibiotic therapy, partly attributed to the intravascular nature of the infection hindering antibiotic penetration into fibrin clots.Citation12 Drawing from this knowledge, we speculate that challenges such as insufficient antibiotic access to lung abscess fibrin clots, bacterial toxin release, and co-infecting bacterial infections could have been contributing factors. Introducing tigecycline to the treatment proved transformative, leading to the alleviation of the patient’s symptoms. This case required an extended antibiotic treatment duration of 12 weeks. Consequently, when clinicians encounter a suboptimal response to antibiotics in treating LS, they should be proactive, considering the abovementioned factors and adjusting the treatment strategy accordingly. This might involve changing the antibiotic regimen and considering surgical intervention for abscess drainage, among other strategies. The duration of antibiotic therapy for LS should be flexible and tailored according to the disease’s severity and the patient’s progress in follow-ups.
Anticoagulation’s role in LS remains debated. While some advocate for its beneficial effects in resolving infections, others argue that clot formation is merely a consequence of the infection and will naturally resolve once it is addressed.Citation16 Numerous studies and meta-analyses have examined the effects of anticoagulation on revascularization and mortality in LS patients. The effect of anticoagulation may be limited to a slight improvement of early in-hospital complications, or may only be found in patients with extensive or bilateral thrombotic extension at the time of diagnosis, although the latter needs to be proved by future studies.Citation17 In the case presented, the patient underwent a complete course of anticoagulation without any associated complications. It’s vital to note that anticoagulation should be considered without contraindications or perceived risks and following clinical anticoagulation guidelines.
Conclusion
Lemierre’s syndrome (LS) is a rare condition with often delayed diagnosis due to limited clinician awareness. Some more advanced or invasive techniques, such as embolic lesion sampling and mNGS of alveolar lavage fluid, enhance early detection. Recommended antibiotic regimens for LS often include piperacillin/tazobactam with metronidazole. If the patients display a delayed clinical response, longer intravenous antibiotics, a reassessment of antibiotic regimens for co-infections, and surgical interventions may be considered. For LS patients without specific contraindications, the inclusion of anticoagulation therapy is generally endorsed.
Statement of Ethics
Compliance with the CARE guidelines was ensured for this case report, and ethical approval was not required as per national regulations. Written informed consent for publication was secured from the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
Data Sharing Statement
Data from this study are available from either of the corresponding authors upon justified request.
Additional information
Funding
References
- Lemierre A. On certain septicæmias due to anaerobic organisms. Lancet. 1936;227(5874):701–703. doi:10.1016/S0140-6736(00)57035-4
- Citron DM. Update on the taxonomy and clinical aspects of the genus fusobacterium. Clin Infect Dis. 2002;35(Suppl 1):S22–S27. doi:10.1086/341916
- Hagelskjaer Kristensen L, Prag J. Lemierre’s syndrome and other disseminated Fusobacterium necrophorum infections in Denmark: a prospective epidemiological and clinical survey. Eur J Clin Microbiol Infect Dis. 2008;27(9):779–789. doi:10.1007/s10096-008-0496-4
- Nygren D, Holm K. Invasive infections with Fusobacterium necrophorum including Lemierre’s syndrome: an 8-year Swedish nationwide retrospective study. Clin Microbiol Infect. 2020;26(8):1089.e7–1089.e12. doi:10.1016/j.cmi.2019.12.002
- Lu MD, Vasavada Z, Tanner C. Lemierre syndrome following oropharyngeal infection: a case series. J Am Board Fam Med. 2009;22(1):79–83. doi:10.3122/jabfm.2009.01.070247
- Valerio L, Zane F, Sacco C, et al. Patients with Lemierre syndrome have a high risk of new thromboembolic complications, clinical sequelae and death: an analysis of 712 cases. J Intern Med. 2021;289(3):325–339. doi:10.1111/joim.13114
- Jones C, Siva TM, Seymour FK, O’Reilly BJ. Lemierre’s syndrome presenting with peritonsillar abscess and VIth cranial nerve palsy. J Laryngol Otol. 2006;120(6):502–504. doi:10.1017/S002221510600034X
- Shen MF, Chen HJ. Unilateral proptosis in Lemierre’s syndrome variant. Thorax. 2020;75(9):815–816. doi:10.1136/thoraxjnl-2020-214748
- Osowicki J, Kapur S, Phuong LK, Dobson S. The long shadow of lemierre’s syndrome. J Infect. 2017;74(Suppl 1):S47–S53. doi:10.1016/S0163-4453(17)30191-3
- Li N, Cai Q, Miao Q, Song Z, Fang Y, Hu B. High-throughput metagenomics for identification of pathogens in the clinical settings. Small Methods. 2021;5(1):2000792. doi:10.1002/smtd.202000792
- Klasinc R, Lupyr K, Zeller I, et al. Clinical characteristics of a large cohort of patients with positive culture of Fusobacterium necrophorum. GMS Infect Dis. 2018;6:Doc03. doi:10.3205/id000038
- Lee WS, Jean SS, Chen FL, Hsieh SM, Hsueh PR. Lemierre’s syndrome: a forgotten and re-emerging infection. J Microbiol Immunol Infect. 2020;53(4):513–517. doi:10.1016/j.jmii.2020.03.027
- Johannesen KM, Bodtger U. Lemierre’s syndrome: current perspectives on diagnosis and management. Infect Drug Resist. 2016;9:221–227. doi:10.2147/IDR.S95050
- Moretti M, De Geyter D, Goethal L, Allard SD. Lemierre’s syndrome in adulthood, a case report and systematic review. Acta Clin Belg. 2021;76(4):324–334. doi:10.1080/17843286.2020.1731661
- Riordan T. Human infection with Fusobacterium necrophorum (Necrobacillosis), with a focus on Lemierre’s syndrome. Clin Microbiol Rev. 2007;20(4):622–659. doi:10.1128/CMR.00011-07
- Ge J, Zhou P, Yang Y, Xu T, Yang X. Anticoagulation may contribute to antimicrobial treatment of Lemierre syndrome: a case report. Thromb J. 2021;19(1):80. doi:10.1186/s12959-021-00336-0
- Valerio L, Pleming W, Pecci A, Barco S. Management of Lemierre Syndrome. Minerva Med. 2021;112(6):726–739. doi:10.23736/S0026-4806.21.07497-8