Abstract
Aim: The outcome of T-cell acute lymphoblastic leukemia (T-ALL) has improved with the use of pediatric-inspired protocols in the adolescents and young adults (AYA) population. There is limited literature regarding the outcome of T-ALL/lymphoblastic lymphoma (LBL) AYA patients treated with pediatric protocols. Methods: A total of 35 T-ALL/LBL-AYA patients ages between 14 and 55 years were treated with AYA-15 protocol. Results: At a median follow-up of 5 years the overall survival, disease-free survival and event-free survival are 71%, 62% and 49.6% respectively. Toxicities were within the expected range. Conclusion: Our single-center experience real-world data in treating T-ALL/LBL-AYA patients with pediatric-inspired protocol demonstrates encouraging results of high survival rate and excellent tolerability for patients aged 18–55 years.
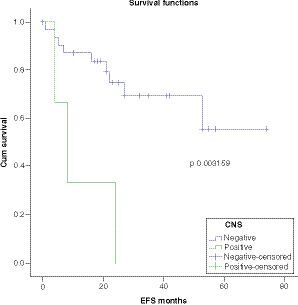
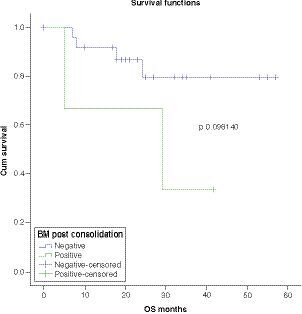
OS was worse in patients with CNS disease at presentation with a statistically significant P-value (p = 0.003159) . There was a trend toward worse survival in patients with MRD-positive disease post-consolidation (p = 0.098140) .
BM: Bone marrow; MRD: Minimal residual disease; OS: Overall survival.
T-cell acute lymphoblastic leukemia (T-ALL) is a malignant proliferation of T-lymphoid precursor cells. T-ALL accounts for 20% of all types of ALL [Citation1]. T-ALL is slightly more common in adolescents and young adults (AYA) than in children, and it affects males more than females. Interestingly, of all cases of lymphoblastic lymphoma (LBL) diagnosed, T-cell phenotype accounts for almost 90% of the cases with a tendency to present with mediastinal mass and involvement of extramedullary areas as well as central nervous system infiltration [Citation2].
Patients with T-ALL/LBL who were treated with adult-protocols had average 5-year overall survival (OS) of 30–60% [Citation3–6].
Historically, T-ALL has been treated similarly to B-ALL. More recently the outcome of T-ALL has improved with the use of pediatric-inspired protocols in the AYA population with intensified chemotherapy including asparaginase and without the use of routine stem cell transplant (SCT) [Citation7]. There is limited literature regarding the outcome of T-ALL/LBL AYA patients treated with pediatric protocols in a real-world fashion. To have a better understanding of efficacy and tolerability of pediatric-inspired protocols in management of T-ALL, we applied our protocol in real-world fashion since it is well known that results come from clinical trials in general are affected by strict inclusion and exclusion criteria which might compromise the ability to reproduce their results in real world [Citation8].
In 2015, our regional tertiary care referral center started using a pediatric-inspired protocol called ‘AYA-15’ protocol, which is a modification of Children’s Cancer Group (CCG) 1900 protocol; a pediatric-based chemotherapy protocol [Citation9], in treating AYA ALL patients. Previously, we reported the outcome of 79 patients treated on this protocol with results showing improvement in disease-free survival (DFS), event-free survival (EFS) and overall survival (OS), as compared with historic adult-oriented protocols (5-year OS, DFS and EFS were 75.8, 69.2 and 57.5%, respectively) [Citation10].
In this paper, we are reporting the real-world outcome of AYA patients diagnosed with T-ALL/LBL treated with the AYA-15 protocol.
Methods
A total of 140 patients with ALL/LBL, both B- and T-cell phenotypes, ages between 14 and 55 years were treated with the AYA-15 chemotherapy protocol. Out of 140 patients, 35 patients were identified as T-ALL/LBL (25%). The study was approved by the institutional review board and electronic records were used to extract data. Exclusion criteria included patients with Philadelphia-positive ALL and patients with biphenotypic leukemia/mixed phenotype leukemia. Data collected on each patient included demographics, specific disease characteristics, treatment complications and clinical outcomes data including disease-free survival (DFS), event-free survival (EFS), overall survival (OS), and toxicities. The pathology lab used multicolor flow cytometry to measure minimal residual disease (MRD). The sensitivity threshold used for MRD was 0.1%. with 2 points check, one at the conclusion of the induction phase and one at the end of the consolidation phase. We defined complete remission (CR) as the achievement of 5% or less bone marrow leukemic blasts at end of induction. OS is defined as survival from time of diagnosis till death for any reason. DFS is defined as survival from date of first remission till disease progression or death. EFS is defined as survival from the time of diagnosis until one of the following events occur: failure of induction, allogeneic stem cell transplant (allo-SCT), disease relapse, death, or being removed from the protocol for whatever reason.
Patients with T-ALL/LBL were risk stratified into high-risk (HRT-ALL) disease or very high-risk (VHRT-ALL) disease according to the criteria shown in . By definition, the patients were stratified as VHRT-ALL if they had white blood cells (WBC) count of >50,000/ul or central venous system (CNS) at presentation, otherwise, patients were stratified as HRT-ALL. Also, patients who had blasts more than 5% on day 14 of induction were considered late responders and their risk stratification was changed to VHR. It is important to point out that none of our patients are considered low risk as all patients with age >10 years are classified as high risk at minimum as compared with patients <10 years of age according to pediatric risk classification.
Chemotherapy protocol
AYA-15 chemotherapy protocol is a pediatric-inspired protocol, a modified version of Children’s Cancer Group (CCG) 1900 pediatric protocol. We made modifications to the pediatric protocol to be more tailored to the AYA age group (summary of modifications is shown in ). Phases of chemotherapy used in T-ALL/LBL are shown in . All phases of this protocol were done in the outpatient setting, except for the induction phase and interim maintenance phase (high-dose methotrexate [HDMTX] phase) which were done in the inpatient setting. Patients with high-risk disease, received a single delayed intensification (DI) and a single interim maintenance (IM) phases while patients with the very high-risk disease received double DI and double IM phases.
AYA-15 protocol started with a pre-phase prednisone at a dose of 40 mg/m2 daily for 4 days. Pre-phase was followed by the induction phase. On day 14 of induction, a bone marrow biopsy was done and results were used for risk stratification as shown in . Day 28 bone marrow biopsy was needed to document remission status. MRD assessment on day 28 bone marrow was only used for prognostication but did not influence the decision regarding continuing on the protocol. If the patient had induction failure (leukemic blasts >5% at day 28 bone marrow biopsy), then will be taken off the protocol to receive salvage therapy according to the treating physician’s discretion. Patients continued on the protocol proceeded to the consolidation phase with a repeat bone marrow biopsy after conclusion of this phase for MRD assessment. Patients with positive MRD after consolidation were taken off protocol to proceed with allo-SCT. Other indications to proceed with allo-SCT included: induction failure, presence of KMT2A (myeloid/lymphoid or mixed-lineage leukemia [MLL]) gene rearrangement and relapsed disease.
Pegylated-asparaginase (peg-asp) was used during all phases of the protocol, except for the maintenance phase. For patients intolerant to peg-asp or who developed a severe allergic reaction to peg-asp, Erwinia asparaginase was used instead. All patients treated with peg-asp received pre-medications to prevent allergic reaction as well as prophylactic anticoagulation (enoxaparin 40 mg subcutaneous once a day). For the patient with HRT-ALL, HDMTX (5 gm/m2) was given during the interim maintenance phase, while patients with VHRT-ALL received two interim maintenance phases, one using HDMTX and one using Capizzi methotrexate escalating dose strategy. During induction, we used daily prednisone, while we used dexamethasone in the following phases. For patients who completed the full protocol, patients with HRT-ALL received total of 24 intrathecal (IT) chemotherapy, while patients with VHRT-ALL received 29 IT chemotherapy. Patients with CNS disease (CNS 3, see ), received cranial irradiation at a dose of 2400 cGy in 12 divided fractions, while CNS 1 and 2 did not receive radiation.
Statistical methods
The primary end points of this study include OS, DFS and EFS which have been calculated using Kaplan–Meier estimator. Survival curves were compared using log-rank test. Remission rate was also estimated with definition of CR as blast percentage of less than 5% in bone marrow biopsy at end of induction, accompanied by resolution of any extramedullary disease sites and complete recovery of complete blood counts (CBC). Other definitions used include: OS which is defined as survival from time of diagnosis till the time of last follow-up. DFS is defined as survival from time of first evidence of complete remission till relapse. EFS is defined as survival from time of diagnosis till occurrence of any one of the following events: induction failure, relapsed disease, undergoing allogeneic stem cell transplantation or death.
Results
Patient characteristics
A total of 35 consecutive patients with T-ALL/LBL (26 T-ALL, 9 T-LBL) were enrolled in the study. The median age was 20 years (range 14–41), 72% are males. Eleven patients (31.4%) were stratified as HRT-ALL/LBL disease and 24 patients (68.6%) as VHRT-ALL/LBL. The median presenting WBC count was 14 (range 1–350) X 109/L. CD1a was positive in 13 patients (37.1%) and negative in 5 patients (14.3%). Results were missing in 17 patients (48.6%). FISH analysis for high risk cytogenetics (complex, hypodiploidy or MLL) was present in five patients (8.6%), while standard cytogenetics by FISH was present in 23 patients (71.4%). Results were missing in seven patients. Three patients (8.6%) had CNS disease at presentation. A summary of patient characteristics are summarized in ()
Outcomes
Out of 35 patients who were enrolled in the study, 31 patients (88.6%) achieved CR after induction chemotherapy (six patients with CR had positive MRD). After the consolidation phase, three patients remained positive for MRD. Five patients (14.3%) received allo-SCT (three patients with positive MRD postconsolidation, one patient with residual activity on PET scan post-consolidation and one patient intolerant to chemotherapy). Six patients relapsed (17.1%) and eight patients died (all-cause mortality was 22.9%. Two patients (5.7%) had non-relapse mortality (NRM), one patient died from a road traffic accident while was in remission, while the other patient died from septic shock.
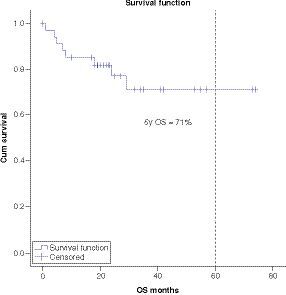
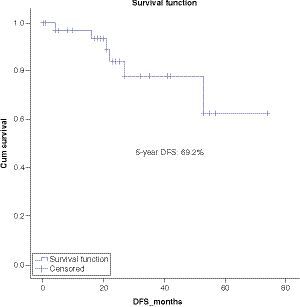
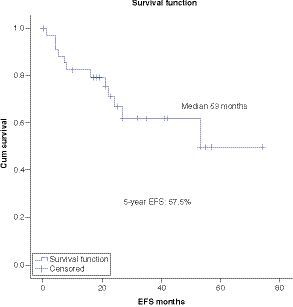
At a median follow-up of 5 years the OS, DFS and EFS are 71, 62 and 49.6% respectively ().
Toxicity & tolerability
The most common side effect was febrile illness (40.6% during induction and 71% during consolidation). Documented infection was present in 28.1% of the patients during induction and 46.9% during consolidation. Asparaginase-related side effects during induction included: thrombosis (25.7%), bleeding (5.7%), hyperglycemia (22.9%) and allergic reaction (22.9%). Those side effects were lower during consolidation (3.2% thrombosis, 3.2% bleeding, and 3.2% hyperglycemia). Allergic reaction during consolidation was similar to induction ().
Liver toxicity in term of elevation of transaminases was observed in 83.2% of patients during induction (grade 1: 37.5%, grade 2: 11.4%, grade 3: 2.9%, grade 4: 2.9%) and 87.1% during consolidation (grade 1: 48.4%, grade 2: 29%, grade 3: 9.7%, grade 4: 0%). Hyperbilirubinemia was mostly grade 1 (during induction: 48.4%, during consolidation: 29%) ().
Discussion
Historically, the outcome of T-ALL/LBL in the adult population has been suboptimal with the use of adult-based chemotherapy protocols that depend heavily on upfront allogeneic –SCT [Citation11]. Different trials including our published data showed an average 5-year OS of 30–60% [Citation3–6]. Literature is showing significant improvement in survival of precursor B-cell ALL-AYA patients treated with pediatric-inspired protocols that involve intensification of chemotherapy with use of asparaginase, excessive CNS prophylaxis and without the routine use of all-SCT [Citation12–15]. The current literature supports the superiority of pediatric-inspired protocol in treatment of precursor B cell ALL-AYA patients as compared with adult-based chemotherapy protocols, with average 5-year OS of 65–70% [Citation16,Citation17]. There are limited data in the literature regarding the outcome of T-ALL/LBL in AYA patients treated with pediatric protocols in a real-world fashion. There is no uniform definition of AYA with variability of cutoffs by different studies, although most protocols used ages between 15 and 39 years [Citation18]. Our protocol accepts ALL patients between the age of 14 and 55 years.
This real-world data from a single-center experience in treating T-ALL/LBL AYA patients with a pediatric-inspired protocol demonstrates a high survival rate and excellent tolerability for patients ages 18–55 years, with results superior to adult-based protocols.
AYA-15 protocol for treatment of T-ALL/LBL AYA patients is an in-house designed pediatric-inspired protocol adopted from CCG 1900 protocol [Citation9]. This protocol is characterized by early use of peg-asp, use of HDMTX during the interim maintenance phase, and intensified use of CNS prophylaxis with an average number of IT chemotherapy treatments between 24 and 29 by the end of the protocol. We used MRD as our most powerful prognostic indicator to decide on proceeding to allo-SCT if MRD continued to be positive by end of consolidation. HR disease patients received the standard protocol, while patients with VHR disease received more intensified protocol with a double interim maintenance phase and double delayed intensification phase. Patients with CNS disease, received cranial XRT (2400 cGy in 12 divided fractions) before initiation of the final maintenance phase.
We use multi-color flow cytometry to measure MRD with a sensitivity of 10-3 (threshold of 0.1%). This sensitivity is below the standard levels used by many centers, the reason for this is because of lack of validity of higher sensitivity due to a small number of patients. We used MRD results post-induction as a prognostication tool, but not as a deciding factor regarding assessment of chemosensitivity since it is well known that T-ALL in general is slow in MRD clearance. If MRD is positive post-consolidation, then the patient will be considered to have a chemo-resistant disease and plan was to proceed with allo-SCT. During the induction phase, we used daily prednisone instead of dexamethasone to reduce toxicity. Dexamethasone was then used in the subsequent phases of the protocol. This approach follows the recommendations of a meta-analysis comparing prednisone and dexamethasone. This shows higher mortality with the use of dexamethasone during induction [Citation19].
Survival analysis showed 5-year OS, DFS and EFS of 71%, 62% and 49.6% respectively. Multivariate analysis showed significantly worse OS in patients with CNS disease as compared with CNS negative patients. Regarding other factors, there was no significant difference among different groups, although there was a trend toward worse survival in patients with MRD-positive disease post-consolidation.
The safety profile of this protocol was analyzed and showed toxicities rates similar to what is published in other international trials. It is important to highlight the tolerability of asparaginase in our patients. Asparaginase-related allergic reaction during induction was high (22.9%). This is likely an overestimation because there was no differentiation between infusion reaction and actual allergic reaction. With regard to elevated risk of thrombosis (25.7%), almost all cases were catheter related and were associated with over correction of fibrinogen.
Conclusion
This is a report of real-world outcomes of the AYA-15 protocol in treatment of T-ALL in AYA patients 14–55 years of age, with results showing significant improvement in 5-years OS, EFS and DFS compared with traditional adult-oriented protocols. Tolerance is excellent with very low TRM. We believe the main reason for this improvement is because of treatment protocol details including intensification, use of MRD and early use of asparaginase and CNS prophylaxis. This study has a number of limitations including a small sample size and the nature of the study being a retrospective analysis.
Table 1 Risk stratification of T-cell acute lymphoblastic leukemia.
Table 2 Modifications of CCG 1900 protocol.
Table 3 Chemotherapy protocol AYA 15.
Table 4 Patient characteristics.
Table 5 Toxicities.
Table 6 Hepatotoxicity in induction and consolidation phase according to CTCAE grading.
Summary points
Treatment of acute lymphoblastic leukemia (ALL) in adults population is challenging and the outcome is inferior to pediatrics population.
Outcome of ALL in adolescents and young adults (AYA) population has improved with use of pediatric-inspired chemotherapy protocols.
There is limited literature regarding the outcome of T-ALL AYA patients treated with pediatric protocols.
This report shows the real-world outcome of 35 patients with T-ALL, ages 18–55 years, treated with AYA-15 protocol (pediatric-inspired).
Survival analysis showed improved 5-year survival on treated patients with 5-year OS of 71% (compared with average published survival for patients treated with adult-based protocols of 30–60%).
OS is worse in patients with CNS disease at presentation with a trend toward worse survival in patients with MRD-positive disease post-consolidation.
Chemotherapy was well tolerated with toxicities rate similar to other international trials.
Asparaginase toxicities were manageable and the drug was well tolerated in general.
This study has a number of limitations including a small sample size and the nature of the study being a retrospective analysis.
Author contributions
A Hanbali: helped in data collection and wrote the manuscript. A Kotb: helped in data collection and statistics. N Shihata: helped in data collection. M Aljurf: did critical analysis of data and manuscript. R El Fakih: reviewed the manuscript. F Alfraih: reviewed the manuscript. W Rasheed: reviewed the manuscript. S Osman Ahmed: reviewed the manuscript. M Shaheen: reviewed the manuscript. S Alhayli: reviewed the manuscript. A Alahmari: reviewed the manuscript. A Alotaibi: reviewed the manuscript. A Alshaibani: reviewed the manuscript. A Albabtain: reviewed the manuscript. M Alfayez: reviewed the manuscript. M Hassan: helped with data collection. F Alsharif: reviewed the manuscript. N Chaudhri: reviewed the manuscript. F Almohareb: reviewed the manuscript. H Alzahrani: reviewed the manuscript.
Ethical conduct of research
All experimental protocols were approved by research ethics committee of king faisal specialist hospital and research center office of research affair (ORA), research ethics committee (REC) and institutional review board (IRB). Informed consent was obtained from all subjects and/or their legal guardians. All patients and/or legal guardians signed an informed consent approved by our Institution Review Board and according to Declaration of Helsinki.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- DoresGM, DevesaSS, CurtisRE, LinetMS, MortonLM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood 119(1), 34–43 (2012).
- HarrisonCJ. Cytogenetics of paediatric and adolescent acute lymphoblastic leukaemia. Br. J. Haematol. 144(2), 147–156 (2009).
- BakrM, RasheedW, MohamedSYet al. Allogeneic hematopoietic stem cell transplantation in adolescent and adult patients with high-risk T cell acute lymphoblastic leukemia. Biol. Blood Marrow Transplant. 18(12), 1897–1904 (2012).
- JainN, LambAV, O’brienSet al. Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood 127(15), 1863–1869 (2016).
- KozlowskiP, ÅströmM, AhlbergLet al. High relapse rate of T cell acute lymphoblastic leukemia in adults treated with hyper‐CVAD chemotherapy in Sweden. Eur. J. Haematol. 92(5), 377–381 (2014).
- YanadaM, JinnaiI, TakeuchiJet al. Clinical features and outcome of T-lineage acute lymphoblastic leukemia in adults: a low initial white blood cell count, as well as a high count predict decreased survival rates. Leuk. Res. 31(7), 907–914 (2007).
- HuguetF, LeguayT, RaffouxEet al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: the GRAALL-2003 study. J. Clin. Oncol. 27(6), 911–918 (2009).
- MakadyA, DeBoer A, HillegeH, KlungelO, GoettschW. What is real-world data? A review of definitions based on literature and stakeholder interviews. Value Health 20(7), 858–865 (2017).
- AvramisVI, SencerS, PericlouAPet al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children’s Cancer Group study. Blood 99(6), 1986–1994 (2002).
- HanbaliA, KotbA, ElFakih Ret al. Improved survival in adolescents and young adults (AYA) patients aged 14–55 years with acute lymphoblastic leukemia using pediatric-inspired protocol–a retrospective analysis of a real-world experience in 79 of patients treated at a national tertiary care referral center. Leuk. Res. Rep. 16, 100270 (2021).
- AljurfM, ZaidiSZ. Chemotherapy and hematopoietic stem cell transplantation for adult T-cell lymphoblastic lymphoma: current status and controversies. Biol. Blood Marrow Transplant. 11(10), 739–754 (2005).
- RamanujacharR, RichardsS, HannIet al. Adolescents with acute lymphoblastic leukaemia: outcome on UK national paediatric (ALL97) and adult (UKALLXII/E2993) trials. Pediatr. Blood Cancer 48(3), 254–261 (2007).
- BoisselN, AuclercM-F, LhéritierVet al. Should adolescents with acute lymphoblastic leukemia be treated as old children or young adults? Comparison of the French FRALLE-93 and LALA-94 trials. J. Clin. Oncol. 21(5), 774–780 (2003).
- DeBont J, VanDer Holt B, DekkerA, SonneveldP, PietersR. Significant difference in outcome for adolescents with acute lymphoblastic leukemia treated on pediatric vs adult protocols in the Netherlands. Leukemia 18(12), 2032–2035 (2004).
- StockW, LugerSM, AdvaniASet al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10.03. Blood 133(14), 1548–1559 (2019).
- MufflyL, CurranE. Pediatric-inspired protocols in adult acute lymphoblastic leukemia: are the results bearing fruit?Hematology Am. Soc. Hematol. Educ. Program 2019(1), 17–23 (2019).
- SiegelSE, StockW, JohnsonRHet al. Pediatric-inspired treatment regimens for adolescents and young adults with philadelphia chromosome–negative acute lymphoblastic leukemia: a review. JAMA Oncol. 4(5), 725–734 (2018).
- SawyerSM, AzzopardiPS, WickremarathneD, PattonGC. The age of adolescence… and young adulthood–Authors’ reply. Lancet Child Adolesc. Health 2(4), e7 (2018).
- InabaH, PuiC-H. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 11(11), 1096–1106 (2010).