Abstract
A 96-well filtration-based 5-HT1A receptor-radioligand binding assay was optimized and adapted to carry out a bioassay-guided fractionation of the methanol extract of the bark of Popowia odoardi Diels (Annonaceae). This purification led to the isolation of two compounds identified as O-methyldauricine (1) and popisidine (2), which bind competitively to 5-HT1A receptors at high concentrations with Ki values of 4.5 and 5.8 μM, with [3H]8-OH-DPAT as the radioligand. These results suggest that both of these bisbenzyltetrahydroisoquinoline alkaloids influence the activity of 5-HT1A receptors through competitive binding as agonists or antagonists and may possess anxiolytic and anti-depressive effects.
Introduction
5-Hydroxytryptamine (5-HT, serotonin) mainly functions as a neurotransmitter for tryptaminergic neurons and its corresponding receptors in the brain, mediating sleep, feeding, mood, temperature regulation, and sexual behavior. To date, seven 5-HT receptor families, designated 5-HT1 to 5-HT7, have been identified and classified into at least 14 subtypes (CitationHoyer et al., 2002).
The 5-HT1A receptor is an important therapeutic target for the treatment of CNS disorders such as anxiety, depression, and schizophrenia, and potentially Alzheimer’s disease (CitationHensler, 2003; CitationHoyer et al., 2002; CitationSchechter et al., 2002). For example, the psychoactive agents buspirone and gepirone exert both anxiolytic and anti-depressant effects. In addition to depression and anxiety, 5-HT1A receptor ligands have therapeutic utility in schizophrenia. This has led to the development of the antipsychotic drugs ziprasidone and aripiprazole (CitationGunasekara et al., 2002). Neurochemical and behavioral data suggest that receptor blockage by a 5-HT1A antagonist appears to enhance activation and signaling through heterosynaptic neuronal circuits known to be involved in cognition processes. This suggests that the 5-HT1A receptor antagonists may have therapeutic potential in Alzheimer’s disease and other diseases associated with cognition dysfunction (CitationSchechter et al., 2002).
In the search for plant extracts with G-protein-coupled receptor and antimicrobial activities, more than 200 Malaysian plants were screened; the plants were chosen based on availability and similarity to species with ethno-botanical uses in medicine (CitationChung et al., 2004, Citation2005a, Citation2005b, Citation2006, Citation2008). The crude methanol extract of Popowia odoardi Diels (Annonaceae) showed pronounced competitive 5-HT receptor1A binding activity. The competitive inhibition of GABAB receptor, dopamine receptor (D2S), muscarinic receptor, chemokine (CXCR2) receptor, and chemotactic C5a-anaphylatoxin receptor binding activity was low or minimal. P. odoardi, a small tree that grows widely in the lowland or hill forests of Borneo, was therefore selected for further bioactivity (5-HT1A receptor binding) guided fractionation studies.
The genus is known as “pisang-pisang” in Peninsula Malaysia, and species such as P. nervifolia Maing., P. nervosa Ridl. and P. ramosissima Hook. f. were used in house construction (CitationBurkill, 1935). Although P. odoardi is not known in indigenous medicine, related species have been used in other parts of the world. For example, P. fornicata Baill. (synonym, Monanthotaxis fornicata Baill.) is indicated for the treatments of mental diseases and snake bites in East Africa (CitationHedberg et al., 1982), while P. herenthas is used in Madagascar as febrifuge (CitationRasoanaivo et al., 1992). A phytochemical screening survey reported that P. odoardi contain several alkaloids which have not been isolated and identified (CitationTeo et al., 1990).
However, isoquinoline-derived alkaloids (CitationChuah et al., 2001; Josang et al., 1986a,b) and flavonoids (CitationWaterman & Pootakahm, 1979) were isolated from other members of the Popowia genus. We now report the isolation of two bisbenzyltetrahydroisoquinoline alkaloids from the crude methanol extract of P. odoardi and their 5-HT1A receptor binding activities.
Materials and methods
Materials
8-Hydroxy-2-[2,3-3H]di-n-(propylamino)tetralin ([3H]8- OH-DPAT; 220 Ci/mmol) was obtained from GE Healthcare Life Sciences (Little Chalfont, Buckinghamshire). Tris(hydroxygethyl)aminomethane (Tris base), Bradford reagent, bovine serum albumin type V, 5-hydroxytryptamine hydrochloride (5-HT.HCl), (±)-8-hydroxy-2-(di-n-propylamino)-tetralin ([±]-8-OH-DPAT), buspirone hydrochloride, dl-propanolol hydrochloride, and (–)scopolamine methyl bromide were obtained from Sigma-Aldrich (St. Louis, MO).
Ninety-six-well flat-bottom Falcon plates were obtained from Becton Dickinson Labware (Franklin Lakes, NJ). Microscint-O scintillation cocktail, TopSeal A, and UniFilter GF/C plates were supplied by Perkin Elmer (Wellesley, MA). Silica gel 60 (mesh 230–400) for column chromatography and silica gel 60 F254 (0.5 mm thick, glass plate) for TLC were obtained from Merck (Darmstadt, Germany). All solvents were of analytical grade and purchased from Fisher Scientific (Loughborough, Leicestershire).
General experimental procedures
1H and 13C Nuclear magnetic resonance (NMR) spectra were obtained on a 500 MHz Varian Unity INOVA spectrometer (Palo Alto, CA). Correlation spectroscopy (COSY), Heteronuclear multiple-bond correlation (HMBC), Heteronuclear multiple quantum correlation (HMQC), and Nuclear overhauser effect spectroscopy (NOESY) NMR experiments were also conducted. Deuterated chloroform (CDCl3) was used as the solvent and tetramethylsilane (TMS) as the internal reference. Mass spectrometry (MS) data were recorded on a Finnigan Polaris Q (Wattham, MA) through direct injection.
Plant material
The bark of P. odoardi Diels (Annonaceae), previously reported as P. odoardoi Diels, was collected from Sabah, Malaysia (CitationChung et al., 2005a, Citation2005b, Citation2006), and identified at the Sandakan (SAN) Herbarium of the Forest Research Center, Sepilok, Sandakan, Malaysia (voucher no.: SAN 143395).
Extraction and isolation
The air-dried bark (200 g) was powdered and extracted exhaustively with methanol, and after removal of the solvent in vacuo, yielded 19.8 g (9.9%) (5-HT1A receptor binding inhibition at 10 μg/well, 71.1% ± 5.9%). The crude extract (10 g) was dissolved in CHCl3/MeOH (1:1) and partitioned with 5% aqueous hydrochloric acid. The aqueous acid phase obtained was basified with 30% ammonium hydroxide to pH 10, followed by partitioning with CHCl3. The chloroform fraction was then dried in vacuo to give a crude alkaloid extract (2.1 g) (5-HT1A receptor binding inhibition at 10 μg/well, 102.1% ± 1.2%; 1 μg/well, 63.5% ± 1.3%). The CHCl3/MeOH fraction containing non-alkaloid materials showed low inhibition on 5-HT1A receptor binding (1 μg/well, 14.6% ± 4.4%).
The CHCl3 extract (600 mg) was fractionated on a silica gel chromatography column (silica gel 60, mesh 230–400) using a CHCl3/MeOH step gradient system (100% CHCl3, 98:2, 96:4, 94:6, 92:8, 90:10, 80:20, 50:50), and eight fractions (F-1 to F-8) were collected. Fraction F-4 (47.3 mg) and fraction F-7 (74.3 mg) showed 84.4% ± 1.1% and 52% ± 7.5% inhibition (1 μg/well), respectively. 15 mg of fraction F-4 was further separated by preparatory scale TLC. Purification by TLC on a silica gel 60 F254 plate using CHCl3/MeOH (95:5) saturated with ammonium hydroxide gave compound 1 (Rf 0.4; 6.6 mg; 0.22% of the powdered bark). For fraction F-7, 28 mg was subjected to further separation as described above. Purification by TLC using CHCl3/MeOH (97:3) saturated with ammonium hydroxide yielded compound 2 (Rf 0.4; 13.5 mg; 0.12% of the powdered bark).
Preparation of 5-hydroxytrptamine receptor crude membrane
Rat brain membranes (cerebral cortices) from male Sprague-Dawley rats (300–400 g) were prepared according to the methods described by CitationChung et al. (2008) with minor modifications (CitationSoo, 2006), and in accordance to the guideline promulgated by the Ethics Committee on Animal Welfare and Experimentation, Faculty of Medicine, University of Malaya, Malaysia (Ref. No.: FAR/21/10/2005/MRM (R)). Rat cerebral cortices were removed on ice, weighed, and placed in 10 volumes (w/v) of ice-cold 50 mM Tris-HCl buffer, pH 7.4, The tissue was homogenized with an Ultra-Turrax homogenizer (IKA Labortechnik, Staufen, Germany) and glass-teflon homogenizer (Terre Haute, Indiana, USA). The homogenate was then centrifuged at 40,000 g for 15 min (Sorvall® Ultra Pro 80, Kendro, Newtown, CT). The resulting supernatant was discarded, and the pellet was resuspended in the same volume of ice-cold buffer and centrifuged at 40,000 g for another 15 min. This procedure was repeated, and the resuspension was incubated in a water bath at 25°C for 20 min to destroy endogenous serotonin (CitationHarms et al., 2000) before being centrifuged as above. All centrifugation steps were performed at 4°C. The final membrane preparation was suspended in 2-3 volumes of ice-cold 50 mM Tris-HCl buffer, pH 7.4, and stored at −86°C until use. Membrane protein concentration was determined using the Bradford protein reagent (Sigma-Aldrich) with bovine serum albumin as the standard, and the protein content corresponded to about 20 mg protein/mL.
96-Well microplate 5HT1A receptor binding assay
Crude extract and partitioned fractions dissolved in DMSO (final concentration, 5% v/v) were tested at 50, 10, and/or 1 μg/well. The isolated compounds and standards (buspirone and (±)-8-OH-DPAT, serotonin, propanolol) were tested at 11–12 concentrations (10−2–10−13 M) in order to obtain Ki values. All assays were performed in triplicate and inhibition results shown are the mean ± SD (n = 3).
The 96-well microplate 5-HT1A receptor assay was optimized and performed as described by CitationSoo (2006). Around 200 μL of the membrane suspension per well (35 μg of protein per well) were added to [3H]8-OH-DPAT (final concentration, 0.4 nM) to determine total binding (TB). Non-specific binding (NSB) was determined by adding 25 μL of 5-HT (final concentration, 10 μM) to the reaction mixture. TB was subtracted by NSB to give specific binding (SB). To determine the inhibition of binding by [3H]8-OH-DPAT in the presence of plant extracts, isolated compounds, and known ligands, 25 μL of the respective compound was added instead of 5-HT. The reaction mixture was incubated at 25°C at pH 7.4 for 90 min. After incubation, the reaction mixtures were rapidly filtered through pre-wetted GF/C filter plates using the FilterMate Cell Harvester (Perkin Elmer) and washed with 200 μL/well of ice-cold 50 mM Tris-HCl buffer pH 7.4 (four times). The seal was then applied to the bottom of the plate, and 25 μL aliquots of [3H]8-OH-DPAT were spotted directly onto the GF/C filter plate in triplicate to determine the total radioactivity per well. The plate was then air-dried and 25 μL of scintillation cocktail were added to each well (MicroScint-O). The top side of the plate was then sealed with TopSeal A, and the plate was agitated at 400 rpm for 5 min before counting each well for 1 min with a TopCount NXT microplate scintillation counter (Perkin Elmer).
Data analysis on saturation experiments using the non-linear least-squares regression method was performed using PRISM® software, version 3.03 (GraphPad Software, San Diego, CA), and the results were presented as the mean ± SD (n = 3). The Ki was calculated according to the Cheng-Prussof equation:
where D is the concentration of the radioligand and KD is the dissociation constant of the radioligand (CitationCheng & Prussof, 1973). The quality of the assays was assessed by performing screening window factor (z’ factor) analysis according to the equation: 1-(3SDTB+3SDNSB)/(MeanTB-MeanNSB) as described by CitationZhang et al. (1999), where SDTB is the standard deviation of total binding, SDNSB is the standard deviation of non-specific binding, MeanTB is the mean of total binding, and MeanNSB is the mean of non-specific binding.
The percentage of inhibition of specific binding to 5-HT1A receptors in the presence of the test compounds or extracts was calculated using a standard data reduction algorithm as shown below:
where TB is the total binding, B is the binding in the presence of test sample, and NSB is the non-specific binding in the presence of excess reference ligand. The data was expressed as the mean ± SD (n = 3) and analyzed statistically using Student’s t test.
Results and discussion
The agonist, 8-hydroxy-2-[2,3-3H]di-n-(propylamino)tetralin ([3H]8-OH-DPAT), was used as the radioligand as it exhibited saturation binding with high affinity to 5-HT1A receptors. The binding data were fitted to the one-site and two-site binding models using the nonlinear regression analysis in Prism® software. The statistical F test indicated that the two-site binding model gave the best fit (p <0.05) with a Kd(H) of 0.15 nM and a Kd(L) of 10.5 nM; Bmax(H) was 143.4 fmol/mg protein and Bmax(L) was 750.1 fmol/mg protein (). These values were similar to those of CitationCarli et al. (1997) and CitationNénonéné et al. (1996). The inhibition constant (Ki) for the competitive reference ligand (5-HT) was 3.25 (Kd(H)) and 3940 (Kd(L)) nM, and the values were similar to those of CitationNénonéné et al. (1994). In the time-course experiment, association of [3H] 8-OH-DPAT was completed in less than 90 min, clearly demonstrating that the 90-min incubation time was adequate (). The quality of the assay was assessed using z’ factor analysis; A z’ factor of 0.91 for the 5-HT1A receptor binding assay demonstrated the suitability and robustness of the data quality of the assay for screening (CitationZhang et al., 1999).
Figure 1. (A) Saturation curve for the 5-hydroxytryptamine1A receptor with [3H]8-OH-DPAT as the radioligand. Receptor membrane (35 μg protein/well) was incubated with increasing concentrations of [3H]8-OH-DPAT (0–10 nM) at 25°C for 90 min. The binding was fitted to the two-site binding model. The Rosenthal plot is shown as the inset of the saturation binding curve. (B) Time course of association of [3H]8-OH-DPAT to the 5-hydroxytryptamine1A receptor. Receptor membrane (35 μg protein/well) was incubated with [3H]8-OH-DPAT (0.2 nM) at 25°C for different time periods (0–180 min). Each data point is expressed as mean ± SD (n = 3).
![Figure 1. (A) Saturation curve for the 5-hydroxytryptamine1A receptor with [3H]8-OH-DPAT as the radioligand. Receptor membrane (35 μg protein/well) was incubated with increasing concentrations of [3H]8-OH-DPAT (0–10 nM) at 25°C for 90 min. The binding was fitted to the two-site binding model. The Rosenthal plot is shown as the inset of the saturation binding curve. (B) Time course of association of [3H]8-OH-DPAT to the 5-hydroxytryptamine1A receptor. Receptor membrane (35 μg protein/well) was incubated with [3H]8-OH-DPAT (0.2 nM) at 25°C for different time periods (0–180 min). Each data point is expressed as mean ± SD (n = 3).](/cms/asset/872603c4-7f92-4aa6-91e5-f759035eb12c/iphb_a_401040_f0001_b.gif)
In the bioassay-guided fractionation of P. odoardi using the optimized 5-HT1A receptor-radioligand binding assay, the crude chloroform extract gave a percentage of inhibition of 71.1% ± 5.9% (10 μg sample/well) (Student’s t-test, P <0.05 compared to the control), and the crude alkaloid extract gave a percentage of inhibition of 102.1% ± 1.2% (10 μg sample/well) and 63.5% ± 1.3% (1 μg sample/well) (Student’s t-test, P <0.05 compared to the control). The alkaloid-containing fractions with 5-HT1A receptor binding activities were separated further to obtain pure compounds. By comparison of the spectral data (EI-MS, 1H-, 13C-NMR, COSY, HMQC, HMBC, NOESY, DEPT) with those in the literature (CitationChuah et al., 2001; CitationJossang et al., 1986a), compounds 1 and 2 were identified to be the bisbenzyltetrahydroisoquinoline alkaloids O-methyldauricine (1) and popisidine (2), respectively ().
Figure 2. Chemical structures of compounds isolated from P. odoardi as competitive inhibitors on [3H]8-OH-DPAT binding to 5-hydroxytryptamine1A receptor.
![Figure 2. Chemical structures of compounds isolated from P. odoardi as competitive inhibitors on [3H]8-OH-DPAT binding to 5-hydroxytryptamine1A receptor.](/cms/asset/8541aaff-faf8-459a-a261-193b3c3aa59b/iphb_a_401040_f0002_b.gif)
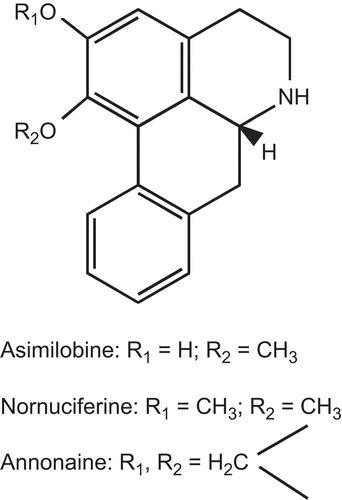
O-Methyldauricine (1) and popisidine (2) showed moderate inhibition with a Ki of 4.5 μM and 5.8 μM, respectively (); the Ki values were calculated using the best fit one-site binding model. This is higher than the inhibition constants of (±)-8-hydroxy-2-(di-n-propylamino)-tetralin [(±)-8-OH-DPAT] buspirone, and 5-hydroxytryptamine (5-HT) (). The values are several-fold higher than that of propanolol (0.14 μM), a 5HT1A/5HT2 receptor antagonist, but are similar to those of other structurally related isoquinoline alkaloids, such as annonaine, nornuciferine and asimilobine (less than 10 μM) () (CitationHasrat et al., 1997a).
Figure 3. Competitive inhibition on the binding of [3H]8-OH-DPAT to the 5-hydroxytryptamine1A receptor by O-methyldauricine and popisidine. Receptor membrane (35 μg protein/well) was incubated with [3H]8-OH-DPAT (0.4 nM) in the presence of O-methyldauricine and popisidine at 25°C for 90 min. Each data point is expressed as mean ± SD (n = 3).
![Figure 3. Competitive inhibition on the binding of [3H]8-OH-DPAT to the 5-hydroxytryptamine1A receptor by O-methyldauricine and popisidine. Receptor membrane (35 μg protein/well) was incubated with [3H]8-OH-DPAT (0.4 nM) in the presence of O-methyldauricine and popisidine at 25°C for 90 min. Each data point is expressed as mean ± SD (n = 3).](/cms/asset/5d45ebfc-1c64-43f4-a6d2-b528a34749aa/iphb_a_401040_f0003_b.gif)
Table 1. Ki values obtained from the competition experiments with standard unlabelled ligands on 5-HT1A receptor binding activity with [3H]8-OH-DPAT as the radioligand.
Indigenous medicine, related species have been used in other parts of the world. For example, P. fornicata Baill. (synonym, Monanthotaxis fornicata Baill.) is indicated for the treatments of mental diseases and snake bites in East Africa (CitationHedberg et al., 1982), while P. herenthas is used in Madagascar as febrifuge (CitationRasoanaivo et al., 1992). A phytochemical screening survey reported that P. odoardi contain several alkaloids which have not been isolated and identified (CitationTeo et al., 1990).
Both O-methyldauricine (1) and popisidine (2) belong to the group of bisbenzyltetrahydroisoquinoline alkaloids, which are derived biogenetically via phenol-oxidative coupling of two units of benzyltetrahydroisoquinoline alkaloids. The bisbenzlyisoquinoline alkaloids constitute one of the best-studied classes of natural products. They are reported to have a variety of biological activities, including antimalarial (CitationFrappier et al., 1996), cytotoxic (CitationAngerhofer et al., 1999), anti-HIV (CitationKashiwada et al., 2005), antitumor (CitationJossang et al., 1996), dopamine inhibitory (CitationCortes et al., 1992; CitationProtais et al., 1995), antihypertensive (CitationQian, 2002), antibacterial (CitationLohombo-Ekomba et al., 2004), and smooth muscle relaxant activity (CitationZafra-Polo et al., 1993). However, 5-HT1A receptor binding activity by bisbenzylisoquinoline alkaloids has not been reported. Structurally related isoquinoline derivatives, such as annonaine, nornuciferine, and asimilobine, isolated from the fruit of Annona muricata, have been shown to inhibit the binding of [3H]rauwolscine to 5-HT1A receptors in calf hippocampus (). The IC50 values for inhibition of receptor binding for annonaine, nornuciferine, and asimilobine were 3, 9, and 5 μM, respectively (CitationHasrat et al., 1997b), which are close to the Ki values of O-methyldauricine (1) (4.5 μM) and popisidine (2) (5.8 μM). In the functional assay, based on the inhibition of the accumulation of cAMP in NIH-3T3 cells stably transfected with 5-HT1A receptor from human, the Ki values for annonaine, nornuciferine, and asimilobine were estimated to be less than 10 μM (CitationHasrat et al., 1997a). In another study, the IC50 for the interaction of annonaine with the dopamine reuptake pump was reported to be 0.8 μM (CitationProtais et al., 1995). Therefore, these aporphines were suggested to be non-selective 5-HT1A receptor agonists. The IC50 of O-methyldauricine in a 3H-dopamine uptake binding assay has been reported to be 5.8 μM (CitationProtais et al., 1995), but O-methyldauricine is less active in the D1 and D2 dopamine receptor binding assays, with an IC50 of 100 μM (CitationCortes et al., 1992).
In summary, the findings suggest that O-methyldauricine (1) and popisidine (2) have moderate affinity towards cerebral cortical 5-HT1A receptors acting as agonists or antagonists, and may contribute to their CNS activities. Therefore, these compounds warrant further investigation for their potential therapeutic activity.
Acknowledgements
This project was partly supported by the Biotechnology Directorate, Ministry of Science, Technology and Innovation, Malaysia (IRPA 26-02-06-0127).
Declaration of interest: The authors alone are responsible for the content and writing of the paper.
References
- Angerhofer CK, Guinaudeau H, Wongpanich V, Pezzuto JM, Cordell GA (1999): Antiplasmodial and cytotoxic activity of natural bisbenzylisoquinoline alkaloids. J Nat Prod 62: 59–66.
- Burkill IH (1935): A Dictionary of the Economic Products of the Malay Peninsula. London, Crown Agents for the Colonies, pp. 1799–1800.
- Carli M, Afkhami-Dastjerdian S, Reader TA (1997): Effects of a chronic lithium treatment on cortical serotonin uptake sites and 5-HT1A receptors. Neurochem Res 22: 427–435.
- Cheng YC, Prusoff WH (1973): Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22: 3099–3108.
- Chuah C-H, Lee K-H, Goh S-H (2001): (–)-Popisabahnine, a bisbenzyltetrahydroisoquinoline alkaloid from Popowia pisocarpa (Annonaceae). Malays J Chem 3: 20–23.
- Chung PY, Chung LY, Ngeow YF, Goh SH, Imiyabir Z (2004): Antimicrobial activities of Malaysian plant species. Pharm Biol 42: 292–300.
- Chung LY, Goh SH, Imiyabir Z (2005a): Central nervous system receptor activities of some Malaysian plant species. Pharm Biol 43: 280–288.
- Chung LY, Goh SH, Majalap N (2006): Activity profiles of Malaysian plant species on some G-protein coupled receptors, in: Singh VK, Govil JN, Ahmad K, Sharma RKR, eds. Recent Progress in Medicinal Plants, Vol. 15, Natural Products. Houston, TX, Studium Press, pp. 291–308.
- Chung LY, Yap KF, Mustafa MR, Goh SH, Imiyabir Z (2005b): Muscarinic receptor activity of some Malaysian plant species. Pharm Biol 43: 672–682.
- Chung LY, Yap KF, Goh SH, Mustafa MR, Imiyabir Z (2008): Muscarinic receptor binding activity of polyoxygenated flavones from Melicope subunifoliolata. Phytochemistry 69: 1548–1554.
- Cortes D, Figadere B, Saez J, Protais P (1992): Displacement activity of bisbenzylisoquinoline alkaloids at striatal 3H-SCH 23390 and 3H-raclopride binding sites. J Nat Prod 55: 1281–1286.
- Frappier F, Jossang A, Soudon J, Calvo F, Rasoanaivo P, Ratsimamanga-urverg, Saez J, Schrevel J, Grellier P (1996): Bisbenzylisoquinolines as modulators of chloroquine resistance in Plasmodium falciparum and multidrug resistance in tumor cells. Antimicrob Agents Chemother 40: 1476–1481.
- Gunasekara NS, Spencer CM, Keating GM (2002): Ziprasidone: A review of its use in schizophrenia and schizoaffective disorder. Drugs 62: 1217–1251.
- Harms A, Gündish D, Müller C, Kovar K (2000): Development of a 5-hydroxytryptamine2A receptor binding assay for high throughput screening using 96-well microfilter plates. J Biomol Screen 5: 269–277.
- Hasrat JA, De Bruyne T, De Backer JP, Vauquelin G, Vlietinck AJ (1997a): Isoquinoline derivatives isolated from the fruit of Annona muricata as 5-HTergic 5-HT1A receptor agonists in rats: Unexploited antidepressive (lead) products. J Pharm Pharmacol 49: 1145–1149.
- Hasrat JA, Pieters L, De Backer J-P, Vauquelin G, Vlietinck AJ (1997b): Screening of medicinal plants from Suriname for 5-HT1A ligands: Bioactive isoquinoline alkaloids from the fruit of Annona muricata. Phytomedicine 4: 133–140.
- Hedberg I, Hedberg O, Madati PJ, Mshigeni KE, Mshiu EN, Samuelsson G (1982): Inventory of plant used in traditional medicine in Tanzania. I. Plants of the families Acanthaceae–Cucurbitaceae. J Ethnopharmacol 6: 29–60.
- Hensler JG (2003): Regulation of 5-HT1A receptor function in brain following agonist or antidepressant administration. Life Sci 72: 1665–1682.
- Hoyer D, Hannon JP, Martin GR (2002): Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav 71: 533–554.
- Jossang A, Cavé A, Saez J, Bartoli MH, Cavé A, Jossang P (1996): Two highly populated conformations at room temperature of Monterine and Granjine, antitumor bisbenzylisoquinoline alkaloids origin and tridimensional structures. J Org Chem 61: 3023–3030.
- Jossang A, Leboeuf M, Cavé A, Sevenet T (1986a): Alkaloids of the Annonaceae. Part 65. Alkaloids of Popowia pisocarpa, Part 1. New bisbenzylisoquinolines. J Nat Prod 49: 1018–1027.
- Jossang A, Leboeuf M, Cavé A, Sevenet T (1986b): Alkaloids of the Annonaceae. Part 66. Alkaloids of Popowia pisocarpa, Part 2. New bisaporphinoids. J Nat Prod 49: 1028–1035.
- Kashiwada Y, Aoshima A, Ikeshiro Y, Chen Y-P, Furukawa H, Itoigawa M, Fujioka T, Mihashi K, Cosentino LM, Morris-Natschke SL, Lee K-H (2005): Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera and structure-activity correlations with related alkaloids. Bioorg Med Chem 13: 443–448.
- Lohombo-Ekomba ML, Okusa PN, Penge O, Kabongo C, Choudhary IM, Kasende OE (2004): Antibacterial, antifungal, antiplasmodial and cytotoxic activities of Albertisia villosa. J Ethnopharmacol 93: 331–335.
- Nénonéné EK, Radja F, Carli M, Grondin L, Reader TA (1994): Heterogeneity of cortical and hippocampal 5-HT1A receptors: A reappraisal of homogenate binding with 8-[3H]Hydroxydipropylaminotetralin. J Neurochem 62: 1822–1834.
- Nénonéné EK, Radja F, Carli M, Van Gelder NM, Afkhami-Dastjerdian S, Reader TA (1996): Alklylation of [3H]8-OH-DPAT binding sites in rat cerebral cortex and hippocampus. Neurochem Res 21: 167–176.
- Protais P, Arbaoui J, Bakkali EH, Bermejo A, Cortes D (1995): Effect of various isoquinoline alkaloids on in vitro 3H-dopamine uptake by rat striatal synaptosomes. J Nat Prod 58: 1475–1484.
- Qian JQ (2002): Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol Sin 23: 1086–1092.
- Rasoanaivo P, Petitjean A, Ratsimamanga-Urverg S, Pakoto-Ratsimamanga A (1992): Medicinal plants used to treat malaria in Madagascar. J Ethnopharmacol 37: 117–127.
- Schechter LE, Dawson LA, Harder JA (2002): The potential utility of 5-HT1A receptor antagonists in the treatment of cognitive dysfunction associated with Alzheimer’s disease. Curr Pharm Des 8: 139–145.
- Soo WK (2006): Development and application of 96-well microplate lipoxygenase and 5-HT1A receptor screening assays for bioactive extracts and compounds from Malaysian plants. MSc Thesis. Department of Pharmacy, Faculty of Medicine, University of Malaya, pp. 109–147.
- Teo LE, Pachiaper G, Chan KC, Hadi HA, Weber JF, Deverre JR, David B, Sevenet T (1990): A new phytochemical survey of Malaysia. V. Preliminary screening and plant chemical studies. J Ethnopharmacol 28: 63–101.
- Waterman PG, Pootakahm K (1979): Chemical studies on the Annonaceae. V. The flavonoids of the fruit of Popowia cauliflora Chipp. Planta Med 35: 366–369.
- Zafra-Polo MC, Tormos MJ, Cortes D, Anselmi E (1993): Comparative study of the rat uterine smooth muscle relaxant activity of three bisbenzyltetrahydroisoquinolines with tetrandrine. J Pharm Pharmacol 45: 563–566.
- Zhang JH, Chung TDY, Oldenburg KR (1999): A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4: 67–73.