Abstract
Even with the development of advanced catheter-based mapping systems, there remain several challenges in the electrophysiological evaluation and elimination of atrial arrhythmias. For instance, atrial tachycardias with irregular rates cannot be reliably mapped by systems that require stability in order to sequentially gather data points to be organized thereafter. Separately, these arrhythmias often arise following initial ablation for atrial fibrillation, posing logistic challenges. Here, we present the available literature summarizing the use of a non-contact mapping catheter, the AcQMap catheter, in conjunction with SuperMap, an algorithm that compiles a large number of non-contact data points from multiple catheter positions within the atria. These studies demonstrate the efficiency, safety and accuracy of this technology.
Plain language summary
Irregular heart rhythms (arrhythmias) are often treatable with medications, but sometimes require expert evaluation in a cardiac electrophysiology laboratory. They are often studied and treated using thin, flexible catheters which enter the body through blood vessels in the leg and reach the internal walls of the heart. Time, expertise and specialized equipment are necessary to identify characteristics specific to each patient’s arrhythmia. For each arrhythmia, a unique electrical blueprint is created before trying to eliminate it. The fleeting nature of certain arrhythmias can make it difficult to generate these blueprints, and many take a lot of time to accurately identify, leading to procedural challenges.
Here we evaluate studies discussing the use of a new catheter (AcQMap) and its accompanying strategy for identifying arrhythmias. Unlike traditional catheters that require direct contact with the internal walls of the heart, the AcQMap catheter floats within these blood-filled chambers and does not touch the walls when obtaining data points. Instead, using ultrasound waves and electrical signals, it can generate data points to create blueprints. This technology also uses a new algorithm that enables the catheter to move freely within the heart, obtaining numerous data points and grouping them together to create maps efficiently and safely, even for fleeting or challenging arrhythmias.
Twitter/X abstract
Mapping arrhythmias can be challenging and time consuming with the current technology. A new non-contact catheter and its novel SuperMap algorithm can safely solve these issues.
An increasing use of catheter ablation for the management of atrial fibrillation (AF) has led to a parallel rise in atrial tachycardias and atrial flutters [Citation1–6]. Some of these arrhythmias are found as mechanisms of recurrence, following initial attempts to ablate and eliminate AF. Often, these correspond with gaps in pulmonary vein isolation, an evidence-based method used for treatment of AF [Citation7,Citation8]. Others may arise from new substrate development, possibly due to creation of a scar during ablation [Citation6]. With this knowledge, some operators may equate the conversion of AF to a nonfibrillatory rhythm during ablation with acute procedural ’success’.
While differing in their modes of presentation, these atrial arrhythmias can often be more symptomatic than AF, upon presenting either for de novo ablation or repeat ablation following prior attempts [Citation9]. These require dedicated strategies to localize their mechanisms and to guide therapy. Logistically, these arrhythmias can present some of the greatest challenges and longest case-times in atrial ablation. Hence, novel strategies are under continuous development and evaluation to aid the operator [Citation10–12]. One of these strategies is termed ’superposition mapping’ and will be reviewed in this article. There are three main challenges associated with the management of these arrhythmias: first, they are frequently transient in nature; second, they may demonstrate variable or fully irregular cycle lengths; and third, there may be multiple distinct mechanisms which exist concurrently [Citation13–20].
First, transient atrial arrhythmias confound mapping attempts, as points acquired sequentially will only generate propagation data if a sufficient number of cycle lengths and anatomical chamber area are covered by the mapping catheter. If a rhythm self-terminates, or is ’bump-terminated’ due to catheter contact during mapping, the map will become clinically useless unless the exact same tachycardia can be reinduced (based on cycle length using current mapping systems). This, in part, was the motivation for the development of non-contact mapping systems, which can acquire global atrial data in a quicker fashion than traditional contact-based methods.
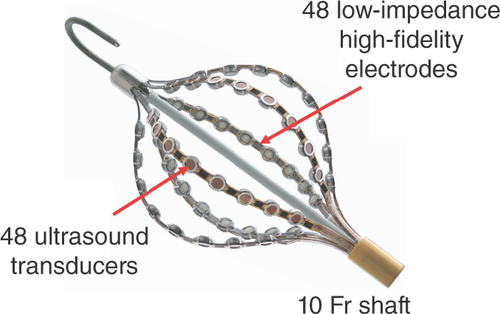
Second, the irregularity of an arrhythmia makes it particularly difficult to create an activation map with currently available, traditional contact-based systems, which require the arrhythmia to be relatively constant in its cycle length. These traditional systems, which collect data points from a mapping catheter in reference to a single point on a separate catheter typically placed in the coronary sinus, cannot reliably annotate arrhythmias with significant variation in their cycle length. Thus, the AcQMap System (Acutus Medical, CA, USA) () [Citation21] was developed in order to quickly generate 3D maps of heart chambers by using ultrasound transducers, while pairing concurrently obtained electrical activation data from adjacent electrodes. This system, which pairs 48 ultrasound transducers and 48 electrodes on six splines that expand to form a balloon-shaped recorder, allows for the rapid collection of a large number of data points without coming into direct contact with the myocardium [Citation21–23].
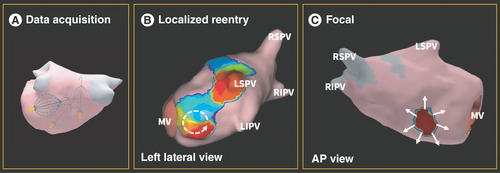
Third, irregular cycle lengths commonly occur when there are multiple arrhythmia mechanisms or circuits that coexist. An example is the presence of a focal beat interacting with a localized re-entrant circuit or a macro-re-entrant flutter () that can make mapping efforts even more challenging. Owing to the multifactorial mechanisms underlying atrial tachycardias, they are inefficient to map with current technology due to the same problems that irregularity in cycle lengths poses, as previously described. Therefore, these rhythms are frequently treated with direct current cardioversion at the end of a long procedure, but can recur clinically, because cardioversion is not a substrate-altering procedure in the same manner that catheter ablation is.
(A) Sample positions of the AcQMap catheter in relation to the walls of the left atrium. (B) SuperMap-derived display depicting a localized re-entrant circuit, with broken white arrow denoting directionality of the re-entrant circuit. (C) SuperMap-derived display depicting a focal source of automaticity, with white arrows denoting centrifugal depolarization.
AP: Anterior–posterior; LIPV: Left inferior pulmonary vein; LSPV: Left superior pulmonary vein; MV: Mitral valve; RIPV: Right inferior pulmonary vein; RSPV: Right superior pulmonary vein.
As a result, an algorithm was derived to aggregate data from the AcQMap catheter in multiple positions, originally named ’superposition mapping’ and now termed ’SuperMap’. After creating an anatomical map using the aforementioned ultrasound transducers, the algorithm compiles data points as the catheter is rotated about in the chamber of interest, sampling through the electrodes at a rate of approximately 9 million data points/min [Citation24]. As a result, an anatomical map demonstrating arrhythmia propagation and chamber depolarization can be created in minutes and is able to effectively address multiple cycle lengths and activation patterns on the reference catheter [Citation25–27].
Aside from the Acutus and SuperMap software, this catheter does not require additional, special techniques for access nor positioning of the catheter once deployed. It utilizes a 16 French sheath for percutaneous venous access, and the software is automatically able to accept or reject data points based on proper catheter position. Finally, it is able to generate traditional contact-based amplitude maps without the need for additional catheters. There is a growing sample of literature describing the use of the AcQMap catheter, and specifically its use with the SuperMap algorithm to map complex arrhythmias [Citation28–30]. We sought to evaluate the available literature and present a summary of the current understanding, followed by updated data from our clinical experience with the catheter and algorithm. We then compare this with the current standard of care in contact mapping algorithms.
Materials & methods
We conducted a review of the current literature to identify available publications focusing on the use of non-contact mapping in the management of atrial arrhythmias, specifically including articles which aggregated data from multiple positions, an algorithm known as SuperMap. The search identified published works available on PubMed through June 2023. Following an initial manual search, a list of key terms and phrases were used to direct subsequent search iterations (’SuperMap’ and ’non-contact mapping’). Separate searches of the references included within each publication were conducted to identify any potentially eligible articles for inclusion.
Results
Study & patient selection
We included four publications in our study, all of which are original works [Citation31–34]. The sample sizes within each study ranged from 7 to 31, with a total of 79 individuals evaluated (). Two studies (Pope et al. [Citation31] and Shi et al. [Citation32]) used blinding methods to compare the mapping characteristics and accuracy of non-contact maps generated by SuperMap with contact maps, while two other studies (Liebregts et al. [Citation34] and Ramak et al. [Citation33]) described the clinical feasibility and safety of this technology. In all studies, termination of arrhythmia(s) without further inducibility was considered a clinically significant end point.
Table 1. Comparison of studies and patient selection.
In the study by Ramak et al., patients were selected for inclusion if they demonstrated recurrence of atrial tachycardia following successful pulmonary vein isolation for AF using cryoballoon ablation [Citation33]. In conjunction with the traditional reference catheters (quadripolar in the inferior vena cava and decapolar in the coronary sinus), mapping was performed with the AcQMap catheter in the right atrium as well as left atrium following trans-septal puncture.
Liebregts et al. selected patients for inclusion if they were treated for persistent AF or atypical atrial flutter in a procedure employing the AcQMap catheter, in conjunction with a quadripolar reference catheter in the coronary sinus [Citation34].
In the study by Shi et al., patients were selected for inclusion if they were referred for catheter ablation of medication-refractory atrial tachycardias [Citation32]. AcQMap catheters were used to generate SuperMap results as part of the study, in conjunction with the traditional reference catheters as in Ramak et al. [Citation33]. Mapping using the AcQMap catheter preceded the use of high-density contact mapping methods (HD Grid and NavX Ensite, Abbott EP, IL, USA) prior to ablation. For comparison of the activation maps generated by each catheter, five blinded reviewers were asked to evaluate the results of all 50 maps (25 for each SuperMap and contact map).
Finally, Pope et al. selected patients for inclusion if they were referred for ablation of atrial tachycardias either de novo or occurring following prior AF ablation procedures [Citation31]. AcQMap catheters were used to generate SuperMap results, in conjunction with reference decapolar catheters in the coronary sinus. In a subgroup of patients, concurrent mapping was performed using contact mapping techniques. For comparison of the depolarization maps generated by each catheter, distinct operators were tasked with completion of SuperMaps and contact maps to ensure blinding of each operator to the opposing technique.
Safety & feasibility
In all studies, complications were reported as observed. Out of a total sample size of 79 patients, a total of two patients experiencing complications were reported by Liebregts et al. [Citation34] in the treatment of atrial flutter. One individual developed hemiparesis for which acute thrombolysis was required. The same individual and another developed complete atrioventricular block, for which a permanent pacemaker was required. Otherwise, no complications related to vascular access, catheter manipulation, or ventricular arrhythmias were reported. This group’s reported incidence of complications (18.2%) is likely higher due to a relatively small sample size. Feasibility was quantified, where possible, using measured times required for creation of anatomical and electrical maps and total procedural time ().
Table 2. Study demonstration of procedural feasibility and safety.
Efficiency, accuracy & comparison with traditional contact mapping
In two studies, maps generated by SuperMap and AcQMap acquisition were compared directly with maps generated by current standard-of-care contact-based catheters and algorithms (). In the study by Shi et al., blinded independent reviewers assessed the accuracy of the maps without knowledge of the clinical outcomes [Citation32]. These blinded reviewers reached a majority concordance (at least three out of five) in identifying the arrhythmia’s mechanism in all AcQMap series (26/26, 100%) and in identifying the site of arrhythmia termination in 76% of series using AcQMap (by comparison, 78% in the same patients using matched contact maps). In the other study, by Pope et al. [Citation31], primary operators were blinded to the initial results of the non-contact map prior to proceeding with contact mapping. These blinded operators independently performed SuperMap data acquisition and contact map data acquisition, resulting in a majority concordance between the compared methods in 79% of cases.
Table 3. Procedural success and blinded comparison with traditional contact-based mapping.
Novel data from clinical experience
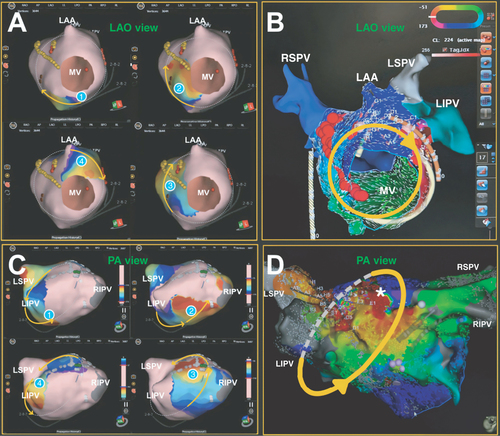
Extending from these previous studies, applications of a dual-mapping approach involving SuperMap and CARTO (Biosense Webster, CA, USA) have yielded novel data confirming agreement between the two mapping techniques. In the first case of an atypical flutter (seen in A), SuperMap detected a re-entrant circuit revolving around the mitral isthmus with a cycle length of 228 ms. Consistently, a similar circuit with a cycle length of 224 ms was seen in the CARTO vector map (B). Delivering the radiofrequency ablation to an area traversing the circuit terminated the flutter. In the second case, an atypical roof flutter was detected in the left atrium using both SuperMap and CARTO with cycle lengths of 230 and 228 ms, respectively (C & D). Roof line and anteroseptal linear ablations terminated the flutter.
(A) Numerically ordered panels of SuperMap displaying the activation sequence of an atypical mitral flutter chronologically. (B) Corresponding CARTO vector map. (C) Numerically ordered panels of SuperMap portraying the activation sequence of an atypical roof flutter in the left atrium chronologically. (D) Corresponding CARTO activation map showing early activation meets latest activation in the left atrium (asterisk). Anatomical surfaces in red correspond to early activation, and surfaces in blue indicate late activation. Yellow arrows denote direction of flutter waves.
CL: Cycle length; LAA: Left atrial appendage; LAO: Left anterior oblique; LIPV: Left inferior pulmonary vein; LSPV: Left superior pulmonary vein; MV: Mitral valve; PA: Posterior–anterior; RIPV: Right inferior pulmonary vein; RSPV: Right superior pulmonary vein.
Discussion
The AcQMap catheter and software is a distinct paradigm shift from traditional contact maps when mapping atrial arrhythmias. The AcQMap catheter is capable of quickly obtaining an ultrasound-based anatomical view of the chamber of interest, followed by acquisition of electrophysiological data points from distinct positions within the same chamber without direct contact with the myocardium. Despite its panoramic view of the entire chamber, its spatiotemporal resolution is equal to or greater than that of many traditional conventional voltage maps, demonstrating that the rapidity with which these maps are generated does not come at the cost of decrement in map density nor accuracy.
When directly comparing SuperMap-generated maps with traditional contact-based voltage maps, two studies demonstrated a significant difference in the amount of time required to generate a depolarization map: approximately 10 min in Shi et al. and 4 min in Pope et al. [Citation31,Citation32]. The comparison also revealed accuracy in the software’s ability to identify the mechanism of arrhythmia in nearly all cases. Shi et al. demonstrated that blinded reviewers of SuperMap results were able to correctly identify the mechanism of arrhythmia in 80% of all cases, and Pope et al. demonstrated that the concordance between SuperMap and contact mapping results approached 80% when performed by blinded, separate operators [Citation31,Citation32]. Overall, these findings highlight the advantage of the SuperMap in decreasing procedure time while maintaining high accuracy.
Additionally, the assumption of a given atrial arrhythmia’s stability is required to maintain the fidelity of a sequentially generated depolarization map. Traditional 3D mapping systems are vulnerable to variations in the cycle length of clinical arrhythmias and changes in the activation patterns within stable cycle lengths. In contrast, the AcQMap catheter is able to generate a large amount of usable data within a short period of time, and the SuperMap algorithm enables distinction between multiple types of arrhythmias as they occur during a single electrophysiological study. Fundamental to this difference between non-contact and contact mapping is the need to sequentially data in a ’point-by-point’ manner in traditional contact mapping. This sequential approach increases procedural duration, fluoroscopy exposure and potential complications. In contrast, the ability to simultaneously gather a large number of data points using non-contact mapping minimizes the susceptibility to cycle length variations, sudden arrhythmia termination or co-occurring arrhythmias.
Furthermore, roving the AcQMap catheter around the chamber confers an increased accuracy in stable rhythms. In the Shi et al. study, a computational modeling approach demonstrated that placing the AcQMap catheter in 60 different locations with the chamber of interest is sufficient to generate a depolarization map of maximal accuracy, measured by reaching an asymptote in the root mean square error of the values of interest. This was demonstrated to be achievable within 60–180 s in the patients studied [Citation32]. These findings set the parameters for SuperMap in its current clinical use.
Finally, SuperMap and CARTO propagation maps displayed a high degree of visual similarity in the two cases described in , which is consistent with the findings described in Shi et al. Qualitatively, both mapping techniques demonstrated concordance in mapping atypical flutters, in which ablation targeting the areas terminated the arrhythmias. Further studies comparing SuperMap and automated CARTO algorithms for processing maps are needed to further explore their similarities, differences and clinical utilities.
Even with these advantages and demonstrated non-inferiority, prior studies have noted that in spite of a high success rate in the immediate postprocedural setting, recurrence of persistent atrial tachyarrhythmias, particularly AF, remained high, suspected to be in part due to limited experience in the use of the AcQMap catheter. What remains to be further explored is the combination of non-contact mapping with roving techniques, in an effort to optimize the use of non-contact characterization of arrhythmia mechanisms, while simultaneously generating depolarization maps that are immune to the effects of transient, variable or confounding arrhythmia mechanisms. We believe that accumulated real-world experience with AcQMap and the SuperMap algorithm will optimize procedural times, outcomes and sustained freedom from arrhythmias.
Conclusion
Future research should continue to include blinded comparisons of mapping acquisition by non-contact SuperMap methods with traditional contact-based methods, as well as blinded comparisons of map interpretation by independent reviewers in addition to software-based identification of arrhythmias’ characteristics. Separately, patient outcomes should be evaluated in a longitudinal fashion, in order to identify any potential differences in the most clinically relevant end point of interest: arrhythmia recurrence.
Background
Certain arrhythmias may be irregular and transient in nature, posing challenges in the approach to catheter-based ablation. This is especially true in individuals who have already undergone catheter-based ablation previously. The AcQMap catheter is able to quickly and safely obtain anatomic and electrical maps without contacting the myocardium directly.
Study & patient selection
Four studies have been published describing the use of SuperMap with the AcQMap catheter describing its feasibility and safety.
Safety & feasibility
The SuperMap algorithm is able to quickly aggregate data from multiple catheter positions, generating maps of significantly higher resolution than those created by traditional, contact-based mapping.
Efficiency, accuracy & comparison with traditional contact mapping
Two studies have compared maps generated by SuperMap against traditional contact-based voltage maps, demonstrating equivalence in accuracy and superiority in efficiency.
Novel data from clinical experience
Several individual patients have undergone successful ablation of complex arrhythmias using the AcQMap catheter and SuperMap technology.
Author contributions
C Lin: literature review, text composition and manuscript finalization. A Nguyen: data collection and comparative analysis. I Ling, R Partow-Navid, S Leung, A Zadeh, ’I Ho’: manuscript review. J Zaman: text editing, literature review, data summarization and project oversight.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- Gerstenfeld EP , CallansDJ , DixitSet al. Mechanisms of organized left atrial tachycardias occurring after pulmonary vein isolation. Circulation110(11), 1351–1357 (2004).
- Wasmer K , MönnigG , BittnerAet al. Incidence, characteristics, and outcome of left atrial tachycardias after circumferential antral ablation of atrial fibrillation. Heart Rhythm9(10), 1660–1666 (2012).
- Chugh A , OralH , LemolaKet al. Prevalence, mechanisms, and clinical significance of macroreentrant atrial tachycardia during and following left atrial ablation for atrial fibrillation. Heart Rhythm2(5), 464–471 (2005).
- Sághy L , TutuianuC , SzilágyiJ. Atrial tachycardias following atrial fibrillation ablation. Curr. Cardiol. Rev.11(2), 149–156 (2015).
- Biviano AB , CiaccioEJ , FleitmanJet al. Atrial tachycardias after atrial fibrillation ablation manifest different waveform characteristics: implications for characterizing tachycardias. J. Cardiovasc. Electrophysiol.26(11), 1187–1195 (2015).
- Chae S , OralH , GoodEet al. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J. Am. Coll. Cardiol.50(18), 1781–1787 (2007).
- Cosedis Nielsen J , JohannessenA , RaatikainenPet al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N. Engl. J. Med.367(17), 1587–1595 (2012).
- Perez FJ , SchubertCM , ParvezB , PathakV , EllenbogenKA , WoodMA. Long-term outcomes after catheter ablation of cavo-tricuspid isthmus dependent atrial flutter: a meta-analysis. Circ. Arrhythm. Electrophysiol.2(4), 393–401 (2009).
- Markowitz SM , ThomasG , LiuC , CheungJW , IpJE , LermanBB. Atrial tachycardias and atypical atrial flutters: mechanisms and approaches to ablation. Arrhythm. Electrophysiol. Rev.8(2), 131–137 (2019).
- Wijffels MC , KirchhofCJ , DorlandR , AllessieMA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation92(7), 1954–1968 (1995).
- Verheule S , TuylsE , van HunnikA , KuiperM , SchottenU , AllessieM. Fibrillatory conduction in the atrial free walls of goats in persistent and permanent atrial fibrillation. Circ. Arrhythm. Electrophysiol.3(6), 590–599 (2010).
- Saoudi N , CosioF , WaldoAet al. Classification of atrial flutter and regular atrial tachycardia according to electrophysiologic mechanism and anatomic bases: a statement from a joint expert group from the Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. J. Cardiovasc. Electrophysiol.12(7), 852–866 (2001).
- Haissaguerre M , HociniM , DenisAet al. Driver domains in persistent atrial fibrillation. Circulation130(7), 530–538 (2014).
- Nattel S , DobrevD. Controversies about atrial fibrillation mechanisms: aiming for order in chaos and whether it matters. Circ. Res.120(9), 1396–1398 (2017).
- Willems S , VermaA , BettsTR. Targeting nonpulmonary vein sources in persistent atrial fibrillation identified by noncontact charge density mapping: UNCOVER AF Trial. Circ. Arrhythm. Electrophysiol.12(7), e007233 (2019).
- Roberts-Thomson KC , KistlerPM , KalmanJM. Atrial tachycardia: mechanisms, diagnosis, and management. Curr. Probl. Cardiol.30(10), 529–573 (2005).
- Kim YH , ChenSA , ErnstSet al. 2019 APHRS expert consensus statement on three-dimensional mapping systems for tachycardia developed in collaboration with HRS, EHRA, and LAHRS. J. Arrhythm.36(2), 215–270 (2020).
- Rostock T , DrewitzI , StevenDet al. Characterization, mapping, and catheter ablation of recurrent atrial tachycardias after stepwise ablation of long-lasting persistent atrial fibrillation. Circ. Arrhythm. Electrophysiol.3(2), 160–169 (2010).
- Hayashi K , MathewS , HeegerCHet al. Pace mapping for the identification of focal atrial tachycardia origin: a novel technique to map and ablate difficult-to-induce and nonsustained focal atrial tachycardia. Circ. Arrhythm. Electrophysiol.9(7), e003930 (2016).
- Wieczorek M , SaliliAR , KaubishS , HoeltgenR. Catheter ablation of non-sustained focal right atrial tachycardia guided by virtual non-contact electrograms. Europace13(6), 876–882 (2011).
- Zaman JA , GraceAA , NarayanSM. Future directions for mapping atrial fibrillation. Arrhythm. Electrophysiol. Rev.11, e08 (2022).
- Bala G , DeAsmundis C , ChierchiaG. A novel noncontact high-resolution charge density mapping system to guide ablation of complex atrial arrhythmias: overview of device technology and application. Expert Rev. Med. Devices18(4), 343–350 (2021).
- Shi R , ChenZ , ButcherCet al. Diverse activation patterns during persistent atrial fibrillation by noncontact charge-density mapping of human atrium. J. Arrhythm.36(4), 692–702 (2020).
- Grace A , VermaA , WillemsS. Dipole density mapping of atrial fibrillation. Eur. Heart J.38(1), 5–9 (2017).
- Grace A , WillemsS , MeyerCet al. High-resolution noncontact charge-density mapping of endocardial activation. JCI Insight4(6), e126422 (2019).
- Gagyi RB , NotenAME , LesinaKet al. Single-beat global atrial mapping facilitates the treatment of short-lived atrial tachycardias and infrequent premature atrial contractions. J. Interv. Card. Electrophysiol.66(4), 951–959 (2023).
- Gagyi RB , NotenAME , WijchersSet al. Dipole charge density mapping integrated in remote magnetic navigation: first-in-human feasibility study. Int. J. Cardiol. Heart Vasc.42(2022), 101095 (2022).
- Pope MTB , BettsTR. Global substrate mapping and targeted ablation with novel gold-tip catheter in de novo persistent AF. Arrhythm. Electrophysiol. Rev.11(2022), e06 (2022).
- Shi R , ParikhP , ChenZet al. Validation of dipole density mapping during atrial fibrillation and sinus rhythm in human left atrium. JACC Clin. Electrophysiol.6(2), 171–181 (2020).
- Shi R , NormanM , ChenZ , WongT. Individualized ablation strategy guided by live simultaneous global mapping to treat persistent atrial fibrillation. Future Cardiol.14(3), 237–249 (2018).
- Pope MTB , LeoM , Briosae Gala A , BettsTR. Clinical utility of non-contact charge density ’SuperMap’ algorithm for the mapping and ablation of organized atrial arrhythmias. Europace24(5), 747–754 (2022).
- Shi R , ZamanJAB , ChenZet al. Novel aggregated multiposition noncontact mapping of atrial tachycardia in humans: from computational modeling to clinical validation. Heart Rhythm19(1), 61–69 (2022).
- Ramak R , ChierchiaG , Paparellaet al. Novel noncontact charge density map in the setting of post-atrial fibrillation atrial tachycardias: first experience with the Acutus SuperMap Algorithm. J. Interv. Card. Electrophysiol.61(1), 187–195 (2021).
- Liebregts M , WijffelsMCEF , KlaverMN , van DijkVF , BaltJC , BoersmaLVA. Initial experience with AcQMap catheter for treatment of persistent atrial fibrillation and atypical atrial flutter. Neth. Heart J.30(5), 273–281 (2022).

