The convergence of artificial intelligence (AI) and machine learning (ML) with modern medicine has not only opened up unprecedented opportunities for innovation but also gained recognition from prominent publications, including Nature and Science of Sleep, for their transformative potential in sleep medicine research and clinical practice. As such, Nature and Science of Sleep welcomes explorations into the applications and implications of AI in the field.
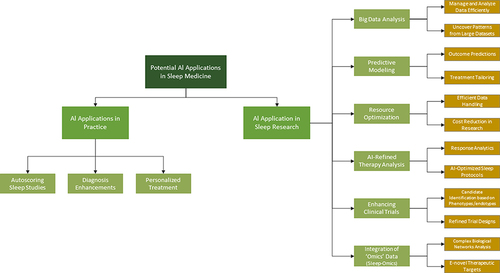
AI enables machines and software to perform tasks such as problem-solving, pattern recognition, learning, and understanding language, which traditionally required human cognition. Meanwhile, ML—a subset of AI—enhances these capabilities further with algorithms that learn and improve from data over time. The field of sleep medicine stands at the threshold of a transformative revolution, propelled by swift progress in AI and ML. Due to the inherently digital nature of data collected in this field, sleep medicine is uniquely positioned to harness AI and ML. Polysomnography, the gold standard diagnostic tool, produces extensive physiological data in digital formats such as EEG, ECG, EMG, and respiratory signals, making it ripe for AI analysis. This wealth of structured digital data makes sleep medicine an ideal candidate for the application of AI and ML algorithms, which excel at identifying complex patterns and relationships within large datasets. Additionally, the widespread availability of consumer sleep technologies, like wearables, expands these opportunities, allowing ML to extract novel insights from extensive real-world sleep data.Citation1,Citation2 This editorial aims to highlight the current uses of AI in sleep medicine and research, validation and ethical challenges, and the exciting future prospects. illustrates the breadth of potential AI applications in sleep medicine practice and research.
Automating Sleep Study Scoring
The application of AI to automate sleep study scoring was one of the earliest and most promising use cases in sleep medicine, primarily due to the inherently digital nature of collected data.Citation3 Manual scoring of sleep studies is both time-consuming and labor-intensive, and liable to inter-scorer variability. By contrast, ML algorithms trained on large datasets have demonstrated sleep staging accuracy comparable to interrater reliability among human scorers, with reported Cohen’s kappa (κ) values reaching up to 0.80.Citation3 Such high-performing algorithms can streamline the sleep staging process, potentially reducing the time and cost associated with manual scoring. Moreover, standardizing sleep staging through autoscoring can enhance the consistency and reliability of research findings across various studies and institutions. AI does not have to necessarily replace human scoring, but it can help in saving time. A recent study showed that semi-automated sleep scoring systems significantly reduce the workload on clinicians by directing their attention to the most critical areas, thereby enhancing overall efficiency.Citation4 This advancement highlights the potential of AI in standardizing sleep scoring and reducing interrater variability—an important development as it provides independent verification of the real-world accuracy of these tools. Nevertheless, thoroughly validating AI models using diverse clinical data as a vital step for their adoption in clinical settings is still needed.Citation5
Building on these advancements, the FDA has cleared a few auto-scoring software systems for use,Citation6 and the American Academy of Sleep Medicine (AASM) has initiated a two-year pilot program to certify autoscoring software, aiming to further validate these tools against expert manual scoring.Citation7 This certification program aims to independently assess the real-world accuracy of autoscoring software by comparing its performance to manual scoring by sleep experts. By doing so, it provides accredited sleep facilities with confidence in the reliability of autoscored results.
Enhancing Diagnosis and Personalized Treatment
Beyond autoscoring, AI can potentially revolutionize the field of sleep medicine by enabling early detection, personalized treatment, and improved management of sleep disorders. AI is being utilized to improve the diagnosis of sleep disorders such as obstructive sleep apnea (OSA), going beyond just the apnea-hypopnea index.Citation8 Traditionally, OSA is diagnosed using polysomnography, which requires an overnight stay in a sleep lab or home sleep apnea testing. Now, researchers are developing AI models that can detect OSA using more accessible and affordable methods. For example, smartwatches can now monitor blood oxygen levels (SpO2) with high accuracy, offering a potential tool for early OSA detection.Citation9–11 Moreover, AI-based predictive models have shown promising accuracy in identifying individuals at risk of developing OSA using simple predictors like age, sex, and body mass index, with reasonable success.Citation12,Citation13 Additionally, AI has demonstrated promise in aiding the diagnosis and subtyping of narcolepsy by analyzing clinical and polysomnographic data.Citation14,Citation15
The application of AI and ML in personalizing treatments for sleep disorders offers a promising method for enhancing patient adherence and outcomes.Citation16 AI algorithms have the capability to analyze extensive datasets to identify trends and relationships among patient traits, sleep physiology, and responses to treatment. This detailed analysis facilitates precise phenotyping and endotyping of OSA, paving the way for the creation of customized treatment strategies that focus on the unique aspects of the disorder.
ML models are capable of predicting essential characteristics linked to OSA, including upper airway collapsibility, reduced muscle responsiveness, a low arousal threshold, and abnormal ventilatory control.Citation17,Citation18 A recent study introduced a new data-driven approach, employing unsupervised multivariate principal component analyses and supervised machine learning, to explore how four key OSA endotypes affect the severity of OSA as defined by polysomnography.Citation19 The study also aimed to understand how typical polysomnographic and clinical variables might predict these endotypes.Citation19 Understanding a patient’s specific endotype can directly tailor treatment strategies. For instance, a machine learning model was employed to forecast the results of different treatment methods, such as oral appliances,Citation20 allowing patients to be matched with the therapy that would likely be most effective for them.
ML algorithms have demonstrated high accuracy in predicting CPAP therapy adherence in OSA patients.Citation21,Citation22 By analyzing large datasets, AI can identify correlations between patient characteristics and treatment responses, enabling clinicians to tailor interventions to individual needs. This personalized approach to sleep therapy could revolutionize patient care, improving outcomes and quality of life.
Randomized controlled trials have historically expressed skepticism regarding the efficacy of CPAP therapy in reducing cardiovascular events among patients with OSA, often yielding debated and disappointing results.Citation23 However, recent advancements and the utility of ML have introduced new hope for discerning the efficacy of CPAP by recognizing heterogeneity within the OSA population. A recent study utilized ML in the ISAACC trial dataset to identify subgroups exhibiting varied responses to CPAP therapy.Citation24 The investigators found that CPAP therapy was associated with fewer cardiovascular events in patients who have shorter durations of apnea events in OSA, compared to those with longer durations who experienced higher risks.Citation24 This study underscores the importance of additional research utilizing ML to create customized treatment approaches tailored to individual patient characteristics. Consequently, ML could potentially redefine the approach to precision medicine in OSA management, highlighting the importance of incorporating personalized treatment protocols in future trials to overturn previously negative outcomes.Citation16 Similar approaches can be investigated in other sleep disorders.
Furthermore, the application of AI/ML methods could significantly enhance the prognostic assessment of OSA, thereby improving patient care and outcomes. For instance, by employing advanced ML techniques to analyze the full ventilatory distribution histogram in conjunction with other critical measures of OSA severity—such as hypoxic burden and arousal indices—these sophisticated algorithms can offer a more comprehensive and precise evaluation of the disease outcome.Citation25,Citation26 Such detailed assessments are crucial for understanding the complete effects of OSA on patients and tailoring treatment to individual needs more effectively.
In the field of chronotherapy, mathematical modeling has shown promise in optimizing the timing of chemotherapy treatments based on the circadian rhythms of patients to improve efficacy and minimize toxicity. These models streamline the interpretation of complex drug interactions and timings, making it far more feasible to align treatments with circadian cycles for improved survival outcomes. For instance, studies applying mathematical modeling in metastatic colorectal cancer have found that treatments timed according to patient-specific circadian rhythms significantly improved survival compared to traditional schedules.Citation27 Recently, an ML method was developed to predict circadian time accurately using just a few blood samples.Citation28 This innovation facilitates personalized chronotherapy in clinical settings by accurately determining each patient’s internal physiological clock state. Such advancements highlight the potential of AI to tailor treatment schedules, thereby enhancing the efficacy and safety of therapies.Citation29
AI and Advancing Sleep Medicine Research with Big Data
Integrating AI and ML with big data presents exciting opportunities for advancing sleep medicine research and improving patient care.Citation30 Due to the wealth of digital physiological data collected through sleep studies, wearable devices, and self-quantification systems, data analysis in sleep medicine research is particularly well-suited for AI/ML applications.Citation31 The selection of appropriate training data is vital for developing accurate and generalizable AI models in sleep medicine research, as the size, quality, and representativeness of the dataset can significantly affect model performance and the likelihood of overfitting or underfitting, which are issues related to how well the model predicts new, unseen data compared to how it performs on the training data.Citation5 To mitigate these risks, researchers should strive to use large, diverse, and representative datasets for training AI models, employ random sampling methods to guarantee that the data accurately reflects the target population, and apply iterative model training along with independent validation to evaluate the stability and generalizability of the models developed.Citation32
Initiatives such as the National Sleep Research Resource (NSRR) and the UK Biobank have made large sleep datasets publicly available, enabling researchers worldwide to leverage this valuable data for analysis. The proposed Human Sleep Project aims to gather real-world sleep data from millions of people using online questionnaires, sleep diaries, and recording devices to establish the true role of sleep and answer fundamental questions about sleep duration, quality, and the impact of genetic and environmental factors.Citation33 These publicly available resources open promising new avenues for impactful real-world research on sleep medicine using AI. However, challenges remain regarding data standardization, privacy issues, and the integration of multi-omics data (eg, transcriptomics, proteomics, metabolomics). Furthermore, ensuring optimal data quality and having independent validation datasets are necessary, given that most existing polysomnogram datasets originate from research studies with specific inclusion criteria and may not be applicable to real clinical practice.Citation34 Addressing these challenges will be necessary for realizing the complete capacity of large datasets and AI/ML in sleep medicine research and clinical applications, clearing the path for personalized sleep medicine and improved patient outcomes.Citation16
AI in Sleep Neuroscience
In sleep neuroscience, AI has greatly improved the analysis of EEG signals, taking its use beyond just routine clinical applications. Machine learning classifiers of EEG signals during sleep do more than diagnose sleep-wake disorders and categorize different sleep stages. They also provide a valuable method for studying the functions of sleep. Additionally, ML models that analyze baseline EEG features have demonstrated potential in predicting various neurological diseases associated with sleep disorders. Specifically, they have shown effectiveness in determining the timing and subtype of phenoconversion in patients with idiopathic Rapid Eye Movement sleep behavior disorder, a condition frequently preceding neurodegenerative diseases.Citation35,Citation36 Similarly, an ML algorithm analyzing sleep structure, frequency band powers, spindles, slow oscillations, and coherence in 10,784 sleep studies across 8044 participants effectively distinguished between those with dementia, mild cognitive impairment, and no cognitive impairments.Citation37 This ability underscores the significant potential of AI in detecting early markers of neurodegenerative disorders linked to sleep abnormalities, underscoring the need for more comprehensive international research to refine these predictive models.
Ethical Considerations and Recommendations
As we explore the new frontier of AI applications in sleep medicine, addressing ethical and legal considerations is essential. The AASM, in their position statement, has raised concerns about AI potentially exacerbating existing healthcare inequities, such as sex disparities in evaluating sleep-disordered breathing.Citation3 It is vital to train AI algorithms on diverse datasets to prevent bias and promote equitable care. While AI tools can enhance clinical decision-making and data analysis, the ultimate responsibility for patient diagnosis, treatment, and research integrity rests with sleep medicine providers and researchers. The AASM also offers several recommendations for sleep disorder centers considering AI-based tools. These include demanding transparency from manufacturers about the intended population, goals, and characteristics of datasets used for developing AI programs. Furthermore, AI applications should be subjected to thorough testing on independent, standardized datasets representative of the target patient population to ensure generalizability and performance on par with expert evaluations. Lastly, manufacturers should support sleep centers in assessing the real-world efficacy and utility of AI software within their specific settings, ensuring that physician review and options for manual rescoring of sleep studies are available to guarantee accurate diagnoses and optimal patient care. Moreover, it is evident that we must validate and standardize ML methodologies in research, particularly in healthcare applications like sleep medicine.Citation16 Robust data handling, model development, validation, and reporting guidelines should be established to warrant the reliability, fairness, and reproducibility of ML-based findings, facilitating their responsible translation into clinical practice for personalized sleep disorders diagnosis and management.Citation38
In summary, the dawn of AI and ML in sleep medicine marks a pivotal era of enhanced diagnostic precision, personalized treatment, and rapid advancements in quality research. This transition, driven by extensive research and big data analytics that identify different endotypes of sleep disorders, opens new avenues for groundbreaking research while necessitating rigorous ethical oversight to ensure its equitable implementation across diverse populations. As AI technologies become increasingly integral to sleep medicine, ongoing research, interdisciplinary collaboration, and thorough evaluation are essential to maximize their transformative potential globally.
Disclosure
Professor BaHammam is the Editor-in-Chief for Nature and Science of Sleep. The author reports no other conflicts of interest in this work.
References
- Khosla S, Deak MC, Gault D, et al. Consumer sleep technology: an American academy of sleep medicine position statement. J Clin Sleep Med. 2018;14(5):877–880. doi:10.5664/jcsm.7128
- BaHammam AS, Pandi-Perumal SR, Hunasikatti M. Wearable Technologies/Consumer Sleep Technologies in Relation to Sleep Disorders Developments in the Last Decade. In: BaHammam AS, Hunasikatti M, editors. Sleep Apnea Frontiers: Pathophysiology, Diagnosis, and Treatment Strategies. Singapore: Springer Nature Singapore; 2023:145–160.
- Goldstein CA, Berry RB, Kent DT, et al. Artificial intelligence in sleep medicine: an American academy of sleep medicine position statement. J Clin Sleep Med. 2020;16(4):605–607. doi:10.5664/jcsm.8288
- Bechny M, Monachino G, Fiorillo L, et al. Bridging AI and clinical practice: integrating automated sleep scoring algorithm with uncertainty-guided physician review. Nat Sci Sleep. 2024;2024:1.
- Alattar M, Govind A, Mainali S. Artificial intelligence models for the automation of standard diagnostics in sleep medicine—a systematic review. Bioengineering. 2024;11(3):206. doi:10.3390/bioengineering11030206
- Choo BP, Mok Y, Oh HC, et al. Benchmarking performance of an automatic polysomnography scoring system in a population with suspected sleep disorders. Front Neurol. 2023;14:1123935. doi:10.3389/fneur.2023.1123935
- American Academy of Sleep Medicine. American academy of sleep medicine. AASM autoscoring certification: sleep stage pilot program. Darien, IL: American Academy of Sleep Medicine; 2023. Available from: https://aasm.org/about/industry-programs/autoscoring-certification/#:~:text=The%20AASM%20is%20excited%20to,the%20performance%20of%20autoscoring%20systems. Accessed April 15, 2024.
- Ferreira-Santos D, Amorim P, Silva Martins T, Monteiro-Soares M, Pereira Rodrigues P. Enabling early obstructive sleep apnea diagnosis with machine learning: systematic review. J Med Internet Res. 2022;24(9):e39452. doi:10.2196/39452
- Serrano Alarcón Á, Martínez madrid N, Seepold R. A minimum set of physiological parameters to diagnose obstructive sleep apnea syndrome using non-invasive portable monitors. a systematic review. Life. 2021;11(11). doi:10.3390/life11111249
- Zhao R, Xue J, Zhang X, et al. Comparison of ring pulse oximetry using reflective photoplethysmography and PSG in the Detection of OSA in Chinese adults: a pilot study. Nat Sci Sleep. 2022;14:1427–1436. doi:10.2147/NSS.S367400
- Almarshad MA, Al-Ahmadi S, Islam MS, BaHammam AS, Soudani A. Adoption of transformer neural network to improve the diagnostic performance of oximetry for obstructive sleep apnea. Sensors. 2023;23(18):7924. doi:10.3390/s23187924
- Yang H, Lu S, Yang L. Clinical prediction models for the early diagnosis of obstructive sleep apnea in stroke patients: a systematic review. Syst Rev. 2024;13(1):38. doi:10.1186/s13643-024-02449-9
- Kuan YC, Hong CT, Chen PC, Liu WT, Chung CC. Logistic regression and artificial neural network-based simple predicting models for obstructive sleep apnea by age, sex, and body mass index. Math Biosci Eng. 2022;19(11):11409–11421. doi:10.3934/mbe.2022532
- Shen N, Luo T, Chen C, et al. Towards an automatic narcolepsy detection on ambiguous sleep staging and sleep transition dynamics joint model. J Neural Eng. 2022;19(5):056009. doi:10.1088/1741-2552/ac8c6b
- Cesari M, Egger K, Stefani A, et al. Differentiation of central disorders of hypersomnolence with manual and artificial-intelligence-derived polysomnographic measures. Sleep. 2023;46(2). doi:10.1093/sleep/zsac288
- BaHammam A. Personalizing obstructive sleep apnea therapy using machine learning: insights from the ISAACC trial. Ann Am Thorac Soc. 2024. doi:10.1513/AnnalsATS.202403-308ED
- Nemati S, Orr J, Malhotra A. Data-driven phenotyping: graphical models for model-based phenotyping of sleep apnea. IEEE Pulse. 2014;5(5):45–48. doi:10.1109/MPUL.2014.2339402
- Brennan HL, Kirby SD. The role of artificial intelligence in the treatment of obstructive sleep apnea. J Otolaryngol. 2023;52(1):7. doi:10.1186/s40463-023-00621-0
- Dutta R, Delaney G, Toson B, et al. A novel model to estimate key obstructive sleep apnea endotypes from standard polysomnography and clinical data and their contribution to obstructive sleep apnea severity. Ann Am Thorac Soc. 2021;18(4):656–667. doi:10.1513/AnnalsATS.202001-064OC
- Dutta R, Tong BK, Eckert DJ. Development of a physiological-based model that uses standard polysomnography and clinical data to predict oral appliance treatment outcomes in obstructive sleep apnea. J Clin Sleep Med. 2022;18(3):861–870. doi:10.5664/jcsm.9742
- Eguchi K, Yabuuchi T, Nambu M, et al. Investigation on factors related to poor CPAP adherence using machine learning: a pilot study. Sci Rep. 2022;12(1):19563. doi:10.1038/s41598-022-21932-8
- Scioscia G, Tondo P, Foschino Barbaro MP, et al. Machine learning-based prediction of adherence to continuous positive airway pressure (CPAP) in obstructive sleep apnea (OSA). Inform Health Soc Care. 2022;47(3):274–282. doi:10.1080/17538157.2021.1990300
- Qasrawi SO, BaHammam AS. Role of Sleep and Sleep Disorders in Cardiometabolic Risk: a Review and Update. Current Sleep Med Rep. 2024;10(1):34–50. doi:10.1007/s40675-024-00276-x
- Cohen O, Sánchez-de-la-Torre M, Al-Taie Z, et al. Heterogeneous effects of CPAP in Non-Sleepy OSA on CVD outcomes: post-hoc machine learning analysis of the ISAACC Trial (ECSACT Study). Ann Am Thorac Soc. 2024;21:1.
- Parekh A, Kam K, Wickramaratne S, et al. Ventilatory burden as a measure of obstructive sleep apnea severity is predictive of cardiovascular and all-cause mortality. Am J Respir Crit Care Med. 2023;208(11):1216–1226. doi:10.1164/rccm.202301-0109OC
- Wickramaratne S, Kam K, Tolbert TM, et al. Combination of ventilatory, hypoxic, and arousal burden predicts short- and long-term consequences of OSA better than the apnea-hypopnea index [abstract]. Am J Respir Crit Care Med. 2023;207:A5968.
- Hesse J, Martinelli J, Aboumanify O, Ballesta A, Relogio A. A mathematical model of the circadian clock and drug pharmacology to optimize irinotecan administration timing in colorectal cancer. Comput Struct Biotechnol J. 2021;19:5170–5183. doi:10.1016/j.csbj.2021.08.051
- Braun R, Kath WL, Iwanaszko M, et al. Universal method for robust detection of circadian state from gene expression. Proc Natl Acad Sci U S A. 2018;115(39):E9247–e56. doi:10.1073/pnas.1800314115
- Zaki NFW, Yousif M, BaHammam AS, et al. Chronotherapeutics: recognizing the importance of timing factors in the treatment of disease and sleep disorders. Clin Neuropharmacol. 2019;42(3):80–87. doi:10.1097/WNF.0000000000000341
- Leppanen T, Varon C, de Zambotti M, Myllymaa S. Machine learning and wearable technology in sleep medicine. Front Digit Health. 2022;4:845879. doi:10.3389/fdgth.2022.845879
- Göktepe-Kavis P, Aellen FM, Alnes SL, Tzovara A. Sleep research in the era of AI. Clin Transl Neurosci. 2024;8(1):13. doi:10.3390/ctn8010013
- Millard K, Richardson M. On the importance of training data sample selection in random forest image classification: a case study in peatland ecosystem mapping. Remote Sensing. 2015;7(7):8489–8515. doi:10.3390/rs70708489
- Roenneberg T. The human sleep project. Nature. 2013;498:427–8. doi:10.1038/498427a
- Bandyopadhyay A, Goldstein C. Clinical applications of artificial intelligence in sleep medicine: a sleep clinician’s perspective. Sleep Breath. 2023;27(1):39–55. doi:10.1007/s11325-022-02592-4
- Jeong E, Shin YW, Byun JI, et al. EEG-based machine learning models for the prediction of phenoconversion time and subtype in iRBD. Sleep. 2024. doi:10.1093/sleep/zsae031
- Cesari M, Christensen JAE, Muntean ML, et al. A data-driven system to identify REM sleep behavior disorder and to predict its progression from the prodromal stage in Parkinson’s disease. Sleep Med. 2021;77:238–248. doi:10.1016/j.sleep.2020.04.010
- Adra N, Sun H, Ganglberger W, et al. Optimal spindle detection parameters for predicting cognitive performance. Sleep. 2022;45(4). doi:10.1093/sleep/zsac001
- Klement W, El Emam K. Consolidated reporting guidelines for prognostic and diagnostic machine learning modeling studies: development and validation. J Med Internet Res. 2023;25:e48763.