Abstract
Spontaneous intracranial hypotension (SIH) linked to the leakage of cerebrospinal fluid typically manifests as positional headaches. The connection between connective tissue disorders and SIH is widely recognized. Nevertheless, instances of SIH related to systemic lupus erythematosus are infrequently reported. We report a 42-year-old female with systemic lupus erythematosus and antiphospholipid syndrome who presented with severe orthostatic headaches. Imaging revealed bilateral subdural hematomas and diffuse pachymeningeal enhancement. Conservative treatment was administered, and the patient showed improvement with resolution of symptoms. It is important to note that not all imaging studies consistently reveal all the characteristic features of SIH. Due to these diagnostic limitations, it is crucial for clinicians to consider a combination of patient history, clinical evaluation and imaging findings meticulously.
Introduction
SIH may occur in SLE patients who present with orthostatic headaches.
Case Presentation
We reported a 42-year-old female with SLE and APS presenting with a severe orthostatic headache. The patient's medical history, current medications, and diagnostic findings, including laboratory results and imaging, are discussed.
Discussion
There is no single definitive test to diagnose IH. It often requires a combination of clinical evaluation, imaging, and other diagnostic procedures, which can be inconclusive. Furthermore, it is important to note that not all imaging studies consistently reveal all the characteristic features of SIH.
Conclusion
Due to these diagnostic limitations, it is crucial for healthcare providers to meticulously consider a combination of clinical evaluation, patient history, and imaging findings when suspecting SIH, especially in the context of a SLE patient presenting with headaches.
Antiphospholipid syndrome (APS), one of the systemic autoimmune diseases, is primarily diagnosed based on clinical manifestations related to various vascular accidents and presence of antiphospholipid antibodies. It can be categorized into two groups of primary or secondary which can co-occur with other autoimmune diseases, especially systemic lupus erythematosus (SLE) [Citation1].
In neurological complaints, headache has a high prevalence among patients with rheumatological diseases [Citation2,Citation3]. In patients with SLE and APS, headaches may arise from diverse etiologies, including vascular diseases such as cerebral venous sinus thrombosis (CVST) and vasculitis, as well as infectious conditions like meningitis, and spontaneous intracranial hypotension (SIH) [Citation4].
Intracranial hypotension (IH) is a rare clinical manifestation with an incidence of 5 per 10,000 per year and is linked to cerebrospinal fluid (CSF) leakage, mainly located at the thoracic or cervicothoracic junction level [Citation5]. Typically, this condition manifests as a headache that worsens when a person is in an upright position and gets better when they lie down. Patients with this condition may also experience symptoms such as nausea, vomiting, neck pain, dizziness, facial paresthesia, radiculopathies, and, in some cases, galactorrhea. This occurrence can be broadly divided into two categories: primary (occurring spontaneously) and secondary (resulting from medical procedures or trauma). This phenomenon could rarely mix with a severe neurological complication known as subdural hematoma (SDH) [Citation6]. Nevertheless, the concurrence of SIH related to SLE has rarely been reported.
Herein, we report a rare case of IH and Bilateral SDH concurrence with APS and SLE. In addition, we discuss the current literature on the association between these clinical manifestations.
Case Presentation
A 42-year-old female with a history of SLE, and APS was presented to the Neurology Emergency Department of Shahid Rajaee Hospital in Karaj (Iran) with a severe orthostatic headache that started three days before her admission. The patient was diagnosed with SLE and APS approximately 21 years ago due to complaints of interphalangeal and metacarpophalangeal joints swelling. She had been under the supervision of her rheumatologist periodically. The patient's past medical history included three abortions at the first trimester, CVST approximately eight years ago, and a transient ischemic attack (TIA) about 13 years ago, which presented with dysarthria and left hemiplegia. She also had a history of recurrent deep vein thrombosis (DVT) occurrences within the last twenty years. There was no history of seizures, diabetes, hyperlipidemia, hypertension, or other significant diseases. No recent head trauma was reported. The patient had been on a daily long-standing of 2.5 mg warfarin, 50 mg azathioprine, 80 mg aspirin and 5 mg prednisolone.
At the time of admission, the patient complained about a severe headache that get worse by sitting, leaning forward, and standing. She had no fever, nausea, vomiting, tinnitus, vertigo, blurred vision, facial paresthesia, or altered mental status. Blood pressure was 140/90 mmHg, and her other vital signs were in normal range. The Glasgow Coma Scale (GCS) score was 15/15. The patient's optic disc examination was unremarkable, showing no signs of papilledema. On neurological examination cranial nerves, muscle forces, and reflexes were all normal. There was no sensory deficit. Laboratory data showed: hemoglobin (Hgb): 11.8 g/dl, white blood cell (WBC): 8.8 × 103 cells/mm3, platelet (Plt): 204 × 103 cells/mm3, prothrombin time (PT): 23.7 s, international normalized ratio (INR): 2, partial thromboplastin time (PTT): 50 s, alanine transaminase (ALT): 38 U/l, aspartate aminotransferase (AST): 31 U/l, alkaline phosphatase (ALP): 98 U/l, creatinine: 0.88 mg/dl, and lactate dehydrogenase (LDH): 240 U/l. C reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were normal. The patient's electrocardiography (ECG) was normal, and the ejection fraction (EF) was 55%. Based on her previous records; complementary hematological test findings revealed: antithrombin III: 111 (75–120), protein C: 105 (70–140), protein S: 110 (60–140), factor V Laden 153.2 (>120), D-dimer: 155 ng/ml (<500 ng/ml), and normal level of Von Willebrand factor-cleaving protease (ADAMTS13). Other serological and immunological tests were positive for antinuclear antibody (ANA), anti-ds-DNA, anticardiolipin, anti-phospholipid, and anti-beta-2-glycoprotein antibody.
Computed Tomographic (CT) scan of her brain revealed bilateral isodense to hypodense crescent-shaped masses overlying and compressing the brain, suggestive of subacute bilateral subdural hematoma. Magnetic Resonance Imaging (MRI) of her brain revealed diffuse pachymeningeal enhancement and extra-axial Cerebrospinal Fluid (CSF) excess ( & ). No evidence of acute intraparenchymal hemorrhage was observed. Magnetic Resonance Venography (MRV) was normal.
Figure 1. Bilateral subdural hematoma and diffuse pachymeningeal enhancement.
T1-weighted MRI images with gadolinium enhancement displayed the presence of bilateral subdural hematoma and widespread pachymeningeal enhancement, as observed in both coronal (A) and axial (B) sections. Additionally, axial sections of Flair MRI exhibited bilateral subdural hematoma (C).
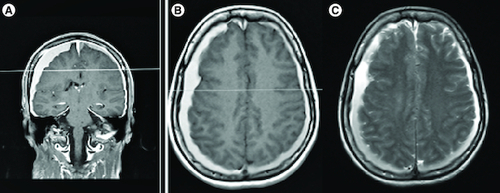
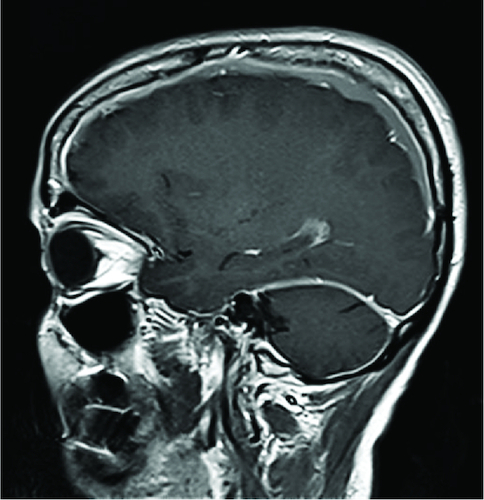
Figure 2. In the sagittal section of T1-weighted MRI with gadolinium enhancement showed diffuse pachymeningeal enhancement of the dura mater in the posterior fossa surrounding the cerebellum.
The clinical presentation and imaging findings raised suspicions of IH. Brain CT scan excluded the skull base as a probable site of a CSF leak. Additionally, CT myelogram investigations were normal. Lumbar Puncture (LP) was initially withheld due to an elevated INR, and it was canceled later as the patient was still considered high-risk for the procedure.
She received conservative treatment, including complete bed rest, fluid therapy, analgesics, anticonvulsants, dexamethasone, and holding anticoagulation therapy. Serum mannitol therapy was also administered. After four days of treatment, the patient showed improvement, and subsequently transferred from the ICU to the inpatient department and later discharged home without any headache. The Warfarin dosage was temporarily withheld but restarted on the 7th day in a minimum dosage. The INR levels were closely monitored, and the goal of reaching an INR range of 2.0–2.5 was achieved after a month. After 3 months of follow-up the patient was doing well without any new events of her APS or new SIH occurrence.
Discussion
The pathophysiological aspect of the headache reported in the SLE and APS comprises a wide range of psychological or structural complications. Intracranial hypotension (IH) refers to a condition where the CSF pressure is lower than 7 mm H2O [Citation7]. It is characterized by a positional headache that worsens when in an upright position and decreases when lying flat. There are more rare symptoms such as nausea, vertigo, neck pain, facial paresthesia, upper limb radiculopathy, and galactorrhea. IH can be broadly classified into two categories: primary (spontaneous) and secondary (iatrogenic or post-traumatic) [Citation8].
IH diagnosis is established by performing a lumbar puncture (LP), which indicates a reduced opening pressure [Citation7]. However, a challenging aspect in our case was the contraindication for LP due to the patient's INR levels. As a result, we had to rely on the clinical and imaging findings to establish the diagnosis.
In our case, imaging findings showed diffused patchy meningitis with bilateral subdural hematoma. Based on the Monro–Kellie theory, CSF leakage results an expansion in vascular spaces, primarily venous, due to their higher compliance. This leads to increased blood flow to these vessels. The dural vessels lack a blood-brain barrier, allowing the increased blood flow to be accompanied by effusion into the dural spaces. These processes manifest as diffuse dural enhancement, observed through contrast imaging, as well as the presence of subdural hematomas and hygromas in spontaneous intracranial hypotension (SIH). The pituitary gland, which also lacks a blood-brain barrier, experiences compensatory hyperemia in the event of a CSF leak [Citation8–10].
SIH is usually caused by a CSF leak in the spine. Dural dehiscence in the presence of a meningeal diverticulum, tears resulting from degenerative changes, and developmental anomalies are common causes of a CSF leak [Citation11]. The spinal meninges are particularly vulnerable around the spinal nerve roots, where the arachnoid layer may protrude through the dura, forming a diverticulum that is susceptible to tears. The exact cause of the tear remains unclear but may be associated with minor trauma leading to stretching of the nerve root sleeves [Citation12]. Patients with genetic connective tissue disorders, such as Ehlers-Danlos and Marfan syndrome, are speculated to be at a higher risk of CSF leaks than the general population [Citation13]. However, our patient had no documented history of genetical connective tissue disorders, and a CT myelogram showed no evidence of other potential causes for a CSF leak.
We present a case of SIH in a patient with a known history of SLE and APS. To date, based on our knowledge, only five similar cases have been reported previously [Citation14–16]. The CNS manifestations of SLE are diverse, including instances of both intracranial hypotension and hypertension. In SLE-related intracranial hypertension, arachnoid granulations blocking by inflammatory cells or immune complexes within the choroid plexus is believed to play a role. Conversely, there is a potential association between SIH and SLE, possibly resulting from inflammation-associated damage of the dura [Citation17].
Related to our case, there was a report on the IH presented by Cohen Addad et al., with the pathophysiology of inflammatory-related responses and dura perforation [Citation18]. In addition, in a case presented by Garcia et al., the IH was the result of dura matter thickness existing with subdural effusion. Moreover, based on the imaging presentation of hypertrophic pachymeningitis, as well as, CSF hypovolemia, a meningeal inflammation was stated with the explanation of meningeal disruption resulting in CSF leak [Citation19]. Accordingly, CSF leak as the cause of IH was mentioned in another case report with the origin of the cervicothoracic junction bilaterally detected by single-photon emission computed tomography (SPECT/CT) [Citation20].
An important aspect of this case is considering infectious causes indicated in the imaging evidence such as pachymeningeal enhancement, in the case of using immunosuppressive agents [Citation21]. In our case, the absence of fever, normal leukocyte number, and normal serum inflammation indicators was in favor of non-infectious etiologies.
The diagnosis of IH can be challenging due to several diagnostic limitations and factors that healthcare professionals need to consider. Symptoms of IH can present in atypical ways, which may not fit the classic clinical and imaging criteria, making diagnosis more challenging [Citation4]. While MRI is a key tool in diagnosing IH, the radiological findings can be subtle and may require an experienced radiologist to identify characteristic features [Citation22]. Moreover, Imaging studies may not always show clear evidence of IH, especially if the CSF leak is small or intermittent. This can lead to false-negative results [Citation23]. Notably imaging performed early in the course of the condition may not capture all the changes and repeat imaging may be necessary [Citation24]. Detecting a CSF leak can pose challenges, especially in cases of SIH. The leak could appear at any location along the neuroaxis and may vary in flow rate (slow, intermediate, or high flow). The initial stage in diagnosis involves confirming IH through contrast-enhanced brain MRI. Subsequently, different imaging modalities are utilized to detect and locate a spinal CSF leak [Citation25]. Caution is required as pooling of contrast at the C1–C2 vertebral body level may be mistaken for a leak [Citation26]. For slow leaks, high spatial and contrast resolution techniques with varied timing may be necessary due to their variability [Citation25]. In our case CT-myelogram was normal and we couldn't find the source of leakage.
The treatment strategy mentioned in case reports and literature dealing with SIH is a conservative management. This kind of approach includes hydration, caffeine and theophylline, which modulate adenosine receptor activity with the goal of arterial vasoconstriction [Citation27]. In another case report with a more similar clinical manifestation to our case, the IH was presented with pachymeningeal enhancement in an SLE patient, in which methylprednisolone pulse was prescribed with a dosage of 1 gm/day for 3 days, subsequently, the oral regimen was replaced and eventually, Azathioprine was administrated [Citation21]. Based on the literature, there was a remarkable response of SLE patients with IH or subdural hemorrhage to immunosuppressive therapy. Correspondingly, the therapeutic procedure is completed within a week [Citation21,Citation28]. For a long-term immunosuppressive drug regimen, azathioprine could be prescribed [Citation21]. In the context refractory CSF leak and the failure of conservative management, the next step involves the administration of a nontargeted epidural blood patch. If this procedure does not yield satisfactory results, precise localization becomes paramount for the implementation of a targeted blood patch [Citation29].
Conclusion
SIH may occur in SLE patients who present with orthostatic headaches. There is no single definitive test to diagnose IH. It often requires a combination of clinical evaluation, imaging, and other diagnostic procedures, which can be inconclusive. Furthermore, it is important to note that not all imaging studies consistently reveal all the characteristic features of SIH. Due to these diagnostic limitations, it is crucial for healthcare providers to meticulously consider a combination of clinical evaluation, patient history, and imaging findings when suspecting SIH, especially in the context of a SLE patient presenting with headaches.
Author contributions
Arsh Haj Mohamad Ebrahim Ketabforoush, and Nahid Abbasi Khoshsirat involved in study concept and design. Zahra Ahmadian did acquisition of data. Arsh Haj Mohamad Ebrahim Ketabforoush, Zahra Ahmadian, Ali Hosseinpour, and Nahid Abbasi Khoshsirat drafted the manuscript. Nahid Abbasi Khoshsirat, Arsh Haj Mohamad Ebrahim Ketabforoush, Zahra Ahmadian, and Ali Hosseinpour involved in critical revision of the manuscript for important intellectual content. Nahid Abbasi Khoshsirat, Arsh Haj Mohamad Ebrahim Ketabforoush, Zahra Ahmadian, and Ali Hosseinpour involved in administrative, technical, and material support. Nahid Abbasi Khoshsirat supervised the study.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained verbal and written informed consent from the patient/patients for the inclusion of their medical and treatment history within this case report.
Acknowledgments
The authors want to thank the Clinical Research Development Unit (CRDU) of Shahid Rajaei Hospital, Alborz University of Medical Sciences, Karaj, Iran, for their support, cooperation, and assistance throughout this study.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
- Xourgia E, Tektonidou MG. An Update on Antiphospholipid Syndrome. Curr. Rheumatol. Rep. 23(12), 84 (2022).
- Hashami L, Haj Mohamad Ebrahim Ketabforoush A, Nirouei M. Giant cell arteritis with rare manifestations of stroke and internal carotid artery dissection: a case study. Clin. Case Rep. 10(3), e05597 (2022).
- Haj Mohamad Ebrahim Ketabforoush A, Bahadorinia M, Dolatshahi E, Nozarian Z, Abbasi Khoshsirat N. IgG4-related disease associated with the primary manifestation of recurrent cerebral venous thrombosis: a rare case report. Clin. Case Rep. 10(9), e6324 (2022).
- de Oliveira I, Sampaio Rocha-Filho PA. Headache and systemic lupus erythematosus: a narrative review. Headache 63(4), 461–471 (2023).
- Samadian M, Tavassol HH, Eraghi MM et al. A rare case of iatrogenic intracranial hypotension due to a minor CSF leakage. Radiol. Case Rep. 18(8), 2659–2662 (2023).
- Kim BW, Jung YJ, Kim MS, Choi BY. Chronic subdural hematoma after spontaneous intracranial hypotension: a case treated with epidural blood patch on c1–2. J. Korean Neurosurg. Soc. 50(3), 274–276 (2011).
- Lin J-p, Zhang S-d, He F-f, Liu M-j, Ma X-x. The status of diagnosis and treatment to intracranial hypotension, including SIH. J. Headache Pain 18, 1–8 (2017).
- Mokri B, Hunter SF, Atkinson JL, Piepgras DG. Orthostatic headaches caused by CSF leak but with normal CSF pressures. Neurology 51(3), 786–790 (1998).
- Paldino M, Mogilner AY, Tenner MS. Intracranial hypotension syndrome: a comprehensive review. Neurosurg. Focus 15(6), 1–8 (2003).
- Brightbill TC, Goodwin RS, Ford RG. Magnetic resonance imaging of intracranial hypotension syndrome with pathophysiological correlation. Headache 40(4), 292–299 (2000).
- Inamasu J, Guiot BH. Intracranial hypotension with spinal pathology. Spine J. 6(5), 591–599 (2006).
- Thielen KR, Sillery JC, Morris JM, Hoxworth JM, Diehn FE, Wald JT et al. Ultrafast dynamic computed tomography myelography for the precise identification of high-flow cerebrospinal fluid leaks caused by spiculated spinal osteophytes. J. Neurosurg. Spine 22(3), 324–331 (2015).
- Mokri B. Familial occurrence of spontaneous spinal CSF leaks: underlying connective tissue disorder. Headache 48(1), 146–149 (2008).
- Arai H, Yamamoto Y, Maeda Y, Aga F, Dobashi H, Nishiyama Y. SPET/CT imaging in radionuclide cisternography to detect cerebrospinal fluid leakage in spontaneous intracranial hypotension associated with SLE. Eur. J. Nucl. Med. Mol. Imaging 39(7), 1225–1226 (2012).
- Ando D, Nakaya I, Watanabe S, Osawa H, Kubo N, Soma J. Spontaneous intracranial hypotension in a case of systemic lupus erythematosus. Mod. Rheumatol. 25(4), 662–663 (2015).
- Cohen-Addad DI, Efendizade A, Grigorian A, Hewitt K, Velayudhan V. Spontaneous intracranial hypotension in a patient with systemic lupus erythematosus. Radiol. Case Rep. 14(10), 1188–1192 (2019).
- Dave S, Longmuir R, Shah VA, Wall M, Lee AG. Intracranial Hypertension in Systemic Lupus Erythematosus. Semin. Ophthalmol. 23(2), 127–133 (2008).
- Cohen-Addad DI, Efendizade A, Grigorian A, Hewitt K, Velayudhan V. Spontaneous intracranial hypotension in a patient with systemic lupus erythematosus. Radiol. Case Rep. 14(10), 1188–1192 (2019).
- Sanchez-Garcia M, Gomez-Delgado F, Gomez-Garduño A, Blanco-Molina A, Puebla RF. Hypertrophic pachymeningitis associated with cerebral spinal fluid hypovolemia as initial presentation of systemic lupus erythematous. Lupus 23(2), 197–200 (2014).
- Arai H, Yamamoto Y, Maeda Y, Aga F, Dobashi H, Nishiyama Y. SPET/CT imaging in radionuclide cisternography to detect cerebrospinal fluid leakage in spontaneous intracranial hypotension associated with SLE. Eur. J. Nucl. Med. Mol. Imaging 39(7), 1225–1226 (2012).
- Radhakrishnan S, Surendran D, Barathi D, Bammigatti C. Spontaneous intracranial hypotension associated with pachymeningeal enhancement in a patient with systemic lupus erythematosus (SLE): an extremely rare presenting feature. BMJ Case Rep. 12(2), (2019).
- Dobrocky T, Nicholson P, Häni L, Mordasini P, Krings T, Brinjikji W et al. Spontaneous intracranial hypotension: searching for the CSF leak. Lancet Neurol. 21(4), P369–P380 (2022).
- Jones LC, Butteriss D, Scoffings D. Spontaneous intracranial hypotension: the role of radiology in diagnosis and management. Clin. Radiol. 77(3), e181–e194 (2022).
- Santacruz JC, Villota C, Ballesteros JG, Bello JM, Londoño JD. Subdural haematoma as the initial manifestation of systemic lupus erythematosus associated with severe pancytopenia. In: Revista Colombiana de Reumatología (English Edition) (Volume 30, Issue 4). Elsevier, Spain, 337–341 (2023).
- Medina JH, Abrams K, Falcone S, Bhatia RG. Spinal imaging findings in spontaneous intracranial hypotension. Am. J. Roentgenol. 195(2), 459–464 (2010).
- Schievink WI, Maya MM, Tourje J. False localizing sign of C1–2 cerebrospinal fluid leak in spontaneous intracranial hypotension. J. Neurosurg. 100(4), 639–644 (2004).
- Kranz PG, Luetmer PH, Diehn FE, Amrhein TJ, Tanpitukpongse TP, Gray L. Myelographic Techniques for the Detection of Spinal CSF Leaks in Spontaneous Intracranial Hypotension. AJR Am. J. Roentgenol. 206(1), 8–19 (2016).
- Li J, Meng H, Jiang W, Liu J, Cui Z, Miao J. Cerebral venous sinus thrombosis and subdural hematoma in a female patient with systemic lupus erythematosus: a case report and literature review. Ann. Palliat. Med. 10(7), 8454–8459 (2021).
- Kranz PG, Luetmer PH, Diehn FE, Amrhein TJ, Tanpitukpongse TP, Gray L. Myelographic techniques for the detection of spinal CSF leaks in spontaneous intracranial hypotension. Am. J. Roentgenol. 206(1), 8–19 (2016).

