Abstract
Craniopharyngiomas are tumors that arise from the remnants of Rathke's pouch along the nasopharynx to the diencephalon. Current standard of care includes maximal surgical resection versus adjuvant radiation if a maximal resection is unfeasible. Pharmacological therapy with MAPK targeted agents is an emerging therapeutic option for tumors with BRAF V600E mutations. We report a 45-year-old male with a strictly third ventricle papillary craniopharyngioma with a BRAF V600E mutation. After initial surgery with subtotal resection, the patient demonstrated durable response to targeted BRAF and MEK inhibitor therapy with vemurafenib and cobimetinib. Our report suggests that targeted therapy may reduce the need for radiation and impact surgical interventions in select cases.
We report a case of strictly third ventricle papillary craniopharyngioma which is a rare neoplasm accounting for 0.7–11% of all craniopharyngiomas.
Surgical complexities and deferred radiation led to exploration of alternative therapies.
Combination vemurafenib and cobimetinib yielded significant tumor reduction.
Targeting the prevalent BRAF V600E mutation provides a promising alternative to traditional treatments.
Dual BRAF/MEK inhibitors emerge as a potential adjuvant therapy post-surgery in this tumor entity.
Craniopharyngiomas are relatively rare neoplasms, comprising up to 1.2–4% of all intracranial tumors, and strictly third ventricle craniopharyngiomas represent an even more novel subset [Citation1,Citation2]. The two major subtypes of craniopharyngiomas are adamantinomatous and papillary. Though it is generally considered a pediatric neoplasm, craniopharyngiomas have a bimodal distribution. While adamantinomatous craniopharyngiomas tends to be more common, papillary craniopharyngiomas predominantly present in adults during the 4th to 5th decade of life [Citation3]. Papillary craniopharyngiomas have recently been found to harbor mutations in BRAF leading to metabolic derangement of the Ras/Raf/MEK/ERK pathway and papillary craniopharyngioma development [Citation4]. Specifically, BRAF V600E wherein glutamic acid is substituted for valine has been implicated in this pathology.
The proximal location of craniopharyngiomas to critical structures such as the hypothalamus, pituitary, and optic chiasm poses challenges for treatment. Complete resection may be possible if the neoplasm is favorably localized. In cases where the tumor involves hypothalamic involvement or additional complexity, a multi-pronged approach of surgery and radiation is recommended. Notably, radiation poses additional risks including cell damage, secondary malignancy, and adverse clinical symptomology for patients.
BRAF/MAPK inhibitory therapy exists in the literature for neoplasms, notably metastatic melanoma. However, reports of targeted BRAF/MAPK inhibitor treatment remain sparse for papillary craniopharyngiomas with fewer than 10 cases in the literature. This case report focuses on a patient with a complex strictly third ventricle papillary craniopharyngioma that underwent adjuvant BRAF/MAPK inhibitor therapy, with findings that could help with the management of this challenging tumor.
Case presentation
We report a 45-year-old male who initially presented with chronic worsening headaches refractory to NSAIDs. MRI showed a 3.1 cm homogenously enhancing mass within the third ventricle with moderate enlargement of lateral ventricles and mass effect on the optic chiasm (). Preoperative endocrine evaluation indicated an intact hypothalamic-pituitary axis. The patient underwent a right frontal craniotomy via a transcortical intraventricular approach using a minimally invasive tubular system. Intraoperatively, the lesion was found to be firm and originating from the hypothalamus and adherent at the infundibular recess. The tumor appeared almost entirely intraventricular with possible involvement of the stalk. Frozen specimen analysis suggested a papillary rather than adamantinomatous craniopharyngioma, and a subtotal resection was determined to be optimal due to risk of hypothalamic injury, risk of postoperative hypopituitarism and the viable option of chemotherapy for management of the residual tumor. Notably, pathology findings indicated a papillary craniopharyngioma with a BRAF V600E mutation ().
Figure 1. Preoperative and postoperative MRI imaging.
Preoperative mid-sagittal (A) and coronal (B) postcontrast T1 WI showing a lobulated solid enhancing 3rd intraventricular mass, displacing the optic chiasm (Ch) downwards and forwards, bowing the floor of the third ventricle (3V) and sparing the pituitary stalk, infundibulum and the chiasmatic cistern (Ch cs), associated with subsequent obstructive hydrocephalus (asterisks). Serial mid-sagittal postcontrast T1 WI images (C) immediately following debulking of the 3rd intraventricular mass showing residual enhancing component (arrow) and resolution of the hydrocephalus (asterisks), (D) significant progression of the mass without treatment on the 4-month postoperative follow-up scan. Vemurafenib and cobimetinib combination therapy was started within 2 weeks and the 3-month post combination therapy shows significant reduction in the size of the residual mass (E). (F) 11-days after the treatment is held, the residual mass continues to shrink and show cavitary changes. Most recent mid-sagittal (G) and coronal (H) postcontrast T1 WI showing near complete resolution of the mass with a subcentimetric residual enhancing focus which remained stable for 1 year since it attained this size (85 weeks since treatment was withheld).
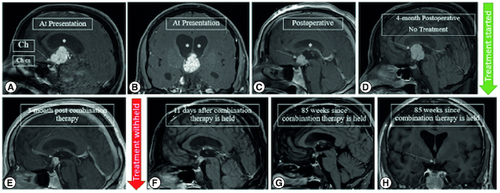
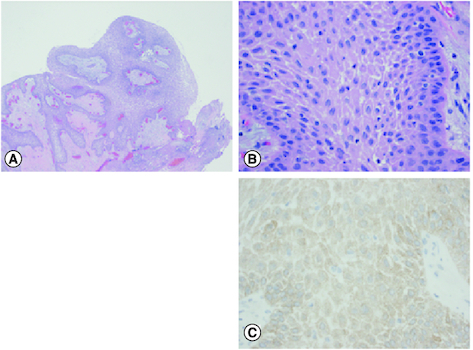
Figure 2. Histopathological findings.
Histopathology of the tumor, with H&E histology at 40× magnification (A), 400× magnification (B) and BRAF V600E immunohistochemistry at 400× magnification (C).
An epithelium-lined mass with papillary configuration is noted (A) consisting of well-differentiated stratified squamous epithelium (B) overall similar to the appearance of a squamous papilloma, and in this anatomic location, characteristic of a papillary craniopharyngioma. Mutation specific immunohistochemistry for BRAF V600E was positive (C), also typical of papillary craniopharyngioma.
After multi-disciplinary evaluation by neurosurgery, radiation therapy, and neuro-oncology, combination therapy with cobimetinib and vemurafenib was recommended rather than adjuvant radiation therapy. The varied perspectives and expertise of the involved consultants contributed to a comprehensive evaluation of the patient's condition, weighing factors such as the patient's young age, overall good health, and the tumor's close proximity to several important structures. These factors placed the patient at increased risk for the long-term toxicities that can be caused by radiation therapy including, but not limited to, second malignancy and damage to nearby structures.
Insurance authorization delayed therapeutic treatment and at a follow-up visit, the patient reported increased headache frequency and the MRI was consistent with disease progression four months after initial surgery (D). MRI findings indicated enlargement of the suprasellar craniopharyngioma from 2.4 × 2.0 × 1.7 cm to 2.3 × 2.3 × 3.0 cm, additional 0.6 cm diameter increase of the third ventricles bilaterally, and mild mass effect on the optic chiasm and infundibulum. Once insurance authorization was obtained, the patient started a 28-day course of the BRAF kinase inhibitor, vemurafenib (960 mg, PO, twice daily) and a 21-day course of the mitogen-activated, extracellular signal-regulated kinase (MEK) inhibitor, cobimetinib (60 mg, PO, daily). Metoclopramide (5 mg) was prescribed to take prior to vemurafenib for the first several days as an anti-emetic and clindamycin cream for rash prevention.
The patient tolerated the combination therapy and the main side effects were diarrhea, nausea, and hypertension. After 1 cycle of the combination therapy, the MRI showed a significant decrease in the size of the lesion from 2.3 × 2.3 × 3.0 cm to 1.0 × 1.0 × 1.2 cm and stable size in bilateral lateral ventricles (E). It was decided to continue this pharmacological therapy for 2 months with follow-up evaluation for possible surgery or radiation therapy pending results of a repeat scan. Despite a 12-day halt to the medications due to lack of supply, the repeat MRI imaging demonstrated further lesion reduction to 0.8 × 0.7 × 0.9 cm, less homogeneity and less solid-appearing. At this time, the patient had no side effects apart from mild nausea. Treatment was withheld and surveillance imaging was conducted on a regular basis. MRI has since demonstrated lesion size stability with the mass measuring 0.4 × 0.3 × 0.3 cm (H) now 29 months from holding treatment.
Discussion
Here, we highlight a rare presentation of a strictly third ventricle craniopharyngioma and build upon the literature of success in BRAF and MEK-targeted therapy. Craniopharyngiomas account for up to 4% of all intracranial tumors [Citation1], and strictly third ventricle craniopharyngiomas account for 0.7–11% of all cases [Citation2]. Unlike suprasellar craniopharyngiomas which tend to present chiefly with visual and/or endocrinologic changes, this rarer subset has been reported to clinically present with headache and raised intracranial pressure (ICP) due to cerebrospinal fluid (CSF) pathway obstruction [Citation1,Citation2,Citation5,Citation6] which is consistent with the case we report.
Prieto et al. describes characteristic T1 and T2-weighted imaging features of strictly intrinsic third ventricle tumors including: (i) a typical round shape, (ii) the downward deviation of the optic chiasm, (iii) a well-observed pituitary stalk, (iv) free chiasmic cistern, (v) mammillary body angle (MBA) is 30–60 degrees, and (vi) the hypothalamus region being situated below the lower third of the tumor [Citation7].
The current treatment plan for craniopharyngiomas consists of surgery and/or a combination of radiation and pharmacological therapy. Surgery can prove challenging due to the location of the craniopharyngioma and the imperative to protect the hypothalamus and adjacent structures. If surgical management is pursued, common approaches heavily depend on tumor location and a variety of approaches have been reported e.g. trans-laminal terminalis [Citation8], transcortical [Citation9], to transcallosal and more [Citation10,Citation11]. Given the difficult location of the tumor, radiation and targeted therapy may be used as primary or adjuvant therapies.
While radiation may help with tumor reduction [Citation12], consequential side effects have been reported in the literature including a dramatic recurrence rate [Citation13], radiation-induced glioma [Citation14], and cerebrovasculopathy [Citation15]. These adverse complications emphasize the need for alternative, more promising therapies. Herein, mitogen-activated protein kinase (MAPK) inhibitors may be such an avenue. Approximately 95% of papillary craniopharyngiomas have been reported to carry the BRAF V600E genetic mutation [Citation16] which constitutively activates the BRAF kinase in the MAPK pathway, leading to proliferation and tumor growth. This mutation is also found in metastatic melanomas and the FDA initially approved treatment of this group with BRAF V600E inhibitors dabrafenib and vemurafenib [Citation17].
This dual therapy has successfully treated other neoplasms but is only beginning to emerge in the literature for papillary craniopharyngiomas. For these brain tumors, therapy includes BRAF and/or MEK inhibitors such as trametinib. To date, there are reports of dual dabrafenib and trametinib therapy [Citation18–24], single-agent vemurafenib [Citation25], and single-agent dabrafenib [Citation26,Citation27] that have reduced tumor size by more than 50%.
Since 2017, there has been a phase II clinical trial to discern the treatment utility of vemurafenib and MEK inhibitor, cobimetinib, for patients with papillary craniopharyngiomas (NCT03224767). Cobimetinib preferentially binds to MEK1 relative to MEK2 while trametinib similarly binds to non-phosphorylated MEK1 and MEK2. Apart from the clinical trial, our report, to the best of our knowledge, is the first case to report impactful results from this combination therapy in a papillary craniopharyngioma.
Conclusion
Strictly third ventricle papillary craniopharyngiomas are rare variants of craniopharyngiomas that are complex to treat given their location. Surgical intervention may be helpful, but radiotherapy may induce hazardous side effects including recurrence, radiation-induced malignancy, and cerebrovascular sequelae. Our case report demonstrates successful tumor reduction with dual BRAF/MEK inhibitor therapy and suggests a potential role of dual BRAF/MEK inhibitor therapy as an adjuvant therapeutic option after surgical resection.
Author contributions
All listed authors participated in the writing of the manuscript and have read and approved the final version.
Financial disclosure
O Aboud is supported in part by the UC Davis Paul Calabresi Career Development Award for Clinical Oncology as funded by the National Cancer Institute/National Institutes of Health through grant #2K12CA138464-11. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval (IRB ID: 2001013-1) or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Acknowledgments
The authors would like to thank R O'Donnell for his critical review of this manuscript.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reference
- Hung ND, Ngan VK, Duc NM. Intrinsic third ventricular papillary craniopharyngioma: a report of five cases and literature review. Int Med Case Rep J 14, 83–87 (2021).
- Behari S, Banerji D, Mishra A et al. Intrinsic third ventricular craniopharyngiomas: report on six cases and a review of the literature. Surg. Neurol. 60(3), 245–252; discussion 252–243 (2003).
- Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers 5(1), 75 (2019).
- Goschzik T, Gessi M, Dreschmann V et al. Genomic alterations of adamantinomatous and papillary craniopharyngioma. J. Neuropathol. Exp. Neurol. 76(2), 126–134 (2017).
- Pascual JM, González-Llanos F, Barrios L, Roda JM. Intraventricular craniopharyngiomas: topographical classification and surgical approach selection based on an extensive overview. Acta Neurochir (Wien) 146(8), 785–802 (2004).
- Tayari N, Etemadifar M, Hekmatnia A, Mahzouni P, Maghzi AH, Rouzbahani R. Intrinsic third ventricular craniopharyngioma: a case report. Int J Prev Med 2(3), 178–185 (2011).
- Prieto R, Pascual JM, Barrios L. Topographic diagnosis of craniopharyngiomas: the accuracy of MRI findings observed on conventional T1 and T2 images. AJNR Am. J. Neuroradiol. 38(11), 2073–2080 (2017).
- Maira G, Anile C, Colosimo C, Cabezas D. Craniopharyngiomas of the third ventricle: trans-lamina terminalis approach. Neurosurgery 47(4), 857–863; discussion 863–855 (2000).
- Iwasaki K, Kondo A, Takahashi JB, Yamanobe K. Intraventricular craniopharyngioma: report of two cases and review of the literature. Surg. Neurol. 38(4), 294–301 (1992).
- Komotar RJ, Roguski M, Bruce JN. Surgical management of craniopharyngiomas. J. Neurooncol. 92(3), 283–296 (2009).
- Jung TY, Jung S, Jang WY, Moon KS, Kim IY, Kang SS. Operative outcomes and adjuvant treatment of purely third ventricle craniopharyngioma after a transcallosal approach. Br. J. Neurosurg. 26(3), 355–360 (2012).
- Ikezaki K, Fujii K, Kishikawa T. Magnetic resonance imaging of an intraventricular craniopharyngioma. Neuroradiology 32(3), 247–249 (1990).
- Bülow B, Attewell R, Hagmar L, Malmström P, Nordström CH, Erfurth EM. Postoperative prognosis in craniopharyngioma with respect to cardiovascular mortality, survival, and tumor recurrence. J. Clin. Endocrinol. Metab. 83(11), 3897–3904 (1998).
- Tolnay M, Kaim A, Probst A, Ulrich J. Subependymoma of the third ventricle after partial resection of a craniopharyngioma and repeated postoperative irradiation. Clin. Neuropathol. 15(2), 63–66 (1996).
- Sagoh M, Murakami H, Hirose Y, Mayanagi K. Occlusive cerebrovasculopathy after internal radiation and bleomycin therapy for craniopharyngioma--case report. Neurol Med Chir (Tokyo) 37(12), 920–923 (1997).
- Brastianos PK, Taylor-Weiner A, Manley PE et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat. Genet. 46(2), 161–165 (2014).
- Long GV, Eroglu Z, Infante J et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J. Clin. Oncol. 36(7), 667–673 (2018).
- Brastianos PK, Shankar GM, Gill CM et al. Dramatic response of BRAF V600E mutant papillary craniopharyngioma to targeted therapy. J. Natl Cancer Inst. 108(2), djv310 (2016).
- Nussbaum PE, Nussbaum LA, Torok CM, Patel PD, Yesavage TA, Nussbaum ES. Case report and literature review of BRAF-V600 inhibitors for treatment of papillary craniopharyngiomas: a potential treatment paradigm shift. J. Clin. Pharm. Ther. 47(6), 826–831 (2022).
- Juratli TA, Jones PS, Wang N et al. Targeted treatment of papillary craniopharyngiomas harboring BRAF V600E mutations. Cancer 125(17), 2910–2914 (2019).
- Bernstein A, Mrowczynski OD, Greene A et al. Dual BRAF/MEK therapy in BRAF V600E-mutated primary brain tumors: a case series showing dramatic clinical and radiographic responses and a reduction in cutaneous toxicity. J. Neurosurg. doi:10.3171/2019.8.Jns19643, 1–6 (2019).
- Rostami E, Witt Nyström P, Libard S, Wikström J, Casar-Borota O, Gudjonsson O. Recurrent papillary craniopharyngioma with BRAFV600E mutation treated with neoadjuvant-targeted therapy. Acta Neurochir (Wien) 159(11), 2217–2221 (2017).
- Roque A, Odia Y. BRAF-V600E mutant papillary craniopharyngioma dramatically responds to combination BRAF and MEK inhibitors. CNS Oncol 6(2), 95–99 (2017).
- Di Stefano AL, Guyon D, Sejean K et al. Medical debulking with BRAF/MEK inhibitors in aggressive BRAF-mutant craniopharyngioma. Neurooncol Adv 2(1), vdaa141 (2020).
- Aylwin SJ, Bodi I, Beaney R. Pronounced response of papillary craniopharyngioma to treatment with vemurafenib, a BRAF inhibitor. Pituitary 19(5), 544–546 (2016).
- Rao M, Bhattacharjee M, Shepard S, Hsu S. Newly diagnosed papillary craniopharyngioma with BRAF V600E mutation treated with single-agent selective BRAF inhibitor dabrafenib: a case report. Oncotarget 10(57), 6038–6042 (2019).
- Himes BT, Ruff MW, Van Gompel JJ et al. Recurrent papillary craniopharyngioma with BRAF V600E mutation treated with dabrafenib: case report. J. Neurosurg. doi:10.3171/2017.11.Jns172373, 1–5 (2018).

