Abstract
Background
Adverse reactions induced by isoosmolar contrast medium (iodixanol) are mostly mild, with rashes and headaches being the most common. Although anaphylactic shock has been reported, no related incidents have been documented on cerebral angiography.
Objective
This article reports a serious case of anaphylactic shock possibly induced by iodixanol and provides an overview of the case report.
Case Summary
A 65-year-old female with persistent headaches for nearly six months and CTA examination revealed multiple intracranial aneurysms. After two treatments, she returned to the hospital for aneurysm of reexamination a month ago. Following a preoperative assessment, cerebral angiography was performed. Three minutes after the procedure, the patient experienced dizziness, increased heart rate, followed by hypotension (BP 90/43 mm Hg), a sudden drop-in heart rate (HR 68 bpm), and a drop in SpO2 to 92%. Intravenous dexamethasone for anti-allergic were administered immediately, along with therapy through oxygen-inhalation. However, the patient then developed limb convulsions, unresponsiveness, and was urgently given diazepam for sedation and sputum aspiration to maintain airway patency. Blood pressure decrease to 53/29 mm Hg, and SpO2 readings were unavailable. Intravenous dopamine to elevates blood pressure, and assists breathing by intubating in the endotracheal. After 3 minutes, as the blood pressure remained undetectable, intermittent intravenous epinephrine 1mg was administered to raise the blood pressure, gradually restoring it to 126/90 mm Hg, and SpO2 increased to 95%. The patient was diagnosed with iodixanol-induced anaphylactic shock and urgently transferred to the NICU for monitoring and treatment. The patient died despite immediate treatment.
Conclusion
A 65-year-old female developed serious anaphylactic shock during cerebral angiography after receiving iodixanol. Although iodixanol is considered one of the safest iodinated contrast mediums (ICM), clinicians should be aware of its the potential for serious hypersensitivity reactions that can lead to fatal and life-threatening events.
Introduction
Cerebral angiography, an X-ray examination method, involves the injection of iodinated contrast mediums (ICM) into the carotid or vertebral arteries, contributing to the visualization of cerebral blood vessels and the diagnosis of conditions such as intracranial aneurysms, vascular malformations, and craniocerebral space-occupying lesions.Citation1 Currently, the common approach for cerebral angiography involves catheterization through the transradial approach and transfemoral approach.Citation2 Meanwhile, ICM used in cerebral angiography are categorized as ionic, nonionic monomeric, and nonionic dimeric compounds.Citation3,Citation4 Among them, nonionic monomeric mediums, with significantly lower osmolality compared to ionic contrast mediums but still approximately twice that of plasma, are termed low-osmolar contrast mediums. These included iohexol, iopromide, iopamidol, iopentol, ioversol, and iomeprol.Citation3,Citation5,Citation6 Nonionic dimeric agents, also known as iso-osmolar contrast mediums, are represented as iodixanol.Citation7 Iodixanol, characterized by its non-disassociation, neutral charge, and isotonicity with plasma, exhibits a lower incidence of adverse reactions and a reduced risk of contrast-induced nephropathy in high-risk patients compare to other ICM.Citation7,Citation8
In recent years, as the use of iodixanol has become more widespread, reports of related adverse reactions have gradually increased, although most reactions remain minor.Citation9 Among these, rash, headache and other hypersensitivity reactions are the most prevalent.Citation10,Citation11 However, adverse events that are life-threatening, such as anaphylactic shock, can occur after the use of ICM. In addition, acute adverse reactions, including nausea, urticaria, bronchospasm, laryngeal edema, and hypotension, generally occur within 1 h of ICM administration. Skin testing is considered the best diagnostic method for determining hypersensitivity reactions to ICM, while administration of adrenaline is typically the preferred treatment for managing related adverse events caused by ICM. However, some severe complications and life-threatening events cannot be completely treated or prevented.Citation12,Citation13 Anaphylactic shock induced by iodixanol in clinical applications is an extremely rare adverse reaction.Citation14 Although preventive measures such as antihistamines, corticosteroids, and epinephrine have been employed,Citation15 but individual variations in hypersensitivity reactions are significant, and preventive measures cannot guarantee complete prevention. Furthermore, currently, there are no specific guidelines for preventing hypersensitivity reactions to ICM in our country.Citation16 Additionally, to date, there have been only a few cases of iodixanol-induced anaphylactic shock have been reported, with no instances documented during cerebral angiography.
This report presents a case of severe anaphylactic shock induced by iodixanol, which was characterized by profound hypotension, metabolic acidosis, respiratory failure, hyperkalemia, and cardiac arrest. This case report describes the potential of iodixanol to induce serious hypersensitivity reactions during cerebral angiography.
Case Presentation
A 65-year-old female was admitted to our hospital in February 2023 due to “persistent headache for six months” and CTA revealed multiple intracranial aneurysms. Subsequently, a digital subtraction angiography (DSA) was performed to identify an aneurysm at the terminal bifurcation of the left middle cerebral artery (M1 segment), left posterior communicating artery aneurysm, left ophthalmic artery aneurysm, right posterior communicating artery aneurysm, and right ophthalmic artery aneurysm. During the injection of Iohexol, a rash appeared on the skin, which quickly resolved with antiallergic treatment. The patient reported no discomfort upon discharge. In April 2023, the patient returned for treatment of the right posterior communicating artery aneurysm and ophthalmic artery aneurysm. And no hypersensitivity reaction occurred with iodixanol injection during the operation. A month ago, she was readmitted for reexamination of the embolization status of the aneurysms.
During the cerebral angiography procedure, approximately 25 mL of iodixanol (Yangtze River pharmaceutical group, Batch No: 23070461) was injected through a 5F Pigtail catheter for angiography of aortic arch. After completing the aortic arch angiography, a 5F VER 135° single-curve catheter was used for cervical-vertebral angiography. Approximately 3 mins after the injection of iodixanol into the ascending aorta through the 5F single-curve catheter via the femoral artery sheath, the patient experienced dizziness, increased heart rate (HR 120 bpm), followed by hypotension (BP 90/43 mm Hg), a sudden drop-in heart rate (HR 68 bpm), and SpO2 decreased to 92%. Intravenous dexamethasone (10 mg) was immediately administered for antiallergic treatment, oxygen therapy via a mask for oxygen-inhalation, accelerated fluid infusion and other anti-shock treatment measures. However, the patient subsequently experienced tics of the limbs, foaming in the mouth and unresponsiveness, emergency administration of intravenous diazepam (5 mg) for sedation, and aspiration of sputum to maintain airway patency. At this point, the blood pressure continued to drop to 53/29 mm Hg, and no SpO2 reading was detected. Intravenous drip saline was accelerated, and dopamine 20 mg was administered to increase the blood pressure. Endotracheal intubation and mechanical ventilation were initiated to assist breathing. After ~3 mins, no blood pressure readings were obtained, and intermittent intravenous boluses of epinephrine (1 mg) were administered immediately to raise the blood pressure (total of 4 mg). The blood pressure gradually recovered to 126/90 mm Hg, and SpO2 gradually increased to 95%. However, the patient had a Glasgow Coma Scale (GCS) score of 3, bilateral dilated pupils with a diameter of approximately 5 mm, no light responsiveness, and no reaction to painful stimuli was observed. The patient was urgently transferred to the NICU for further monitoring and treatment.
The patient remained in a deep coma state with bilateral fixed dilated pupils measuring approximately 5 mm. Spontaneous breathing was not observed, and cardiopulmonary resuscitation was initiated to actively revive the patient’s heartbeat. However, there was still no spontaneous breathing observed (the concentration of K+ dropped to 2.8 mol/L). After continuing with antiallergic, anti-shock, and correction of internal environmental disturbances, the patient was transferred to the Intensive Care Unit (ICU) for further treatment.
After being transfer to the ICU, the patient remained in a deep coma state with bilateral dilated pupils measuring approximately 3.5 mm. She had a delayed response to light reflex and shallow breathing. The patient was supported with ventilator (FiO2, 90%) to maintain SpO2 above 90%. Blood pressure and heart rate were relatively stable after intravenous of norepinephrine (10 mg) and epinephrine (2 mg), with HR was 102 bpm and BP was 108/63 mm Hg. Continuous mild hypothermic brain protection therapy was administered. The patient experienced extensive subcutaneous bleeding in the lower limbs, coarse breath sounds in the lungs, dull heart sounds in the apex, absent bowel sounds, low muscle tone in the limbs, and no lower limb edema. Analysis of arterial gas indicated: pH 7.38, PCO2 20.63 mmHg, PO2 86.71 mmHg, K+ 5.04 mmol/L, Na+ 155 mmol/L, Ca2+ 0.84 mmol/L, Lac 17.60 mmol/L. Coagulation tests showed: INR 1.75, PT 19.40 s, Fbg 0.78 g/L, TT 87.60 s, APTT 55.90 s (Key values of coagulation tests can be found in ). The patient had abnormal coagulation function and received plasma to correct coagulation, the posterior pituitary (36U/50 mL) and carbazochrome sodium sulfonate (80 mg/250 mL) for hemostasis, omeprazole (80 mg/250 mL) to suppress acid, antiallergic and anti-shock treatment, and correction of internal environment disturbances. Laboratory evaluations revealed the white blood cell count of 19.01×10^9/L, with 94.9% being neutrophils, and TNT-HSST was 2210.00 ng/L (Changes in white blood cells and neutrophil percentages can be found in ). The alanine aminotransferase (ALT), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) levels were 317 U/L (normal 7~40 U/L), 562 U/L (normal 13~35 U/L), and 1788 U/L (normal 109~245 U/L), respectively. IL-6 was 9168.38 pg/mL and procalcitonin (PCT) level was 49.71 ng/mL, prompting timely continuous renal replacement therapy (CRRT). Afterwards, the patient’s condition deteriorated further (PH 6.9, K+ 6.72 mmol/L, AST 953 U/L, ALT 1580 U/L, SCR 350 μmol/L), with loss of light reflex and no response to painful stimuli in the limbs. And the patient’s severe metabolic acidosis, persistent hyperlactacidemia, and severe internal environmental disturbances could not be corrected, leading to continuous deterioration (key indicators of blood gas analysis, electrolyte levels, and liver and kidney function tests are shown in ). Finally, the patient was declared dead due to rescue invalid as a result of anaphylactic shock, disseminated intravascular coagulation, and multiple organ dysfunction.
Figure 1 The changes in coagulation test results in the patient during before and after the occurrence of the allergic reaction. (A) Changes in international normalized ratio (INR); (B) Changes in activated partial thromboplastin time (APTT); (C) Changes in thrombin time (TT); (D) Changes in fibrinogen levels. Negative values represent the time before the allergic reaction, while positive values represent the time after the allergic reaction.
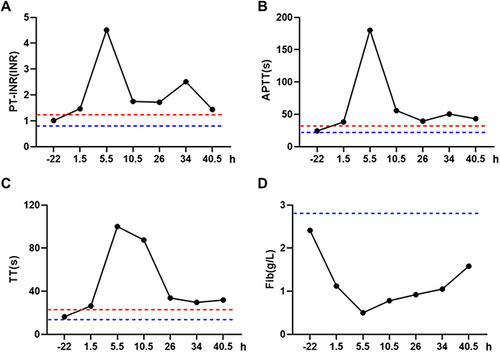
Figure 2 The changes in white blood cell count (A) and percentage of neutrophils (B) in the patient during the before and after the occurrence of the allergic reaction. Negative values represent the time before the allergic reaction, while positive values represent the time after the allergic reaction.
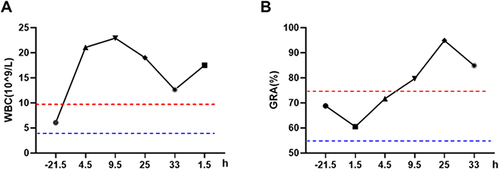
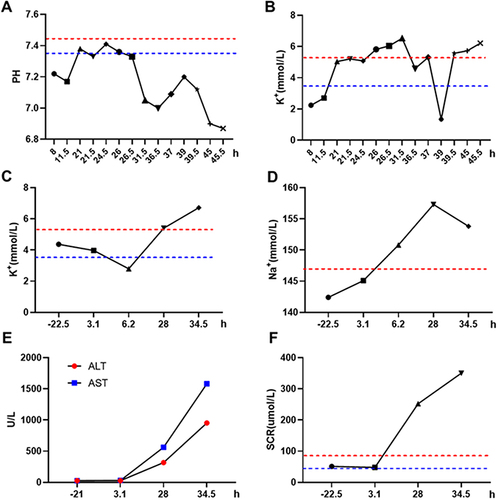
Figure 3 The changes in pH level (A) and K+ concentration (B) measured by blood gas analysis in the ICU after the occurrence of an allergic reaction of the patient. The changes in electrolyte detection of K+ (C) and Na+ (D) in the patient during the before and after the occurrence of the allergic reaction; The change of alanine aminotransferase (ALT) and aspartate transaminase aminotransferase (AST) in liver function (E) and creatinine (SCR) in renal function (F) was measured in the patient during the before and after the occurrence of the allergic reaction. Negative values represent the time before the allergic reaction, while positive values represent the time after the allergic reaction.
Discussion
Drug (including ICM)-induced hypersensitivity is a type of hyperacute reaction that affects multiple organs,Citation17 and it is a rapidly developing, acute, and life-threatening reaction.Citation18 According to the mechanism defined by the hypersensitivity reaction model, drug-induced hypersensitivity reactions can be divided into IgE or IgG-mediated allergies,Citation17 where IgE-mediated hypersensitivity reactions are generally considered as acute and life-threatening systemic allergic reactions.Citation19 Mast cells and eosinophils play important roles in hypersensitivity reactions activated by IgE.Citation20 As effector cells in IgE-mediated allergic inflammatory diseases, mast cells typically cause hypersensitivity reactions through degranulation and release of pro-inflammatory mediators after antigen crosslinking with the IgE receptor FcεRI.Citation21,Citation22 Eosinophils, which account for less than 1% of peripheral blood leukocytes, are prone to produce a large amounts of T helper 2 (Th2) cell cytokines and play a critical role in the development of IgE-mediated chronic allergic inflammation. They act as initiators rather than effectors.Citation23 It is worth noting that eosinophils also play a crucial role in IgG-mediated allergies.Citation24 Anaphylactic shock is the most severe and life-threatening allergic reaction, and although adrenaline can alleviate life-threatening symptoms of hypersensitivity reactions,Citation25 severe anaphylactic shock may develop resistance to adrenaline and ultimately result in patient death.Citation26
The main causes of hypersensitivity reactions are drugs, including ICM, and immediate reactions and non-immediate or delayed reactions caused by ICM are relatively common.Citation27 Research has shown that 37.26% of adverse reactions to ICM occur within 5 mins, and 84.08% occur within 30 mins.Citation28 Immediate reactions are generally associated with severe hypersensitivity reactions.Citation29 Although research has specifically indicated the safety and good early tolerability of iodixanol, with most patients experience mild or no discomfort during its administration.Citation9,Citation10 However, investigations have shown that iodixanol mostly causes immediate reactions, with an overall confirmation rate for delayed reactions of only 15%.Citation30 Therefore, recently reported severe anaphylactic shock caused by iodixanol (including this case) is an immediate reaction, characterized by a short occurrence time, severe symptoms, and even death.Citation12 In general, life-threatening immediate reactions are mediated by mast cells binding to high-affinity IgE receptor FcεRI,Citation31 but some studies suggest that only a few cases reported clinically severe hypersensitivity reactions are IgE-mediated type I immediate reactions.Citation32 Currently, measuring serum total mast cell tryptase is the gold standard for laboratory detection of acute-phase allergies.Citation33 Unfortunately, the tryptase levels were not measured in this study. However, mast cells are key participants in allergic diseases and their activation through the high-affinity IgE receptor FcεRI leads to serious inflammatory reactions.Citation34 Combined with the patient’s history of allergy to iohexol and previous use of iodixanol, the occurrence of severe hypotension and shock during the hypersensitivity reaction, with IL-6 9168.38 pg/mL, the patient also fits the characteristics of IgE-mediated type I immediate reactions.
It is worth noting that the mechanism underlying the severe hypotension accompanying severe anaphylactic shock has not yet been elucidated, but there are measures that can reduce the incidence of hypersensitivity reactions. In particular, in the reported case, the patient had a history of allergy to iohexol, and although there was no hypersensitivity reaction during the second use of iodixanol, taking certain preventive measures can greatly reduce the occurrence of severe medical accidents. For example, preheating the contrast media is useful in avoiding adverse reactions,Citation35 and skin testing is useful for diagnosing ICM allergies, selecting safe ICM, and preventing the recurrence of hypersensitivity reactions caused by the same ICM,Citation27 particularly in relation to immediate reactions associated with severe reactions caused by ICM, which are often confirmed by positive skin test results.Citation29 Especially for patients with a history of ICM allergy, studies have used graded dosing protocols, administering 1% of the total expected dose 1 h before surgery, an additional 10% 30 mins before surgery, followed by the final injection in the operating room, with no adverse reactions occurring specifically.Citation36 These data, based on allergy history and skin testing, are effective in preventing the recurrence of contrast media-induced hypersensitivity reactions. However, further prospective research is necessary to ensure patient safety.Citation37 In addition, it is important to thoroughly assess the patient’s allergy history and contraindications before using ICM. The examination room should also be equipped with emergency medications such as adrenaline, H1 histamine receptor blockers, dexamethasone, atropine, saline or Ringer’s solution, and antiepileptic drugs (such as diazepam). It is also necessary to establish an emergency reinforcing mechanism in collaboration with the emergency department or other relevant clinical departments for the rescue of adverse reactions to iodinated contrast mediums, ensuring that in the event of an adverse reaction, clinical physicians can promptly arrive at the scene for rescue if needed.
Conclusion
Our report suggests that even iso-osmolar, non-ionic, the iodixanol can induce severe anaphylactic shock, which is difficult to predict and prevent. For patients who have received iodinated contrast mediums multiple times, closer monitoring of their condition is warranted. Once a patient experiences anaphylactic shock, early diagnosis and timely, standardized salvage therapies should be implemented. Although skin testing can help detect hypersensitivity reactions mediated by iodinated contrast mediums, doctors should not rely solely on the results of skin test results. Before using iodinated contrast mediums, healthcare personnel should be fully prepared for any potential adverse reactions and be ready to respond to emergency situations.
Consent for Publication
Written informed consent was obtained from the daughter of the deceased patient for the publication of any data included in this work. The case details are open access and can be browsed without institutional approval.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, made diagrams, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to take responsibility and be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- Sahoo A, Abdalkader M, Saatci I, et al. History of Neurointervention. Semi Neurol. 2023;43(03):454–465. doi:10.1055/s-0043-1771455
- Batista S, Oliveira LB, Diniz JBC, et al. Transradial versus transfemoral approach in cerebral angiography: a meta-analysis. Inter Neuroradiol. 2023;2:1.
- Muschick P, Wehrmann D, Schuhmann-Giampieri G, Krause W. Cardiac and hemodynamic tolerability of iodinated contrast media in the anesthetized rat. Invest Radiol. 1995;30(12):745–753. doi:10.1097/00004424-199512000-00009
- Costa N. Understanding contrast media. J Infus Nurs. 2004;27(5):302–312. doi:10.1097/00129804-200409000-00004
- Jost G, Lengsfeld P, Lenhard DC, Pietsch H, Hütter J, Sieber MA. Viscosity of iodinated contrast agents during renal excretion. Eur. J. Radiol. 2011;80(2):373–377. doi:10.1016/j.ejrad.2011.02.003
- D D’Avella RC, Albiero F, Piscitelli G, et al. Effect of intracarotid injection of iopamidol on local cerebral glucose utilization in rat brain. AJNR Am J Neuroradiol. 1989;10(4):797–801.
- Aspelin P, Aubry P, Fransson S-G, Strasser R, Willenbrock R, Berg KJ. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348(6):491–499. doi:10.1056/NEJMoa021833
- Häussler MD. Safety and patient comfort with iodixanol: a postmarketing surveillance study in 9515 patients undergoing diagnostic CT examinations. Acta Radiol. 2010;51(8):924–933. doi:10.3109/02841851.2010.504739
- Zhang BC, Hou L, Lv B, Xu YW. Post-marketing surveillance study with iodixanol in 20 185 Chinese patients from routine clinical practices. Br J Radiol. 2014;87(1034):1034. doi:10.1259/bjr.20130325
- Gharekhanloo F, Torabian S. Comparison of allergic adverse effects and contrast enhancement between iodixanol and iopromide. Iran J Radiol. 2012;9(2):63–66. doi:10.5812/iranjradiol.7696
- Poirier VC, Hecht ST, Nemzek WR, Nemzek WR. Phase III clinical trial comparing iodixanol and iohexol in cerebral angiography. Acad Radiol. 1996;3:S495–S499. doi:10.1016/S1076-6332(05)80365-4
- Matthew S, Davenport RHC, Elaine M, James H. Repeat contrast medium reactions in premedicated patients: frequency and severity. Radiology. 2009;253(2):372–379. doi:10.1148/radiol.2532090465
- Adam N, Williams JMK. Radiocontrast-induced anaphylaxis despite pretreatment and use of iso-osmolar contrast. Ann Allergy Asthma Immunol. 2007;99(5):467–468. doi:10.1016/S1081-1206(10)60574-1
- Qiu L, Cui Q, Gong X, Zhou H. Anaphylaxis following contrast-enhanced CT with iodixanol: a case report and literature review. J Asthma Aller. 2023;16:195–200. doi:10.2147/JAA.S386811
- Messaad D, Sahla H, Benahmed S, Godard P, Bousquet J, Demoly P. Drug provocation tests in patients with a history suggesting an immediate drug hypersensitivity reaction. Ann Intern Med. 2004;140(12):1001–1006. doi:10.7326/0003-4819-140-12-200406150-00009
- Morcos SK, Thomsen HS, Webb JA. Prevention of generalized reactions to contrast media: a consensus report and guidelines. Eur Radiol. 2001;11(9):1720–1728. doi:10.1007/s003300000778
- Bruhns P, Chollet-Martin S. Mechanisms of human drug-induced anaphylaxis. J Allergy Clin Immunol. 2021;147(4):1133–1142. doi:10.1016/j.jaci.2021.02.013
- Cianferoni A. Non–IgE-mediated anaphylaxis. J Allergy Clin Immunol. 2021;147(4):1123–1131. doi:10.1016/j.jaci.2021.02.012
- Bao C, Chen O, Sheng H, et al. A mast cell–thermoregulatory neuron circuit axis regulates hypothermia in anaphylaxis. Sci Immunol. 2023;8(81):eadc9417. doi:10.1126/sciimmunol.adc9417
- Engeroff P, Plattner K, Storni F, et al. Glycan-specific IgG anti-IgE autoantibodies are protective against allergic anaphylaxis in a murine model. J Allergy Clin Immunol. 2021;147(4):1430–1441. doi:10.1016/j.jaci.2020.11.031
- Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T. Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol. 2007;9(1):81–88. doi:10.1038/ni1546
- Bansal G, Xie Z, Rao S, Nocka KH, Druey KM. Suppression of immunoglobulin E–mediated allergic responses by regulator of G protein signaling 13. Nat Immunol. 2007;9(1):73–80. doi:10.1038/ni1533
- Obata K, Mukai K, Tsujimura Y, et al. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110(3):913–920. doi:10.1182/blood-2007-01-068718
- Tsujimura Y, Obata K, Mukai K, et al. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28(4):581–589. doi:10.1016/j.immuni.2008.02.008
- Pennington LF, Gasser P, Brigger D, Guntern P, Eggel A, Jardetzky TS. Structure-guided design of ultrapotent disruptive IgE inhibitors to rapidly terminate acute allergic reactions. J Allergy Clin Immunol. 2021;148(4):1049–1060. doi:10.1016/j.jaci.2021.03.050
- Zimmer J, Kim M-H, Lee S-Y, et al. Anaphylaxis to iodinated contrast media: clinical characteristics related with development of anaphylactic shock. PLoS One. 2014;9(6). doi:10.1371/journal.pone.0108414
- Ahn Y-H, Koh Y-I, Kim J-H, et al. The Potential Utility of Iodinated Contrast Media (ICM) Skin Testing in Patients with ICM Hypersensitivity. J Korean Med Sci. 2015;30(3):245–251. doi:10.3346/jkms.2015.30.3.245
- Chen Q-L, Zhao X-Y, Wang X-M, et al. Retrospective analysis of non-laboratory-based adverse drug reactions induced by intravenous radiocontrast agents in a Joint Commission International-accredited academic medical center hospital in China. Therap Clin Risk Manage. 2017;13:565–573. doi:10.2147/TCRM.S134265
- Doña I, Bogas G, Salas M, et al. Hypersensitivity reactions to multiple iodinated contrast media. Front Pharmacol. 2020;11:575437. doi:10.3389/fphar.2020.575437
- Kvedariene V, Orvydaite M, Petraityte P, Rudyte J, Edvardas Tamosiunas A. Inherent clinical properties of non‐immediate hypersensitivity to iodinated contrast media. Int J Clin Pract. 2021;75(11):e14766. doi:10.1111/ijcp.14766
- Hitomi K, Tahara-Hanaoka S, Someya S, et al. An immunoglobulin-like receptor, Allergin-1, inhibits immunoglobulin E–mediated immediate hypersensitivity reactions. Nat Immunol. 2010;11(7):601–607. doi:10.1038/ni.1886
- Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Invest Allergol Clin Immunol. 2016;26(3):144–155. doi:10.18176/jiaci.0058
- Beck SC, Wilding T, Buka RJ, Baretto RL, Huissoon AP, Krishna MT. Biomarkers in human anaphylaxis: a critical appraisal of current evidence and perspectives. Front Immunol. 2019;10:10. doi:10.3389/fimmu.2019.00010
- Allen JD, Jaffer ZM, Park S-J, et al. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood. 2009;113(12):2695–2705. doi:10.1182/blood-2008-06-160861
- Kim S-R, Park KH, Hong YJ, Oh YT, Park J-W, Lee J-H. Intradermal testing with radiocontrast media to prevent recurrent adverse reactions. Am J Roentgenol. 2019;213(6):1187–1193. doi:10.2214/AJR.19.21547
- Soffer G, Cohen B, Toh J, Edelman D, Garg K, Jariwala S. Successful graded dose challenge to iodixanol radiocontrast media in a patient with delayed anaphylaxis to iohexol. Vascu Endovasc Surg. 2017;52(1):59–60. doi:10.1177/1538574417736420
- Lin X, Yang J, Weng L, Lin W. Differences in hypersensitivity reactions to iodinated contrast media: analysis of the us food and drug administration adverse event reporting system database. J Allergy Clin Immunol. 2023;11(5):1494–1502.e1496. doi:10.1016/j.jaip.2023.01.027

