Abstract
Background
Combined ICS and long-acting bronchodilators (LABD) more effectively reduce COPD exacerbations than LABD therapy alone. Corticosteroid-related adverse effects, including pneumonia, limit ICS use. Previous data suggest this risk is lower for extrafine beclometasone (ef-BDP). We compared pneumonia risk among new users of fixed dose ICS/LABD formulations containing ef-BDP, versus patients initiating LABD without any ICS.
Methods
A propensity-matched historical cohort study design used data from OPCRD. COPD patients with ≥1 year of continuous data who initiated LABD or ICS/LABD formulations containing ef-BDP were matched. Primary outcome was time to pneumonia event, as treated, using either sensitive (physician diagnosed) or specific (physician diagnosed and x-ray or hospital admission confirmed) definitions, with non-inferiority boundary of 15%.
Results
23,898 COPD patients were matched, who were 68±11 years, 54.3% male and 56% current-smokers, while 43% were former-smokers. Initiation of ef-BDP/LABD was not associated with an increased risk of pneumonia versus LABD, for either a sensitive 0.89 (0.78–1.02), P = 0.08 or a specific 0.91 (0.78–1.05), P = 0.18 definition of pneumonia. The probability of remaining pneumonia free 1-year after ef-BDP/LABD was 98.4%, which was comparable to LABD at 97.7%, and was sustained up to 6 years of observation; non-inferiority criterion was met for both definitions. Initiation of ef-BDP/LABD was also associated with a reduced risk of developing LRTIs in the propensity matched cohort.
Conclusion
Risk of pneumonia when using ICS for the management of COPD reported in several randomised controlled trials may not be relevant with ef-BDP in a diverse real-world clinical population.
Introduction
Inhaled long-acting bronchodilators (LABD) include both long-acting beta agonists (LABA) and long-acting muscarinic antagonists (LAMA).Citation1 LABD use in COPD has been shown to improve lung function, dyspnoea, health status and reduce exacerbations rates.Citation2 In patients who experience frequent or moderate to severe exacerbations, inhaled corticosteroids (ICS) can be combined with LABD, forming either a double or triple therapy.Citation1,Citation2
The use of a double therapy containing ICS and LABA has been shown to be more effective than each component in isolation in improving lung function, symptoms and quality of life and reducing exacerbations and their severity and is a commonly used first line treatment for symptomatic patients at risk of exacerbations, especially in those with high blood eosinophil levels.Citation3–11 In the most recent GOLD 2023 iteration, triple therapy with ICS/LABA/LAMA is the preferred treatment of choice in COPD patients that require ICS, particularly with the following thresholds of blood eosinophils: >100 cells/mL - Favors use; >300 cells/mL - Strongly favors use. At these eosinophil thresholds, ICS/LABA/LAMA triple therapy is more effective for reducing exacerbations when compared to combined LABD therapy.Citation2,Citation12 Further, two large one-year randomized controlled trials (IMPACT and ETHOS) provide new evidence on mortality reduction with fixed-dose inhaled triple combinations compared to dual bronchodilation,Citation2 so triple therapy is recommended for patients whose dyspnoea and exacerbations are not managed on dual regimes.Citation2,Citation13
The superior effects of ICS-containing dual and triple therapies in improving COPD outcomes, when compared to each component in isolation have mostly been attributed to their anti-inflammatory effects.Citation14–16 Despite the benefits of ICS, there are risks, among the most notable being an increased incidence of pneumonia that is associated with regular ICS useCitation5,Citation16–23 However, COPD itself is a significant risk factor for the development of pneumonia,Citation24,Citation25 and this is further magnified for patients aged 55 years and older, with severe airflow limitation (FEV1 <50% predicted), a history of prior COPD exacerbations, a poor modified medical research council (mMRC) dyspnoea score, low body mass indexCitation26 and comorbid conditions.Citation27 ICS should also be used with caution in COPD patients with a prior history of pneumonias.Citation28
Recent observations suggest not all ICS carry the same pneumonia risk, and subclass variations may exist. For example, in the PATHOS and UPLIFT studies, patients using fluticasone propionate containing formulations had an increased risk of developing pneumonia when compared to therapies containing other ICS compounds.Citation20,Citation28 More specifically, the use of fluticasone propionate/salmeterol in COPD patients has been associated with an increased risk of pneumonia and severe exacerbations when compared to budesonide/formoterol or beclometasone/formoterol in a retrospective cohort study.Citation29,Citation30 Similarly, a recent meta-analysis suggests the use of fluticasone containing formulations in COPD are linked to an increased pneumonia risk (greater with fluticasone propionate than with fluticasone furoate) which was not observed when using budesonide or beclometasone.Citation31 However, these apparent differences between ICS in terms of pneumonia risk may relate to other factors including patients’ characteristics, diagnostic criteria for pneumonia, or different study designs.
In highlighting these observations, it should be acknowledged that, irrespective of subtype, the use of an ICS containing formulation may carry an increased risk of pneumonia compared to LABD without ICS, as evidenced in the ETHOS, TRINITY, FORWARD and IMPACT studies.Citation21,Citation32–34 A notable exception is the TRIBUTE study, which directly compared extrafine beclometasone-glycopyrronium-formoterol in a single inhaler with glycopyrronium-indacaterol, and showed no difference in the risk of pneumonia over 12 months in patients with severe symptomatic COPD who were at risk of exacerbations.Citation35
Notably, the above reported studies were randomised controlled trials (RCTs) and the pneumonia risk associated with ICS use were reported as adverse outcomes. Thus, they utilised a select, non-real-world cohort of participants and were not designed or necessarily powered to detect the true risk of ICS use association with pneumonia in a real-world population of COPD patients. Previous real-world studies suggested that extra-fine particle formulations of ICS may be associated with lower risk of pneumonia than with other fine-particle formulations, which may relate to differences in lung deposition.Citation36,Citation37 Therefore it would be of interest to assess the risk of pneumonia in a broad real-world matched population of COPD patients who are utilising either ICS/LABD formulations or LABD therapies without ICS. Doing so would enable assessment of pneumonia risk in a large clinical population of COPD patients with diversity in age, comorbidities, risk factors and disease severity, which is different to the homogeneous populations normally incorporated in RCTs. Additionally, the use of a uniform definition of pneumonia would also be advantageous. The aim of the present investigation was to compare the risk of pneumonia in COPD patients who are new users of fixed dose ICS/LABD formulations containing extrafine beclometasone (ef-BDP), administered as either a dual or triple therapy, versus patients initiating LABD, either as mono or dual LABA/LAMA therapy.
Methods
Study Design and Ethical Approval
A historical propensity matched cohort study, including a broad real-life population of patients with active COPD in the UK was conducted. The baseline, reference period was one year prior to the index date, which was defined as the initiation date of either a dual or triple ICS/LABD formulation containing ef-BDP, or either mono or dual LABD therapy containing a LABA and/or a LAMA. Data was obtained from the Optimum Patient Care Research Database (OPCRD), which comprises electronic medical records from over 12 million patients and allows for a long retrospective period, with the median time in the database being 13 years and a large proportion having summary medical data from birth. Data were restricted from 2014 onwards. Additionally, the respiratory-related measures contained within the database have been validated using patient reported outcomes. OPCRD has ethical approval from the National Health Service Research Authority to hold and process anonymised research data (Research Ethics Committee reference: 15/EM/0150).
The protocol for the current investigation was established prior to data extraction and was performed in accordance with the European Network of Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP) code of conduct (2014). The study was also registered with the European Union electronic Register of Post-Authorisation studies (EUPAS35439) and approval was granted by the Anonymised Data Ethics Protocols and Transparency committee, the independent scientific advisory committee for OPCRD (ADEPT0820).
Inclusion and Exclusion Criteria
To be eligible for inclusion, patients were required to be 40 years of age or older at the time of COPD diagnosis, have >1 year of continuous data within their electronic medical record prior to the index date and be commencing either a dual or triple ICS/LABD formulation containing ef-BDP (Foster® and Trimbow®) or a mono or dual LABD therapy (either LAMA, LABA or LAMA/LABA). Patients were also required to have no history of using the class of formulation (ie no ICS before) that they were commencing and were required to have a second prescription of the same medication within 90 days of the first, to avoid brief treatment trials and patients with only temporary medical records.
Exclusion criteria for the current investigation were having a smoking status of “never smoked” or a diagnostic read code for asthma or any other chronic lower respiratory tract condition other than COPD. A diagnosis of bronchiectasis was not established as an exclusion criterion as it is often misdiagnosed and a frequent comorbidity of COPD. Any patients who experienced a pneumonia event or respiratory related bacterial infection in the 28 days prior to the index date also had their medical records excluded.
Outcomes
The primary outcome was time to first pneumonia event, using either a sensitive or a specific definition. A sensitive definition pneumonia was defined as any pneumonia diagnosed by a physician, and a specific definition pneumonia was defined as physician diagnosed pneumonia that was confirmed with a chest radiograph or hospital admission within one month of diagnosis. The secondary outcome was time to first lower respiratory tract infection (LRTI), which was defined as needing an antibiotic prescription with evidence of a LRTI on the same day. A full list of diagnostic READ codes used to define LRTI is available upon request. Exploratory analyses were also conducted to allow for more comprehensive comparisons of the harm related to these treatment regimens. This included the time to first event for acute oral corticosteroid use, antibiotics prescription, exacerbation, primary care recorded hospitalisation and pneumonia-related hospitalisation.
Confounders and Propensity Matching
Full details of the 1:1 matching process are provided in Appendix 1. Briefly, inclusion in the matching process required a covariate to have no more than 20% missing data. The propensity score was generated from logistic regression modelling using all available patient-level baseline characteristics that were expected on clinical grounds to be related to the outcome or to both the outcome and treatment allocation. The logit of the propensity score was used as the matching scale with a calliper width equal to 0.2 of the standard deviation of the logit of the propensity score, in line with the recommendations of Austin.Citation38
Matching rate and multivariable balance were used to establish criteria for accepting a matching set. Residual bias potential after propensity matching was assessed and if bias statistics were at least 2%, baseline variables were added to the outcome model in a forward selection approach, in descending order of highest bias potential.
Statistical Analysis
Non-inferiority of ef-BDP/LABD formulations was assessed against LABD using per protocol analysis. The upper boundary of non-inferiority was a relative difference of 15% corresponding to a hazard ratio at or below 1.15, with the non-inferiority boundary based on a prior randomised controlled trial comparing fluticasone with salmeterolCitation5 and triple therapy containing fluticasone with double bronchodilation.Citation33 The increased risk associated with ICS use in COPD patients was not an expected finding by Calverley et al,Citation5 as such there was no prospective definition of pneumonia in this study. Lipson et alCitation33 however, defined a pneumonia event as a physician diagnosis considering cough, sputum purulence, dyspnoea, physical examination findings and laboratory results, but also required a chest radiograph to be obtained. In the current investigation, patients were censored at the end of data availability, either when leaving the practice or last time data was extracted or on changes to medication regime.
Cox regression was used for time to event analysis. In the primary analysis, this allowed for the assessment of the association between ICS treatment and time to the first pneumonia event following initiation of an ef-BDP/LABD or LABD. Analyses were repeated in unmatched and propensity score matched samples to quantify the impact of measured confounders. To minimise residual confounding, a doubly robust approach was adopted in which the Cox regression models were adjusted for baseline characteristics with bias statistics of at least 2% in propensity score matched samples (Appendix 2). A similar approach was adopted for the secondary and exploratory outcomes. Exacerbations occurring within 28 days of a previous event were considered part of a single episode. From this modelling, hazard ratios and 95% confidence intervals for each effect were generated. All statistical analysis was performed using R,Citation39 with propensity score matching performed using the Matching package.Citation37
Results
Comparison of ef-BDP and LABD Populations
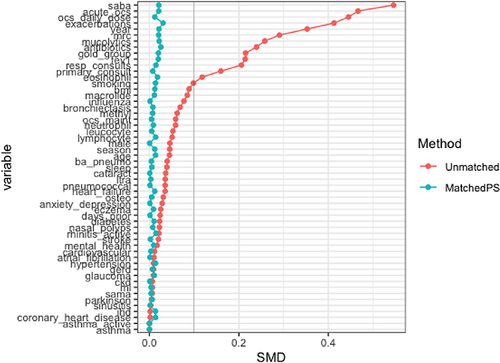
A total of 54,400 patients were eligible for inclusion, 13,485 commencing an ef-BDP containing ICS/LABD and 40,915 commencing LABD (). Of these 23,898 patients were matched, that is 11,949 commencing an ef-BDP based ICS/LABD formulation and 11,949 commencing LABD. Baseline characteristics of the unmatched and propensity matched cohorts for new users of ef-BDP/LABD and LABD are shown in and Appendix 2. Prior to matching, the ef-BDP/LABD cohort had more severe COPD in comparison to the LABD cohort, as indicated by exacerbations, FEV1% and mMRC scores. Following the matching process, irrespective of the cohort, patients were most likely to have experienced ≤1 exacerbations in the previous year, have an FEV1% between 50–80% of predicted value and have an mMRC score of ≥2. The distribution of propensity scores had a broad region of common support (0.03 < propensity score <0.97) () and the standardized mean difference was below the 10% threshold for all baseline characteristics, signifying sufficient balance between groups (). In particular, both groups of patients were 68±11 years, 54.3% were male, and 56% were current-smokers while 43% were former-smokers. By design, both groups were also equally balanced by BMI, blood eosinophils, GOLD stage, FEV1% predicted and mMRC score (all p>0.05).
Table 1 Demographic Features of Unmatched and Matched Populations Commencing Formulations Containing Extrafine Beclometasone/Long-Acting Bronchodilators (Ef-BDP/LABD) or Long-Acting Bronchodilators (LABD)
Figure 1 (A) Flow diagrams for patients commencing (A) LABD or (B) ef-BDP/LABD therapy and their eligibility for matching. (B) Flow diagrams for patients commencing (A) LABD or (B) ef-BDP/LABD therapy and their eligibility for matching.
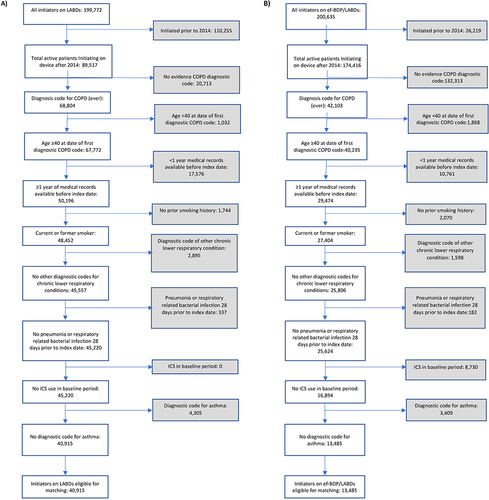
Figure 2 Density plots showing the distribution of propensity scores for patients treated with formulations containing extrafine beclometasone/long-acting bronchodilators (ef-BDP/LABD) or long-acting bronchodilators (LABD) without ICS.
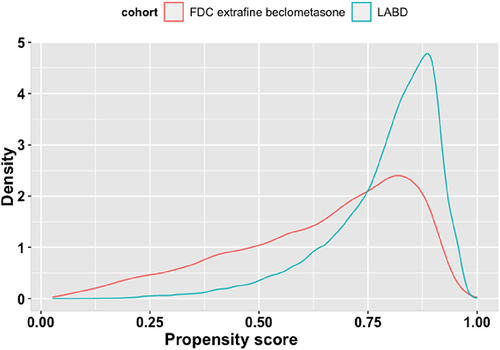
Figure 3 Covariate plot showing standardised mean differences (SMD) for comparison of baseline characteristics for new users of formulations containing extrafine beclometasone/long-acting bronchodilators or long-acting bronchodilators without ICS before and after propensity score matching.
Primary Outcomes
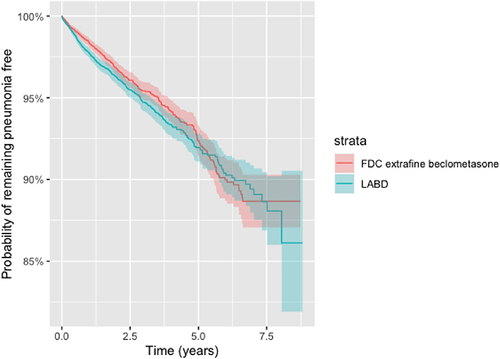
In comparison to LABD, initiation of ef-BDP/LABD was not associated with any significant increase in the hazard risk (HR) and 95% confidence interval of developing a sensitive definition pneumonia, for either matched (HR 0.89 (0.78–1.02), P = 0.08) or unmatched populations (HR 1.09 (0.98–1.21), P = 0.11) ( and ). The probability of remaining pneumonia free 1 year after commencement of ef-BDP/LABD was 98.4%, which was comparable to LABD at 97.7% (). Similar results were also observed 6 years after the commencement of ef-BDP/LABD (90.3%) and LABD (90.0%).
Table 2 Hazard Ratios for Comparing Time-to-Event Outcomes for New Users of Formulations Containing Either Extrafine Beclometasone/Long-Acting Bronchodilators (Ef-BDP/LABD) or Long-Acting Bronchodilators (LABD) in Propensity Score Matched Samples
Figure 4 Hazard ratios for sensitive- and specific-pneumonia definitions for extrafine beclometasone/long-acting bronchodilators or long acting bronchodilators (LABD) in unmatched and propensity score matched analyses.
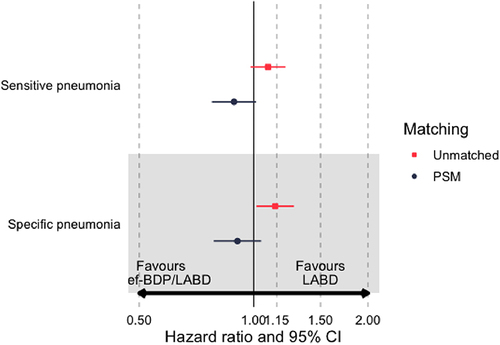
Figure 5 Kaplan-Meier survival curves for the time to pneumonia (sensitive definition) for new users of FDC extrafine beclometasone/long acting bronchodilators or long acting bronchodilators (LABD) in propensity score matched samples (as-treated).
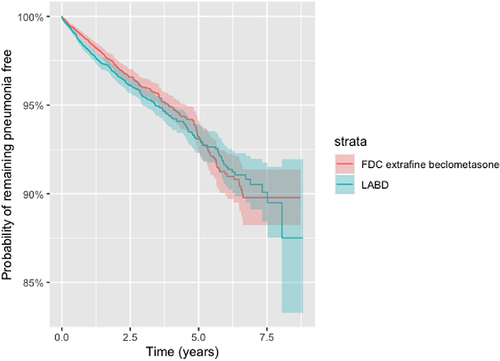
Similarly, the commencement of ef-BDP/LABD was not associated with any significant increase in the risk of developing a specific definition of pneumonia in the matched population 0.91 (0.78–1.05), P = 0.18, but with a marginal significant increase in the unmatched population 1.14 (1.02–1.28), P = 0.02 when compared to LABD. There was no significant variation in the survival function for remaining pneumonia free, from a specific definition pneumonia, one to six-years after the commencement of either ef-BDP/LABD or LABD in the propensity matched as-treated population (ef-BDP pneumonia free 98.6% at 1 year and 91.1% at 6 years; LABD pneumonia free 98.0% at 1 year and 91.5% at 6 years (). The criterion for non-inferiority for those patients commencing ef-BDP/LABD therapy was achieved for both sensitive and specific definitions with the upper 95% CI boundaries below 1.15 ( and ).
Figure 6 Kaplan-Meier survival curves for the time to pneumonia (specific definition) for new users of FDC extrafine beclometasone/long acting bronchodilators or long acting bronchodilators (LABD) in propensity score matched samples (as-treated).
Secondary Outcomes
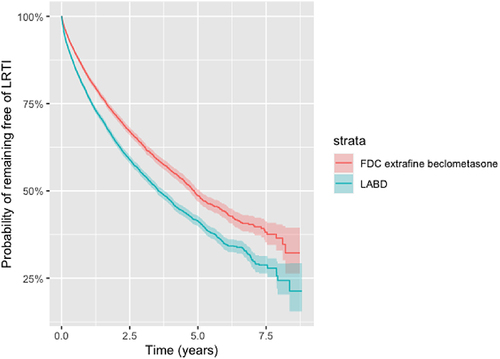
The initiation of ef-BDP/LABD was associated with a reduced risk of developing LRTI in the matched population 0.76 (0.72–0.79), P < 0.001, when compared to LABD (). The probability of remaining free from LRTI after the commencement of ef-BDP/LABD was 82.2%, compared to 80.1% after the commencement of LABD in the propensity matched cohort and remained significant after six years (P < 0.001). ().
Figure 7 Kaplan-Meier survival curves for the time to lower respiratory tract infection (LRTI) for new users of FDC extrafine beclometasone/long acting bronchodilators or long acting bronchodilators (LABD) in propensity score matched samples (as-treated).
Exploratory Outcomes
Assessment of exploratory outcomes indicates ef-BDP/LABD use was associated with a significant reduced risk of requiring antibiotic prescriptions, developing exacerbations, and having a primary care recorded hospitalisation (all p<0.05) in the matched population, when compared to LABD alone (). The use of ef-BDP/LABD was not associated with a different risk of pneumonia-related hospitalisations for matched 0.90 (0.75–1.07), P = 0.23 and unmatched 0.90 (0.75–1.07), P = 0.23 populations ().
Discussion
Compared with LABD, the initiation of ICS/LABD formulations containing extrafine beclometasone was not associated with a significantly increased risk of developing pneumonia, and the criterion for non-inferiority was achieved for both the sensitive and specific definitions of pneumonia. New users of beclomethasone-containing dual or triple therapies had a statistically significant reduced risk of developing lower respiratory tract infections, requiring prescriptions for antibiotics, developing exacerbations, and having a period of hospitalisation recorded within their primary care records. Rates of our study more sensitive definition of pneumonia in both the ef-BDP/LABD and LABD were lower than anticipated, but comparable to prior studies.
Our real-world results are in line with the results of the TRIBUTE RCT.Citation34 Conversely, both the TRINITY and FORWARD RCTs reported the use of extrafine formulations containing beclometasone to be associated with an increased risk of pneumonia when compared to LABD without ICS.Citation31,Citation32 Notably, assessing ICS use and its associated risk of pneumonia was not a primary objective of the TRINITY and FORWARD studies; rather it was reported as an adverse event and had an effect size, measured as an unadjusted absolute risk difference, of 1% and 2% respectively.Citation31,Citation32 In comparison to the FORWARD study which had a one year follow up, the effect size (adjusted absolute risk difference) for ef-BDP use when compared to LABD in the present investigation over the same period of time was −0.4% and −0.3% for our study sensitive and specific definitions of pneumonia, respectively. Further, differences in pneumonia rates between ef-BDP containing ICS/LABD and the LABD in these three RCTs highlight the variability of capturing this event as a TEAE.
Differences in the study populations and how pneumonia was defined may help explain the variation in effects and effect sizes.Citation40
The current study was designed to more closely represent what is observed in broader COPD populations, rather than those included in RCTs. In doing so, observing that extrafine beclometasone formulations do not significantly alter the risk of pneumonia, and in fact an association with a reduced risk of developing lower-respiratory tract infections was observed when compared to LABD alone, suggesting variations in the risk profile of ICS drugs when used for the management of a wider and more diverse clinical population of COPD patients. Potential effect modifiers may explain a reduced risk profile of ef beclometasone, including particle size, potency and dose used.
In relation to particle size, extrafine formulations of ICS more easily enter the small airways than formulations containing larger particles.Citation41 This allows to use lower doses of extrafine formulations to mediate the same desired clinical effect when compared to suspensions with larger particle size,Citation41 which may be advantageous when trying to minimise adverse effects. Other factors including lipophilicity, which influences retention in the airways, have also been suggested.Citation29,Citation42 Collectively, these observations may explain why ef-BDP did not significantly increase the risk of pneumonia when administered with LABDs to COPD patients in the present study.
As reported in the current investigation, the one-year Kaplan-Meier estimates for cumulative incidence of our study sensitive definition of pneumonia for both ef-BDP/LABD and LABD groups was close to 2%. This rate is in line with estimates reported in the TRINITY and FORWARD clinical trials, but is much lower than the annual rate seen in IMPACT.Citation31–33 It is possible this lower rate of pneumonia observed in the present investigation is attributed to the level of COPD severity within the study population. In comparison to IMPACT, LABD patients in the present investigation had milder COPD, as evidenced by higher FEV1% values and lower exacerbation rates, and the matched ef-BDP/LABD patients also had milder symptoms.
A key strength of the current investigation was the design which allowed for direct assessment and quantification of mostly unbiased risk associated with ef-BDP/LABD use when compared to LABD by propensity-matching, minimizing confounding. Additionally, a robust study population with available medical records over an extensive period serves as additional strength. The median time for data collection in a patient’s record in the database was 13 years, and this lengthy follow-up period allowed for sufficient assessment of pneumonia risk associated with extrafine beclometasone containing ICS/LABD formulations. The presentation of as-treated data is also important as it considered the actual period during which patients were at-risk of pneumonia and other respiratory related adverse effects of their treatment. Finally, use of a propensity score matching process with a calliper helped minimise residual confounding bias by ensuring a high level of balance between the groups across a wide range of measured covariates.
With respect to limitations, it should be noted that the current investigation does not allow for exploration of how pneumonia risk varies with the use of mono or dual LABDs in both the ICS/LABD or LABD in isolation groups. Time to event analysis was also only conducted for the first event, meaning any subsequent pneumonia event/s were not captured in the current analysis. The exploratory analysis of time to event for acute OCS use and antibiotics prescriptions were also not qualified specifically for COPD exacerbations and pneumonia respectively. Additionally, COPD patients who had no smoking history were excluded from this study, as they have been shown to have milder COPD symptoms, less inflammation and fewer comorbidities than current or former smokers.Citation43 Finally, this study does not provide direct comparisons between different ICS-containing fixed-dose combinations, or different dosages, and further research on the impact of different ICS on the lung microbiome and immune system are needed to further shed light on these intra-class differences.
Conclusion
In comparison to LABD, commencement ef-BDP/LABD formulations was not associated with a significant increased risk of developing pneumonia in a large propensity matched real-world cohort of COPD patients, and therefore non-inferiority vs LABD was achieved; this effect at 1 year was sustained up to 6 years of observation. New users of ef-BDP/LABD also had a significantly reduced risk of lower-respiratory tract infections compared to LABD therapy. Collectively, this suggests that extrafine beclometasone formulations for the management of COPD are not associated with an increased risk of pneumonia in a diverse real-world clinical population, contrary to what has been reported in some randomised controlled trials. Notably, this could be attributed to molecular potency, lung deposition and formulation characteristics of extrafine beclometasone, as well as differences in the clinical features of the populations involved.
Abbreviations
ADEPT, Anonymised Data Ethics Protocols and Transparency; BMI, Body mass index; CI, Confidence Interval; COPD, Chronic Obstructive Pulmonary Disease; ef-BDP, extrafine beclometasone; ef-FDC-BDP, extrafine particle fixed dose beclometasone; ENCePP, European Network Centers for Pharmacoepidemiology and Pharmacovigilance; FEV1, Forced Expiratory Volume 1; fp-FDC-F, fine-particle fixed dose fluticasone; fp-FDC-FF, fine-particle fixed dose fluticasone furoate; fp-FDC-FP, fine-particle fixed dose fluticasone propionate; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; HR, Hazard Ratio; ICS, inhaled corticosteroids; LABA, long-acting beta-2 agonists; LABD, long-acting bronchodilators; LAMA, long-acting muscarinic antagonists; LRTI, lower-respiratory tract infection; mMRC, modified Medical Research Council; OPCRD, Optimum Patient Care Research Database; RCT, randomized controlled trial; URTI, upper-respiratory tract infection.
Ethics Approval
The study protocol was established prior to data extraction, in accordance with the criteria for the European Network Centers for Pharmacoepidemiology and Pharmacovigilance (ENCePP) and follows the ENCePP code of conduct (2014). Registration of the study with the European Union electronic Register of Post-Authorization studies was also undertaken (EUPAS35439). As noted, the dataset was derived from the OPCRD, which has ethical approval from the National Health Service Research Authority to hold and process anonymised research data (Research Ethics Committee reference: 15/EM/0150). Approval for this study was granted by the Anonymised Data Ethics Protocols and Transparency (ADEPT) committee – the independent scientific advisory committee for the OPCRD (ADEPT0820). The authors do not have permission to give public access to the study dataset; requests to access OPCRD can be made via the OCPRD website (https://opcrd.co.uk/our-database/data-requests/) or via the enquiries email [email protected].
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. All authors took part in drafting the article or revising it critically for important intellectual content. All authors agreed to submit to the current journal. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Disclosure
Leonardo M. Fabbri reports lecture fees and/or consultancies from Alfasigma, AstraZeneca, Chiesi, Boehringer Ingelheim, GlaxoSmithKline, Lusofarmaco, Merck, Novartis, Zambon, and Verona Pharma.
Huib AM Kerstjens reports grants and consultancy/advisory board participation from/for AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Novartis, all outside the submitted work. All were paid to his institution.
Alberto Papi is on the boards for and has received research and travel support and consultancy and lecture fees from Chiesi Farmaceutici, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Merck Sharp & Dohme, Takeda, Mundipharma Research Limited, and Teva; reports grants from Chiesi, AstraZeneca, GlaxoSmithKline and Sanofi; personal fees from Iqvia, Avillion, Elpen Pharmaceuticals, Moderna, Edmond Pharm; has received lecture fees and travel support from Menarini, Novartis, and Zambon; is on the boards for and has received lecture fees and travel support from Pfizer and has received research support from Sanofi.
Nicolas Roche reports research grants and fees from Boehringer Ingelheim, Novartis, Pfizer, GSK and fees for advisory boards, consultation, education, presentations from Austral, Biosency, MSD, AstraZeneca, Chiesi, Menarini, MSD, Nuvaira, Sanofi, and Zambon.
Dave Singh has received personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Teva, Theravance and Verona.
Claus F Vogelmeier gave presentations at symposia and/or served on scientific advisory boards sponsored by Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Grifols, Insmed, Menarini, Novartis, Nuvaira, MedUpdate, Roche, and Sanofi.
Elif Şen reports is a full-time employee of Chiesi Farmaceutici S.p.A.
Joan B Soriano reports grants from Novartis and personal fees for lecturing with AstraZeneca, Chiesi, Grifols, GSK, Novartis, Pfizer, Teva, and Zambon.
José Eduardo Delfini Cançado reports grants and personal fees from Aché, AstraZeneca, Boehringer Ingelheim, Chiesi, Eurofarma, GlaxoSmithkline, Glenmarkpharma, Novartis, Sanofi and Zambon.
Elena Nudo and George Georges are employees of Chiesi Farmaceutici, S.p.A.
Maxim Kots is a full time Chiesi Farmaceutici employee.
William Henley, Derek Skinner, Rebecca Vella and Victoria Carter are employees of Observational and Pragmatic Research Institute who conducted this study.
David Price has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; consultancy agreements with AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Viatris, Teva Pharmaceuticals; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Chiesi, Viatris, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and UK National Health Service; grants from Boehringer Ingelheim; grants, personal fees from Theravance; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Viatris, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme, Teva Pharmaceuticals; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Novartis, Teva Pharmaceuticals; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme, and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. The authors report no other conflicts of interest in this work.
Acknowledgments
Professor Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC). Jaco Voorham is acknowledged for his contribution to protocol development. We would also like to acknowledge Ms. Shilpa Suresh (MSc) of the Observational and Pragmatic Research Institute (OPRI), Singapore, for editorial and formatting assistance which supported the development of this publication.
Additional information
Funding
References
- Mathioudakis AG, Vestbo J, Singh D. Long-acting bronchodilators for chronic obstructive pulmonary disease: which one(S), how, and when? Clin Chest Med. 2020;41(3):463–474. PMID: 32800199. doi:10.1016/j.ccm.2020.05.005
- Global Initiative for Chronic Obstructive Lung Disease. Global initiative for chronic obstructive lung disease global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease; 2023. Available from: http://www.goldcopd.org/. Accessed July 14, 2023.
- Agarwal R, Aggarwal AN, Gupta D, Jindal SK. Inhaled corticosteroids vs placebo for preventing COPD exacerbations: a systematic review and metaregression of randomized controlled trials. Chest. 2010;137(2):318. doi:10.1378/chest.09-1305
- Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297. doi:10.1136/bmj.320.7245.1297
- Calverley PM, Anderson JA, Celli B, et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775. doi:10.1056/NEJMoa063070
- Macie C, Wooldrage K, Manfreda J, Anthonisen NR. Inhaled corticosteroids and mortality in COPD. Chest. 2006;130(3):640. doi:10.1378/chest.130.3.640
- Wise R, Connett J, Weinman G, Scanlon P, Skeans M; Lung health study research group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902.
- Yang IA, Clarke MS, Sim EH, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD002991. doi:10.1002/14651858.CD002991.pub3
- Nannini LJ, Lasserson TJ, Poole P. 2012 Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus long-acting beta(2)-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;9:CD006829. PMID: 22972099; PMCID: PMC4170910. doi:10.1002/14651858.CD006829.pub2
- Nannini LJ, Poole P, Milan SJ, Kesterton A. 2013 Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;30(8):CD006826. PMID: 23990350; PMCID: PMC6486274. doi:10.1002/14651858.CD006826.pub2
- Singh D, Agusti A, Martinez FJ, et al. Blood eosinophils and chronic obstructive pulmonary disease: a global initiative for chronic obstructive lung disease science committee 2022 review. Am J Respir Crit Care Med. 2022;206(1):17–24. doi:10.1164/rccm.202201-0209PP
- NICE Guideline Updates Team (UK). Inhaled Triple Therapy: Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management: Evidence Review I PMID: 32755138. London: National Institute for Health and Care Excellence (UK); 2019.
- Papi A, Petruzzelli S, Vezzoli S, Georges G, Fabbri LM. Triple therapy for all patients with severe symptomatic COPD at risk of exacerbations. Eur Respir J. 2019;53(4):1900147. PMID: 31000665. doi:10.1183/13993003.00147-2019
- Barnes PJ. Scientific rationale for inhaled combination with long acting β2-agonisitslong-acting β 2 -agonists and corticosteroids. Eur Respir J. 2002;19(1):182. doi:10.1183/09031936.02.00283202
- Haque R, Hakim A, Moodley T, et al. Inhaled long acting β2 agonists enhance glucocorticoid receptor nuclear translocation and efficacy in sputum macrophages in COPD. J Allergy Clin Immunol. 2013;132(5):1166. doi:10.1016/j.jaci.2013.07.038
- Usmani OS, Ito K, Maneechotesuwan K, et al. Glucocorticoid receptor nuclear translocation in airway cells after inhaled combination therapy. Am J Respir Crit Care Med. 2005;172(6):704. doi:10.1164/rccm.200408-1041OC
- Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Ann Am Thorac Soc. 2015;12(1):27–34. doi:10.1513/AnnalsATS.201409-413OC
- Festic E, Bansal V, Gupta E, Scanlon PD. Association of inhaled corticosteroids with incident pneumonia and mortality in COPD patients; systematic review and meta-analysis. COPD. 2016;13(3):312. doi:10.3109/15412555.2015.1081162
- Janson C, Johansson G, Ställberg B, et al. Identifying the associated risks of pneumonia in COPD patients: arctic an observational study. Respir Res. 2018;19(1):172. doi:10.1186/s12931-018-0868-y
- Latorre M, Novelli F, Vagaggini B, et al. Differences in the efficacy and safety among inhaled corticosteroids (ICS)/long-acting beta2-agonists (LABA) combinations in the treatment of chronic obstructive pulmonary disease (COPD): role of ICS. Pulm Pharmacol Ther. 2015;30:44. doi:10.1016/j.pupt.2014.10.006
- Morjaria JB, Rigby A, Morice AH. Inhaled corticosteroid use and the risk of pneumonia and COPD exacerbations in the UPLIFT study. Lung. 2017;195(3):281. doi:10.1007/s00408-017-9990-8
- Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax. 2013;68(11):1029. doi:10.1136/thoraxjnl-2012-202872
- Garcia-Vidal C, Carratalà J, Fernández-Sabé N, et al. Aetiology of, and risk factors for, recurrent community-acquired pneumonia. Clin Microbiol Infect. 2009;15(11):1033. doi:10.1111/j.1469-0691.2009.02918.x
- Molinos L, Clemente MG, Miranda B, et al.; for the ASTURPAR group. Community-acquired pneumonia in patient with and without chronic obstructive pulmonary disease. J Infect. 2009;58(6):417. doi:10.1016/j.jinf.2009.03.003
- Crim C, Calverley PMA, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641. doi:10.1183/09031936.00193908
- Mannino DM, Davis KJ, Kiri VA. Chronic obstructive pulmonary disease and hospitalizations for pneumonia in a US cohort. Respir Med. 2009;103(2):224. doi:10.1016/j.rmed.2008.09.005
- Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52(6):1801219. doi:10.1183/13993003.01219-2018
- Janson C, Larsson K, Lisspers KH, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β2 agonist: observational matched cohort study (PATHOS). BMJ. 2013;346(may29 4):f3306. doi:10.1136/bmj.f3306
- Chang TY, Chien JY, Wu CH, Dong YH, Lin FJ. Comparative safety and effectiveness of inhaled corticosteroid and long acting β2 agonist combination in patients with COPD. Chest. 2020;157(5):1117. doi:10.1016/j.chest.2019.12.006
- Zhang Q, Li S, Zhou W, Yang X, Li J, Cao J. Risk of pneumonia with different inhaled corticosteroids in COPD patients: a meta-analysis. COPD. 2020;17(4):462. doi:10.1080/15412555.2020.1787369
- Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919. doi:10.1016/S0140-6736(17)30188-5
- Wedzicha JA, Singh D, Vestbo J, et al.; for the FORWARD Investigators. Extrafine beclomethasone/formoterol in severe COPD patients with history of exacerbations. Resp Med. 2014;108(8):1153. doi:10.1016/j.rmed.2014.05.013
- Lipson DA, Barnhart F, Brealey N, et al.; for the IMPACT Investigators. Once-daily single inhaler triple versus dual therapy in patient with COPD. N Engl J Med. 2018;378(18):1671. doi:10.1056/NEJMoa1713901
- Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391:1076–1084. doi:10.1016/S0140-6736(18)30206-X
- Sonnappa S, Martin R, Israel E, et al.; Respiratory Effectiveness Group, Small Airways Study Group. Risk of pneumonia in obstructive lung disease: a real-life study comparing extra-fine and fine-particle inhaled corticosteroids. PLoS One. 2017;12(6):e0178112. doi:10.1371/journal.pone.0178112
- Price DB, Henley W, Cançado JED, et al. Interclass difference in pneumonia risk in COPD patients initiating fixed dose inhaled treatment containing extrafine particle beclometasone versus fine particle fluticasone. Int J Chron Obstruct Pulmon Dis. 2022;17:355–370. doi:10.2147/COPD.S342357
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multi Behav Res. 2011;46(3):399. doi:10.1080/00273171.2011.568786
- R Core Team. 2020. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org/. Accessed December 28, 2023.
- Wise RA, Bafadhel M, Crim C, et al. Discordant diagnostic criteria for pneumonia in COPD trials: a review. Eur Respir Rev. 2021;30(162):210124. doi:10.1183/16000617.0124-2021
- Derendorf H, Nave R, Drollmann A, Cerasoli F, Wurst W. Relevance of pharmacokinetics and pharmacodynamics of inhaled corticosteroids to asthma. Eur Respir J. 2006;28(5):1042. doi:10.1183/09031936.00074905
- Lipworth B, Kuo CR, Jabbal S. Current appraisal of single inhaler triple therapy in COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3003. doi:10.2147/COPD.S177333
- Thomsen M, Nordestgaard BG, Vestbo J, Lange P. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med. 2013;1(7):543. doi:10.1016/S2213-2600(13)70137-1

